Abstract
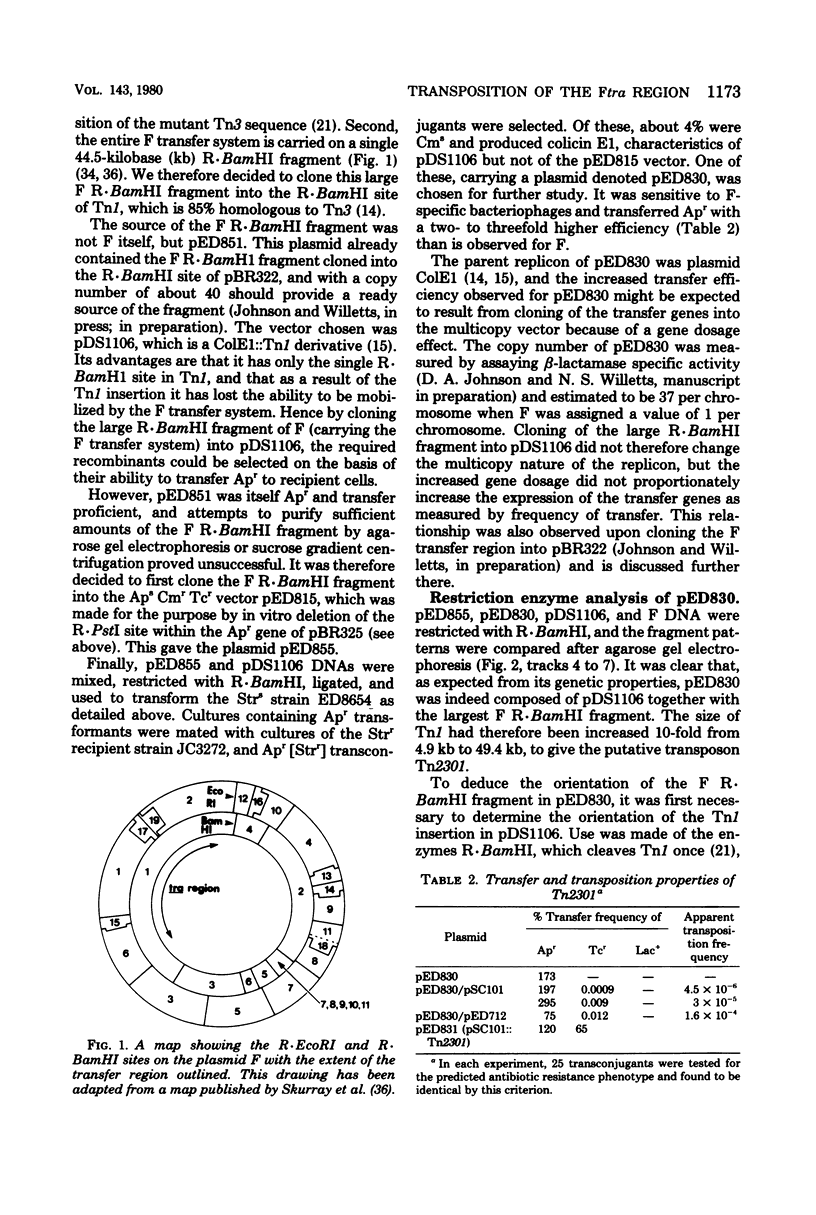
The largest R . BamHI fragment of the plasmid F, which carries the entire F conjugation system, has been cloned into the single R . BamHI site of the ampicillin (Ap) resistance transposon TN1. pDS1106 (ColE1 mob::Tn1) was the vector plasmid, and the resultant conjugative plasmid, pED830, was characterized both genetically and by restriction enzyme analysis. The transposon construct, denoted Tn2301, was transposable at frequencies similar to Tn1 to small nonconjugative plasmids or to the Escherichia coli host chromosome. In the former case, Apr conjugative plasmids were obtained, whereas in the latter case, Hfr strains resulted. Representative Hfr strains were characterized by quantitative and interrupted mating experiments. Extension of this technique for Hfvr formation should aid chromosome mapping both in E. coli and in other bacterial genera.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P. M., Richmond M. H. Translocation of a discrete piece of deoxyribonucleic acid carrying an amp gene between replicons in Eschericha coli. J Bacteriol. 1976 Apr;126(1):1–6. doi: 10.1128/jb.126.1.1-6.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Recircularization and autonomous replication of a sheared R-factor DNA segment in Escherichia coli transformants. Proc Natl Acad Sci U S A. 1973 May;70(5):1293–1297. doi: 10.1073/pnas.70.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deonier R. C., Mirels L. Excision of F plasmid sequences by recombination at directly repeated insertion sequence 2 elements: involvement of recA. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3965–3969. doi: 10.1073/pnas.74.9.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan G., Saul M., Twigg A., Gill R., Sherratt D. Polypeptides expressed in Escherichia coli K-12 minicells by transposition elements Tn1 and Tn3. J Bacteriol. 1979 Apr;138(1):48–54. doi: 10.1128/jb.138.1.48-54.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan G., Sherratt D. The transposon Tn1 as a probe for studying ColE1 structure and function. Mol Gen Genet. 1977 Mar 7;151(2):151–160. doi: 10.1007/BF00338689. [DOI] [PubMed] [Google Scholar]
- Finnegan D. J., Willetts N. S. Two classes of Flac mutants insensitive to transfer inhibition by an F-like R factor. Mol Gen Genet. 1971;111(3):256–264. doi: 10.1007/BF00433110. [DOI] [PubMed] [Google Scholar]
- Finnegan D., Willetts N. The nature of the transfer inhibitor of several F-like plasmids. Mol Gen Genet. 1972;119(1):57–66. doi: 10.1007/BF00270444. [DOI] [PubMed] [Google Scholar]
- Gasson M. J., Willetts N. S. Further characterization of the F fertility inhibition systems of "unusual" Fin+ plasmids. J Bacteriol. 1977 Aug;131(2):413–420. doi: 10.1128/jb.131.2.413-420.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill R., Heffron F., Dougan G., Falkow S. Analysis of sequences transposed by complementation of two classes of transposition-deficient mutants of Tn3. J Bacteriol. 1978 Nov;136(2):742–756. doi: 10.1128/jb.136.2.742-756.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W., Lindenmaier W., Pfeifer F., Schrempf H., Schelle B. Transposition and insertion of intact, deleted and enlarged ampicillin transposon Tn3 from mini-R1 (Rsc) plasmids into transfer factors. Mol Gen Genet. 1977 Nov 29;157(2):119–129. doi: 10.1007/BF00267389. [DOI] [PubMed] [Google Scholar]
- Heffron F., Bedinger P., Champoux J. J., Falkow S. Deletions affecting the transposition of an antibiotic resistance gene. Proc Natl Acad Sci U S A. 1977 Feb;74(2):702–706. doi: 10.1073/pnas.74.2.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Translocation of a plasmid DNA sequence which mediates ampicillin resistance: molecular nature and specificity of insertion. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3623–3627. doi: 10.1073/pnas.72.9.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W. Plasmids that mobilize bacterial chromosome. Plasmid. 1979 Jan;2(1):1–19. doi: 10.1016/0147-619x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- Hu S., Ohtsubo E., Davidson N. Electron microscopic heteroduplex studies of sequence relations among plasmids of Escherichia coli: structure of F13 and related F-primes. J Bacteriol. 1975 May;122(2):749–763. doi: 10.1128/jb.122.2.749-763.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. Temperature-sensitive mutants for the replication of plasmids in Escherichia coli: requirement for deoxyribonucleic acid polymerase I in the replication of the plasmid ColE 1 . J Bacteriol. 1973 Jun;114(3):1116–1124. doi: 10.1128/jb.114.3.1116-1124.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- LOW B., WOOD T. H. A QUICK AND EFFICIENT METHOD FOR INTERRUPTION OF BACTERIAL CONJUGATION. Genet Res. 1965 Jul;6:300–303. doi: 10.1017/s001667230000416x. [DOI] [PubMed] [Google Scholar]
- Lane D., Gardner R. C. Second EcoRI fragment of F capable of self-replication. J Bacteriol. 1979 Jul;139(1):141–151. doi: 10.1128/jb.139.1.141-151.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matney T. S., Goldschmidt E. P., Erwin N. S., Scroggs R. A. A preliminary map of genomic sites for F-attachment in Escherichia coli K12. Biochem Biophys Res Commun. 1964 Oct 14;17(3):278–281. doi: 10.1016/0006-291x(64)90397-3. [DOI] [PubMed] [Google Scholar]
- McIntire S., Willetts N. Plasmid cointegrates of Flac and lambda prophage. J Bacteriol. 1978 Apr;134(1):184–192. doi: 10.1128/jb.134.1.184-192.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G. E., Ausubel F. M., Cannon F. C. Physical map of chromosomal nitrogen fixation (nif) genes of Klebsiella pneumoniae. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2866–2870. doi: 10.1073/pnas.76.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurray R. A., Nagaishi H., Clark A. J. Construction and BamHL analysis of chimeric plasmids containing EcoRL DNA fragments of the F sex factor. Plasmid. 1978 Feb;1(2):174–186. doi: 10.1016/0147-619x(78)90037-9. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., McIntire S. Isolation and characterisation of lambdatra transducing phages from EDFL223 (Flac traB::EDlambda4). J Mol Biol. 1978 Dec 15;126(3):525–549. doi: 10.1016/0022-2836(78)90057-8. [DOI] [PubMed] [Google Scholar]