Abstract
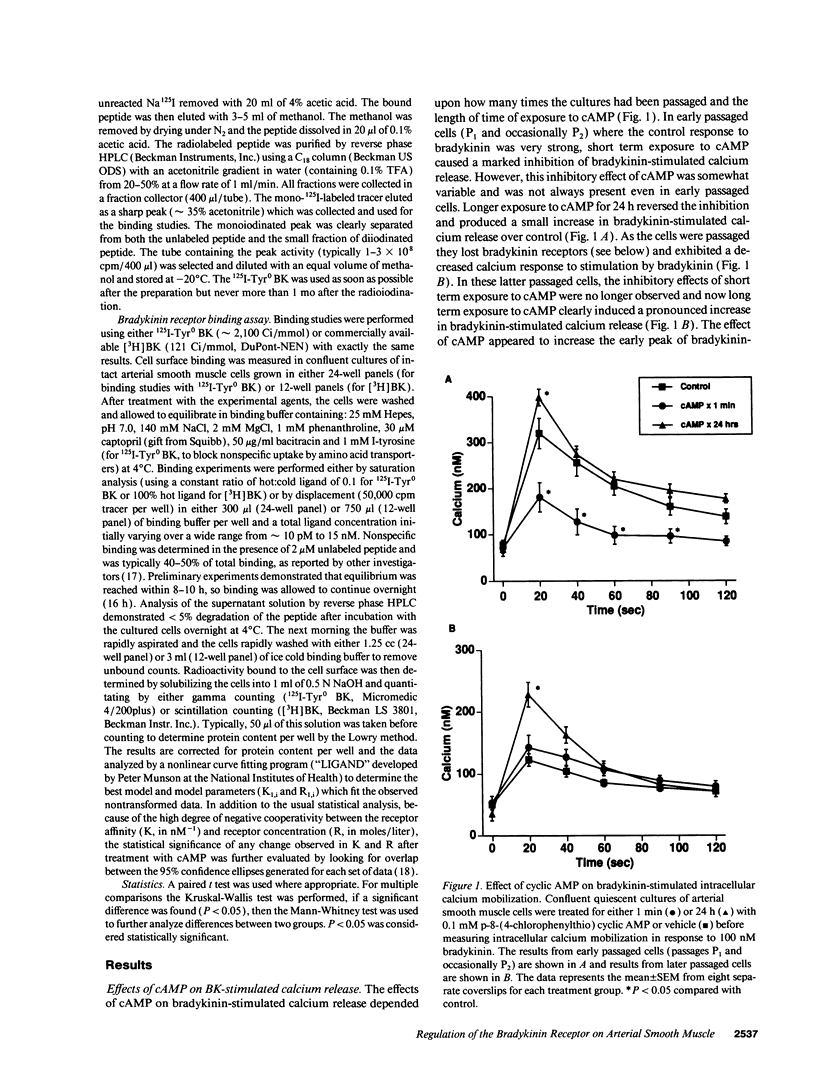
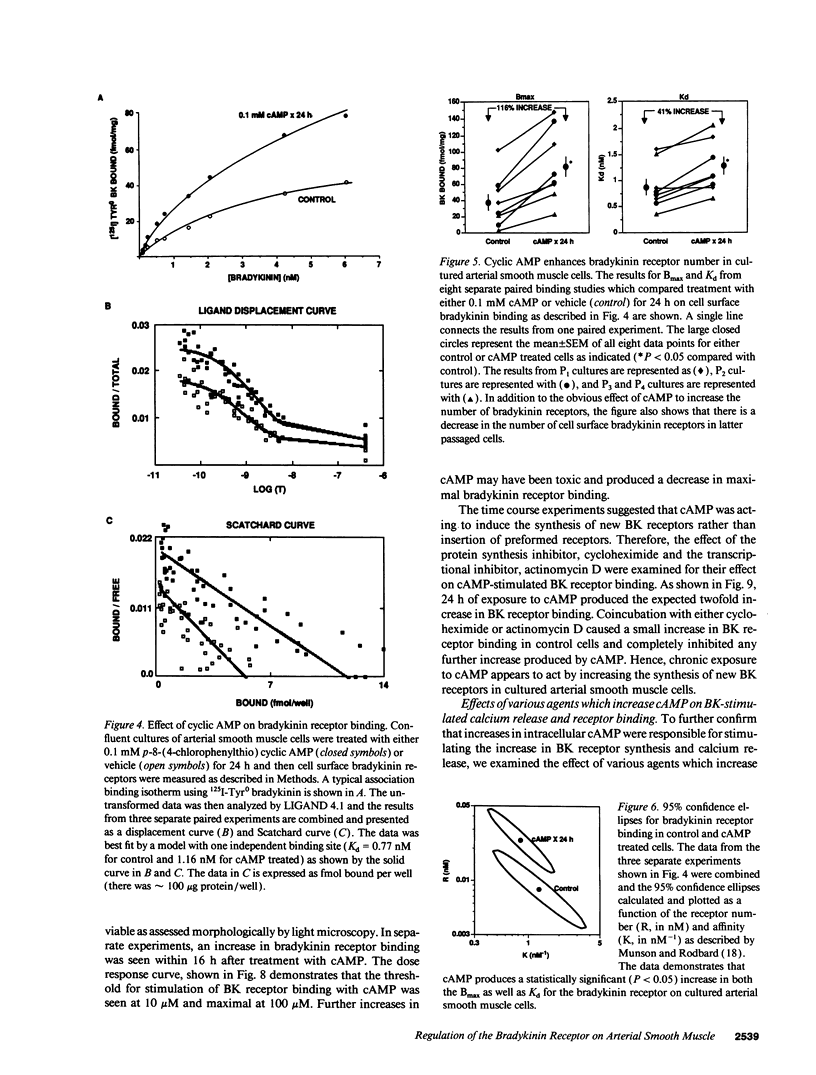
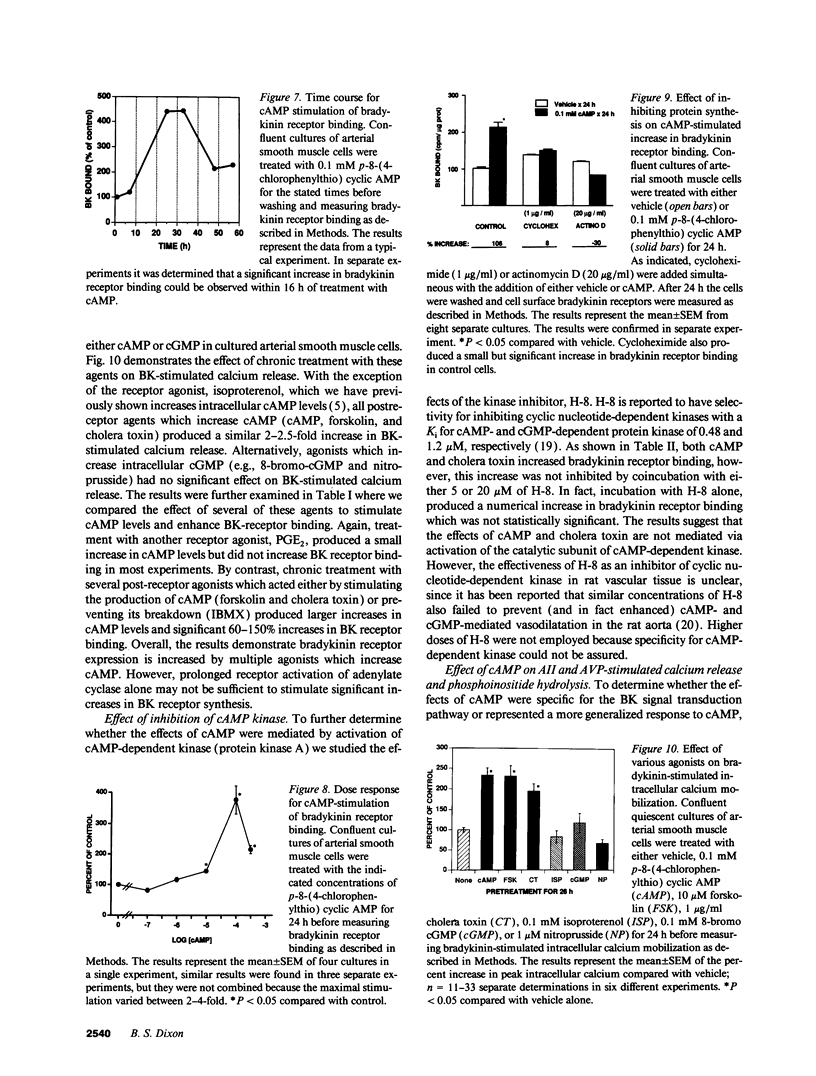
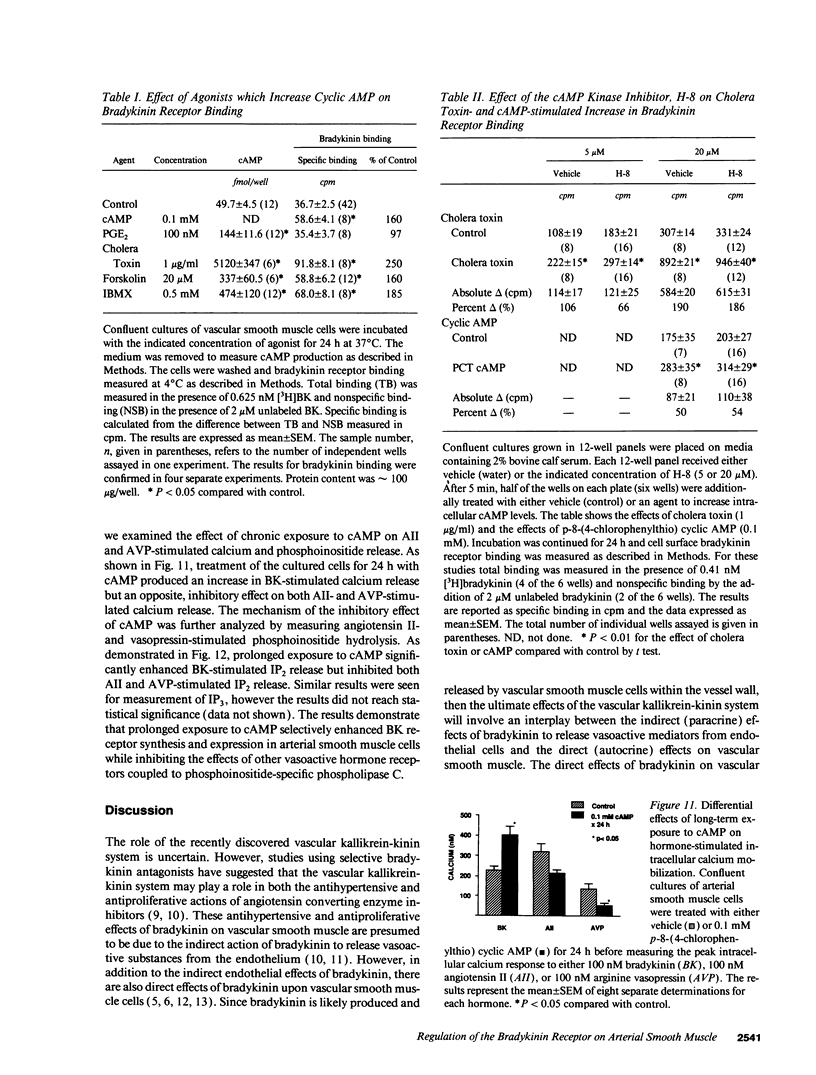
Bradykinin receptors on vascular smooth muscle may play an important role in regulating the endogenous effects of the vascular kallikrein-kinin system. The present study examined the effect of cyclic nucleotides on bradykinin-stimulated responses in cultured arterial smooth muscle cells. Short term stimulation (1 min) with cyclic AMP produced a variable inhibition of bradykinin-stimulated calcium mobilization which was lost in later passaged cells. However, long-term stimulation (24 h) produced a consistent increase in bradykinin-stimulated calcium mobilization in both early and late passaged cells. Further analysis demonstrated that chronic exposure to cAMP produced a twofold increase in both the number of cell surface bradykinin receptors and in bradykinin-stimulated phosphoinositide hydrolysis. The increase in bradykinin receptors was time dependent (> 7 h) and blocked by protein synthesis inhibitors, suggesting that cAMP enhanced the synthesis of new bradykinin receptors. The increase in bradykinin receptor binding and calcium mobilization was also stimulated by cholera toxin, forskolin, and isobutylmethylxanthine, but not isoproterenol or prostaglandin E2. Of considerable interest, prolonged exposure to cAMP inhibited both angiotensin II and arginine vasopressin-stimulated phosphoinositide hydrolysis and intracellular calcium mobilization. In summary, prolonged treatment with cAMP selectively stimulates the synthesis and expression of bradykinin receptors on arterial smooth muscle while decreasing the responsiveness to vasoconstrictor agonists such as angiotensin II and vasopressin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag G., McCormick F. Regulators and effectors of ras proteins. Annu Rev Cell Biol. 1991;7:601–632. doi: 10.1146/annurev.cb.07.110191.003125. [DOI] [PubMed] [Google Scholar]
- Bolton A. E. Comparative methods for the radiolabeling of peptides. Methods Enzymol. 1986;124:18–29. doi: 10.1016/0076-6879(86)24004-5. [DOI] [PubMed] [Google Scholar]
- Carbonell L. F., Carretero O. A., Stewart J. M., Scicli A. G. Effect of a kinin antagonist on the acute antihypertensive activity of enalaprilat in severe hypertension. Hypertension. 1988 Mar;11(3):239–243. doi: 10.1161/01.hyp.11.3.239. [DOI] [PubMed] [Google Scholar]
- Colucci W. S. Adenosine 3',5'-cyclic-monophosphate-dependent regulation of alpha 1-adrenergic receptor number in rabbit aortic smooth muscle cells. Circ Res. 1986 Feb;58(2):292–297. doi: 10.1161/01.res.58.2.292. [DOI] [PubMed] [Google Scholar]
- Daugirdas J. T., Zhou H. L., Tamulaitis V. V., Nutting C. W., Fiscus R. R. Effect of H-8, an isoquinolinesulfonamide inhibitor of cyclic nucleotide-dependent protein kinase, on cAMP- and cGMP-mediated vasorelaxation. Blood Vessels. 1991;28(5):366–371. doi: 10.1159/000158883. [DOI] [PubMed] [Google Scholar]
- Derian C. K., Moskowitz M. A. Polyphosphoinositide hydrolysis in endothelial cells and carotid artery segments. Bradykinin-2 receptor stimulation is calcium-independent. J Biol Chem. 1986 Mar 15;261(8):3831–3837. [PubMed] [Google Scholar]
- Dixon B. S., Breckon R., Fortune J., Vavrek R. J., Stewart J. M., Marzec-Calvert R., Linas S. L. Effects of kinins on cultured arterial smooth muscle. Am J Physiol. 1990 Feb;258(2 Pt 1):C299–C308. doi: 10.1152/ajpcell.1990.258.2.C299. [DOI] [PubMed] [Google Scholar]
- Dixon B. S., Sharma R. V., Dickerson T., Fortune J. Bradykinin and angiotensin II: activation of protein kinase C in arterial smooth muscle. Am J Physiol. 1994 May;266(5 Pt 1):C1406–C1420. doi: 10.1152/ajpcell.1994.266.5.C1406. [DOI] [PubMed] [Google Scholar]
- Downward J., de Gunzburg J., Riehl R., Weinberg R. A. p21ras-induced responsiveness of phosphatidylinositol turnover to bradykinin is a receptor number effect. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5774–5778. doi: 10.1073/pnas.85.16.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhy R. D., Carretero O. A., Ho K. L., Scicli A. G. Role of kinins and nitric oxide in the effects of angiotensin converting enzyme inhibitors on neointima formation. Circ Res. 1993 Jun;72(6):1202–1210. doi: 10.1161/01.res.72.6.1202. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Tawada Y., Shigekawa M. Regulation of the plasma membrane Ca2+ pump by cyclic nucleotides in cultured vascular smooth muscle cells. J Biol Chem. 1988 Jun 15;263(17):8058–8065. [PubMed] [Google Scholar]
- Graier W. F., Groschner K., Schmidt K., Kukovetz W. R. Increases in endothelial cyclic AMP levels amplify agonist-induced formation of endothelium-derived relaxing factor (EDRF). Biochem J. 1992 Dec 1;288(Pt 2):345–349. doi: 10.1042/bj2880345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon G., Gallo-Payet N., Balestre M. N., Lombard C. Cholera-toxin and corticotropin modulation of inositol phosphate accumulation induced by vasopressin and angiotensin II in rat glomerulosa cells. Biochem J. 1988 Aug 1;253(3):765–775. doi: 10.1042/bj2530765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Imai A., Gershengorn M. C. Evidence for tight coupling of thyrotropin-releasing hormone receptors to stimulated inositol trisphosphate formation in rat pituitary cells. J Biol Chem. 1985 Sep 5;260(19):10536–10540. [PubMed] [Google Scholar]
- Lincoln T. M., Cornwell T. L., Taylor A. E. cGMP-dependent protein kinase mediates the reduction of Ca2+ by cAMP in vascular smooth muscle cells. Am J Physiol. 1990 Mar;258(3 Pt 1):C399–C407. doi: 10.1152/ajpcell.1990.258.3.C399. [DOI] [PubMed] [Google Scholar]
- Majerus P. W. Inositol phosphate biochemistry. Annu Rev Biochem. 1992;61:225–250. doi: 10.1146/annurev.bi.61.070192.001301. [DOI] [PubMed] [Google Scholar]
- McAtee P., Dawson G. Phospholipase C activity in NCB-20 cells is inhibited by protein kinase A-mediated phosphorylation of low molecular mass GTP-binding proteins. J Biol Chem. 1990 Apr 25;265(12):6788–6793. [PubMed] [Google Scholar]
- McDaniel N. L., Rembold C. M., Richard H. M., Murphy R. A. Cyclic AMP relaxes swine arterial smooth muscle predominantly by decreasing cell Ca2+ concentration. J Physiol. 1991 Aug;439:147–160. doi: 10.1113/jphysiol.1991.sp018661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachern A. E., Shelton E. R., Bhakta S., Obernolte R., Bach C., Zuppan P., Fujisaki J., Aldrich R. W., Jarnagin K. Expression cloning of a rat B2 bradykinin receptor. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7724–7728. doi: 10.1073/pnas.88.17.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan-Boyd R., Stewart J. M., Vavrek R. J., Hassid A. Effects of bradykinin and angiotensin II on intracellular Ca2+ dynamics in endothelial cells. Am J Physiol. 1987 Oct;253(4 Pt 1):C588–C598. doi: 10.1152/ajpcell.1987.253.4.C588. [DOI] [PubMed] [Google Scholar]
- Nolly H., Scicli A. G., Scicli G., Carretero O. A. Characterization of a kininogenase from rat vascular tissue resembling tissue kallikrein. Circ Res. 1985 Jun;56(6):816–821. doi: 10.1161/01.res.56.6.816. [DOI] [PubMed] [Google Scholar]
- Odya C. E., Goodfriend T. L., Peña C. Bradykinin receptor-like binding studied with iodinated analogues. Biochem Pharmacol. 1980 Feb;29(2):175–185. doi: 10.1016/0006-2952(80)90326-3. [DOI] [PubMed] [Google Scholar]
- Oza N. B., Schwartz J. H., Goud H. D., Levinsky N. G. Rat aortic smooth muscle cells in culture express kallikrein, kininogen, and bradykininase activity. J Clin Invest. 1990 Feb;85(2):597–600. doi: 10.1172/JCI114479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipili E., Manolopoulos V. G., Catravas J. D., Maragoudakis M. E. Angiotensin converting enzyme activity is present in the endothelium-denuded aorta. Br J Pharmacol. 1989 Oct;98(2):333–335. doi: 10.1111/j.1476-5381.1989.tb12599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M. Cyclic guanosine monophosphate inhibition of contraction may be mediated through inhibition of phosphatidylinositol hydrolysis in rat aorta. Circ Res. 1986 Mar;58(3):407–410. doi: 10.1161/01.res.58.3.407. [DOI] [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988 Jul 5;263(19):9063–9066. [PubMed] [Google Scholar]
- Saed G. M., Carretero O. A., MacDonald R. J., Scicli A. G. Kallikrein messenger RNA in rat arteries and veins. Circ Res. 1990 Aug;67(2):510–516. doi: 10.1161/01.res.67.2.510. [DOI] [PubMed] [Google Scholar]
- Supattapone S., Danoff S. K., Theibert A., Joseph S. K., Steiner J., Snyder S. H. Cyclic AMP-dependent phosphorylation of a brain inositol trisphosphate receptor decreases its release of calcium. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8747–8750. doi: 10.1073/pnas.85.22.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda N. Actions of bradykinin on isolated cerebral and peripheral arteries. Am J Physiol. 1977 Mar;232(3):H267–H274. doi: 10.1152/ajpheart.1977.232.3.H267. [DOI] [PubMed] [Google Scholar]
- Toda N., Bian K., Akiba T., Okamura T. Heterogeneity in mechanisms of bradykinin action in canine isolated blood vessels. Eur J Pharmacol. 1987 Mar 31;135(3):321–329. doi: 10.1016/0014-2999(87)90681-9. [DOI] [PubMed] [Google Scholar]
- Twort C. H., van Breemen C. Cyclic guanosine monophosphate-enhanced sequestration of Ca2+ by sarcoplasmic reticulum in vascular smooth muscle. Circ Res. 1988 May;62(5):961–964. doi: 10.1161/01.res.62.5.961. [DOI] [PubMed] [Google Scholar]
- Vane J. R., Anggård E. E., Botting R. M. Regulatory functions of the vascular endothelium. N Engl J Med. 1990 Jul 5;323(1):27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]




