Abstract
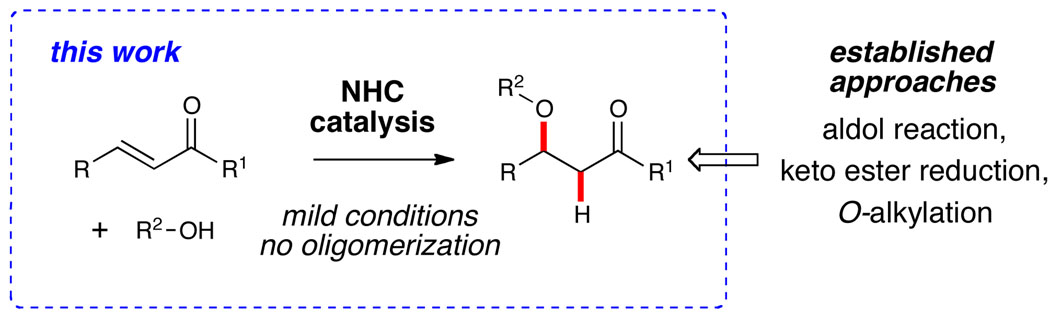
An efficient intermolecular conjugate addition of alcohols to activated alkenes catalyzed by N-heterocyclic carbenes has been developed. With 5 mol % of the free carbene derived from IMes•HCl, unsaturated ketones and esters are competent substrates and a variety of primary and secondary alcohols can be employed as the nucleophile. No oligomerization is observed under these mild conditions for effective hydroalkoxylation. In addition to reactions with activated alkenes, IMes catalyzes the formation of vinyl ethers through the 1,4-addition of alcohols to ynones and promotes tandem conjugate addition/Michael cascade reactions. Preliminary data supports a Brønsted base mechanism with the free carbene.
The carbon-oxygen bond is a key component of biologically active compounds. Classical synthetic approaches to these prevalent linkages include substitution reactions employing a highly nucleophilic alkoxide (e.g., Williamson ether synthesis).1 The direct formation of these bonds through the intermolecular 1,4-addition of alcohols to conjugate acceptors is a logical approach, but remains challenging due to potential oligomerization of the acceptor and the inherent energetic aspects with this process.2 A catalytic approach to this important structural motif is a) desirable due to the reduction of potential waste, and b) opens possibilities to control the overall stereochemistry by means other than traditional approaches. While the addition of metal alkoxides (generated with a full equiv of base) to related nitroalkenes has been successful,3 the catalytic addition of alcohols to α,β-unsaturated ketones in a bimolecular process is much more unusual.4
Verkade has shown that proazaphosphatranes promote this transformation and Toste and Bergman have reported a mechanistically intriguing phosphine-catalyzed hydroalkoxylation of simple activated olefins.5 A broader scope for this process with possibility of stereocontrol and/or chemoselectivity would allow for direct and catalytic access to a wide variety of useful β-alkoxy carbonyl compounds. Recently, N-heterocyclic carbenes (NHCs) have emerged as powerful Lewis bases and our program in this area continues to explore new possibilities with these highly versatile catalysts.6,7 While the Lewis basic properties of NHCs are firmly established, their potential as Brønsted base catalysts is only just emerging.8 Nolan, as well as Waymouth and Hedrick, independently demonstrated that NHCs catalyze transesterification reactions,9 but new processes that capitalize on this Brønsted base characteristic of NHCs remain limited. In this Communication, we report an efficient carbene-catalyzed intermolecular conjugate addition of alcohols to activated alkenes.
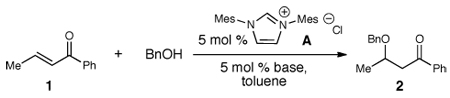
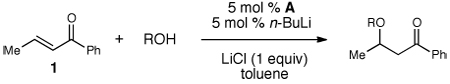
We began our investigation with 5 mol % loading of azolium salt A (IMes•HCl) and then surveying different bases in a 1:1 ratio relative to A.10 Aryl ketone 1 was employed as the conjugate acceptor with benzyl alcohol as the nucleophile in toluene. A weak base such as triethylamine provided no product (entry 1), but KHMDS generated a 70% yield (GC) of β-benzyloxy ketone (2, entry 2). Employing NaH led to a decrease in yield, but n-BuLi in combination with A led to a measurable increase in reaction efficiency (88%). To probe the impact of lithium counter ion further, the addition of 1 equiv of 12-crown-4 was accompanied with an observed decrease in yield (74%). Based on this result, one equiv of LiCl was added (entry 6), which resulted in a 95% yield of the desired β-alkoxy ketone (2).11
With efficient reaction conditions in hand, we surveyed a variety of alcohols in combination with ketone 1. A variety of primary and secondary alcohols are competent nucleophiles in this carbene-catalyzed reaction. The addition of allyl alcohol affords the product in excellent yield. Additionally, p-methoxybenzyl alcohol and 2-(trimethylsilyl)ethanol were also well tolerated. The addition of N-BOC serine tert-butyl ester at the hydroxyl group is successful in the presence of the carbamate (entry 8). Addition of the secondary alcohol 1-(naphthalen-1-yl)ethanol to the parent enone is successful (75% yield) but with no diastereoselectivity observed to date. To date, tertiary alcohols are not competent nucleophiles under the current conditions.
We then set out to determine the substrate scope with respect to conjugate acceptors (Table 3). Replacing the phenyl ring on the ketone with a more electron-deficient aryl rings provided the desired alcohol in good yield (73%, entry 1). Alkyl ketones are also accommodated under the reaction conditions (entry 2), as well are highly reactive conjugate acceptors such as methyl vinyl ketone (entry 3). This particular example underscores the unusual and useful aspects of this reaction given that no polymerization is observed with this highly reactive conjugate acceptor. Additions to esters also proceed in moderate yield, but substrates with aryl substituents in the β-position do not afford desired products (not shown). Based on this profile, a differentially substituted divinyl ketone was subjected to the reaction conditions and provided a regioselective addition in 82% yield (entry 6). Cyclohexenone and cyclopentenone provided ~50% conversion under the reaction conditions (not shown). The differential reactivity exhibited by acyclic and cyclic substrates is currently under investigation.
Table 3.
Survey of Conjugate Acceptorsa
 | ||||
|---|---|---|---|---|
| entry | R1 | R2 | product | yield(%)b |
| 1 | Me | 4-Cl-C6H4 | 12 | 73 |
| 2 | Me | Et | 13 | 82 |
| 3 | H | Me | 14 | 50 |
| 4 | Me | OBn | 15 | 60 |
| 5 | CH3(CH2)4 | Ph | 16 | 70 |
| 6 | Me | 17 | 82 | |
See Supporting Information for reaction details.
Isolated yields.
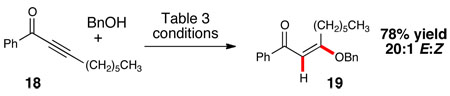
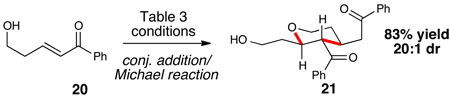
With the reaction components of this reaction initially charted, we sought to capitalize on the unusual aspects of this NHC-alcohol combination and expand the scope beyond general enones. The addition of alcohols to β-substituted ynones to generate vinyl ethers with selectivity favoring one olefin geometry would be a useful transformation.12 Gratifyingly, benzyl alcohol underwent 1,4-addition to ynone 18 in good yield and excellent selectivity (eq. 1). Further exploration found that the initial conjugate addition of alcohol 20 can be coupled to an intramolecular Michael reaction to generate tetrahydropyran 21 with excellent diastereoselectivity (eq. 2).
 |
(1) |
 |
(2) |
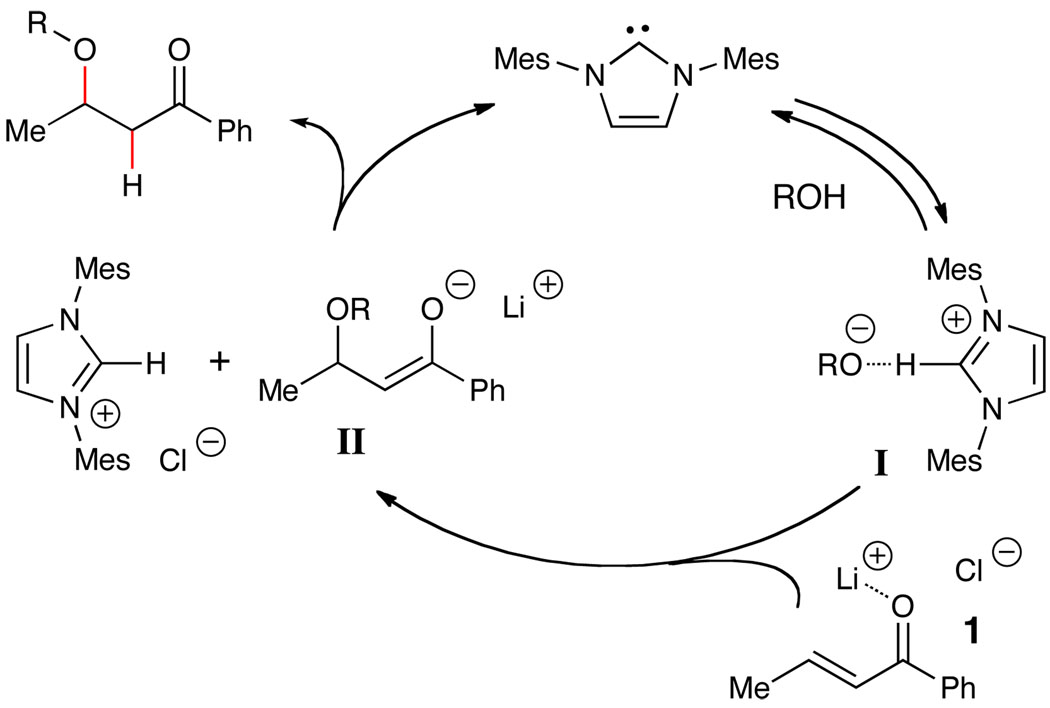
Our current understanding of this reaction is illustrated in Scheme 3. While NHCs are known to undergo additions to activated alkenes via a conjugate addition manifold,13 we currently favor a Brønsted base-catalyzed process. Consideration of previous work by Movassaghi14 in combination with our own studies prompted us to invoke a NHC-alcohol complex as a key intermediate (I, Scheme 2).15 This complex facilitates the 1,4-addition of the alcohol to the conjugate acceptor generating an imidazolium ion and the corresponding enolate (II), presumably stabilized by the addition of a lithium counter ion. Subsequent protonation of the enolate affords the β-alkoxy ketone and regenerates the NHC.16,17 In contrast to reactions performed under anionic conditions, these mild conditions attenuate unwanted side reactions such as oligomerization of the enone or aldol condensations at ambient temperature.18
Scheme 2.
Proposed Pathway
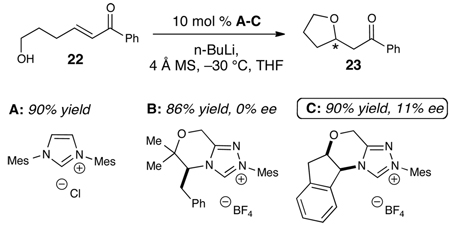
An enantioselective version of this reaction would be useful, but is challenging given the control of the stereochemistry and potential reversibility. To date, all studies with intermolecular variants of this process with chiral NHC catalysts have afforded racemic products. However, we then surveyed a variety of azolium precatalysts (A–C) with a substrate capable of intramolecular cyclization (22). While all the yields for this reaction are high (>85%), the reaction with azolium B19 produces a modest, but reproducible, 10–11% ee at −30 °C for this NHC-catalyzed conjugate addition (eq. 3).20 Reducing the reaction temperature to −78 °C provided much lower levels of conversion and did not improve the enantioselectivity of the process. While this observed selectivity is not synthetically practical as of yet, these results do support a pathway involving the NHC•alcohol complex depicted in Scheme 2. Further exploration and development of this catalytic asymmetric conjugate addition is underway.
 |
(3) |
In summary, we have discovered a novel conjugate addition of alcohols that demonstrates carbenes as efficient Brønsted base catalysts. A free carbene with the addition of lithium chloride allows for the addition of alcohols to enones in good to excellent yields at ambient temperature and with no oligomerization of the substrates. This new carbene-alcohol combination does not require stoichiometric amounts of strong base and also extends beyond additions to enones. The formation of vinyl ethers is possible through the 1,4-addition of alcohols to ynones and a tandem conjugate addition/Michael reaction occurs to generate a tetrahydropyran with good selectivity. Studies directed toward enhancing asymmetric induction and exploring further the mechanism are currently ongoing. This particular development of N-heterocyclic carbenes as efficient and unusual Brønsted base catalysts creates new opportunities for future direct bond forming processes.
Supplementary Material
Scheme 1.
NHC-Catalyzed Conjugate Additions
Table 1.
Conjugate Addition Optimizationa
 | |||
|---|---|---|---|
| entry | base | additive | GC yield (%)c |
| 1 | Et3N | – | NR |
| 2 | KHMDS | – | 70 |
| 3 | NaH | – | 60 |
| 4 | n-BuLib | – | 88 |
| 5 | n-BuLib | 12-crown-4 | 74 |
| 6 | n-BuLib | LiCl (1 equiv) | 95 |
Reactions run on 0.4 mmol scale.
A and base were combined in THF at −78 °C and warmed to 20 °C. THF was then removed under vacuum.
Determined by gas chromatography with dodecane as an internal standard.
Table 2.
Alcohol Addition Scope
 | |||
|---|---|---|---|
| entry | R | product | yield(%)b |
| 1 | Bn | 2 | 89 |
| 2 | Me | 3 | 79 |
| 3 | allyl | 4 | 78 |
| 4 | propargyl | 5 | 85 |
| 5 | 4-MeO-C6H4CH2OH | 6 | 81 |
| 6 | 7 | 80 | |
| 7 | 8 | 75 | |
| 8c | 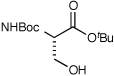 |
9 | 81d |
| 9 | 10 | 75d | |
| 10 | 11 | 89 | |
See Supporting Information for details.
Isolated yields.
CH2Cl2 used as solvent.
1.2:1 dr (determined by 1H NMR spectroscopy).
Acknowledgements
This work was supported by NIGMS (GM73072-01) and (P50 GM086145-01), AstraZeneca, Amgen and GlaxoSmithKline. E.M.P. thanks ACS Division of Organic Chemistry fellowship sponsored by Organic Reactions (2008–2009). Funding for the NU Integrated Molecular Structure Education and Research Center (IMSERC) has been furnished in part by the NSF (CHE-9871268). We thank Sigma-Aldrich and FMCLithium for generously providing reagents and Prof. Dean Tantillo (University of California, Davis) for helpful discussions.
Footnotes
Supporting Information Available: Experimental procedures and spectral data for new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Williamson AW. J. Chem. Soc. 1852;106:229–239. [Google Scholar]
- 2.The calculated heat of reaction for the conjugate addition between an alcohol and α,β-unsaturated ketone is approximately −11 to −16 kcal/mol (semi-empirical/ AM-1). The entropy penalty for combining two molecules is ~7–10 kcal/mol, see: Houk KN, Tucker JA, Dorigo AE. Acc. Chem. Res. 1990;23:107–113.. By a comparison, our calculated heat of a reaction for a Diels–Alder using this same approach is 25–30 kcal/mol and correlates with the reported values, see: Rogers FE. J. Phys. Chem. 1972;76:106–109. Rogers FE, Quan SW. J. Phys. Chem. 1973;77:828–831. For reviews of conjugate additions, see: Perlmutter P. Conjugate Addition Reactions in Organic Synthesis. Oxford, U.K.: Pergamon Press; 1992. Jung ME. In: In Comprehensive Organic Synthesis. Trost BM, Fleming I, editors. Oxford, U.K.: Pergamon; 1991.
- 3.(a) Seagers WJ, Elving PJ. J. Am. Chem. Soc. 1949;71:2947. [Google Scholar]; (b) Fuer H, Markofsky S. J. Org. Chem. 1964;29:929–934. [Google Scholar]; (c) Duffy JL, Kurth JA, Kurth MJ. Tetrahedron Lett. 1993;34:1259–1260. [Google Scholar]; (d) Dulcere JP, Dumez E. Chem. Commun. 1997:971–972. [Google Scholar]; (e) Adderley NJ, Buchanan DJ, Dixon DJ, Laine DI. Angew. Chem. Int. Ed. 2003;42:4241–4244. doi: 10.1002/anie.200352007. [DOI] [PubMed] [Google Scholar]; (f) Hernandez-Juan FA, Richardson RD, Dixon D. J. Synlett. 2006:2673–2675. [Google Scholar]
- 4.For stoichiometric alkoxide conjugate additions to β,γ-unsaturated-α-ketoesters, see: Xiong X, Ovens C, Pilling AW, Ward JW, Dixon DJ. J. Org. Lett. 2008;10:565–567. doi: 10.1021/ol702693m.. For a secondary amine-catalyzed tandem reaction, see: Reyes E, Talavera G, Vicario JL, Badia D, Carrillo L. Angew. Chem. Int. Ed. 2009;48:5701–5704. doi: 10.1002/anie.200901333. For a ruthenium-catalyzed addition of alcohols to acrylonitriles, see: Yi CS, Yun SS, He Z, Guzei IA. Organometallics. 2003;22:3031–3033.
- 5.(a) Kisanga PB, Ilankumaran P, Fetterly BM, Verkade JG. J. Org. Chem. 2002;67:3555–3560. doi: 10.1021/jo010228k. [DOI] [PubMed] [Google Scholar]; (b) Stewart IC, Bergman RG, Toste FD. J. Am. Chem. Soc. 2003;125:8696–8697. doi: 10.1021/ja035232n. [DOI] [PubMed] [Google Scholar]
- 6. Enders A, Balensiefer T. Acc. Chem. Res. 2004;37:534–541. doi: 10.1021/ar030050j. A. Berkessel A, Gröger H. Asymmetric Organocatalysis. Weinheim: Wiley-VCH; 2005. Zeitler K. Angew. Chem. Int. Ed. 2005;44:7506–7510. doi: 10.1002/anie.200502617. Enders D, Niemeier O, Henseler A. Chem. Rev. 2007;107:5606–5655. doi: 10.1021/cr068372z. Marion N, Diez-Gonzalez S, Nolan IP. Angew. Chem. Int. Ed. 2007;46:2988–3000. doi: 10.1002/anie.200603380. Phillips EM, Chan A, Scheidt KA. Aldrichimica Acta. 2009;42:55–66. For a review on Lewis base catalysis, see: Denmark SE, Beutner GL. Angew. Chem. Int. Ed. 2008;47:1560–1638. doi: 10.1002/anie.200604943.
- 7.(a) Mattson AE, Bharadwaj AR, Scheidt KA. J. Am. Chem. Soc. 2004;126:2314–2315. doi: 10.1021/ja0318380. [DOI] [PubMed] [Google Scholar]; (b) Chan A, Scheidt KA. Org. Lett. 2005;7:905–908. doi: 10.1021/ol050100f. [DOI] [PubMed] [Google Scholar]; (c) Chan A, Scheidt KA. J. Am. Chem. Soc. 2007;129:5334–5335. doi: 10.1021/ja0709167. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Reynolds TE, Stern CA, Scheidt KA. Org. Lett. 2007;9:2581–2584. doi: 10.1021/ol0710515. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Chan A, Scheidt KA. J. Am. Chem. Soc. 2008;130:2740–2741. doi: 10.1021/ja711130p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Kawanaka Y, Phillips EM, Scheidt KA. J. Am. Chem. Soc. 2009;131:18028–18029. doi: 10.1021/ja9094044. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Lee JY, Roberts JM, Farha OK, Sarjeant AA, Scheidt KA, Hupp JT. Inorg. Chem. 2009;48:9971–9973. doi: 10.1021/ic901174p. [DOI] [PubMed] [Google Scholar]; (h) Phillips EM, Wadamoto M, Roth HS, Ott AW, Scheidt KA. Org. Lett. 2009;11:105–108. doi: 10.1021/ol802448c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Phillips EM, Roberts JM, Scheidt KA. Org. Lett. 2010;12:2830–2833. doi: 10.1021/ol100938j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Cardinal-David B, Raup DEA, Scheidt KA. J. Am. Chem. Soc. 2010;132:5345–5347. doi: 10.1021/ja910666n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Alder RW, Allen PR, Williams SJ. Chem. Commun. 1995:1267–1268. [Google Scholar]; (b) Kim YJ, Streitwieser A. J. Am. Chem. Soc. 2002;124:5757–5761. doi: 10.1021/ja025628j. [DOI] [PubMed] [Google Scholar]; (c) Amyes TL, Diver ST, Richard JP, Rivas FM, Toth K. J. Am. Chem. Soc. 2004;126:4366–4374. doi: 10.1021/ja039890j. [DOI] [PubMed] [Google Scholar]; (d) Chu Y, Deng H, Cheng JP. J. Org. Chem. 2007;72:7790–7793. doi: 10.1021/jo070973i. [DOI] [PubMed] [Google Scholar]
- 9.(a) Grasa GA, Kissling RM, Nolan SP. Org. Lett. 2002;4:3583–3586. doi: 10.1021/ol0264760. [DOI] [PubMed] [Google Scholar]; (b) Nyce GW, Lamboy JA, Connor EF, Waymouth RM, Hedrick JL. Org. Lett. 2002;4:3587–3590. doi: 10.1021/ol0267228. [DOI] [PubMed] [Google Scholar]
- 10.Additional imidazolium and triazolium salts were evaluated as catalysts, but provided lower yields of 2 than IMes•HCl (A).
- 11.More LiCl did not improve the yield. One equiv of LiCl without the free N-heterocyclic carbene or azolium salt resulted in no conjugate addition reaction. The combination of 5 mol % n-BuLi to BnOH in toluene followed by the addition of 1 afforded <30% of desired product 2 as observed by GC along with multiple additional peaks compared to the NHC-catalyzed process.
- 12.(a) Inanaga J, Baba Y, Hanamoto T. Chem. Lett. 1993;22:241. [Google Scholar]; (b) Kuroda H, Tomita I, Endo T. Polymer. 1997;38:3655–3662. [Google Scholar]; (c) Tejedor D, Santos-Exposito A, Mendez-Abt G, Ruiz-Perez C, Garcia-Tellado F. Synlett. 2009;8:1223–1226. and references cited therein. [Google Scholar]
- 13.(a) Fischer C, Smith SW, Powell DA, Fu GC. J. Am. Chem. Soc. 2006;128:1472–1473. doi: 10.1021/ja058222q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) He L, Jian TY, Ye S. J. Org. Chem. 2007;72:7466–7468. doi: 10.1021/jo071247i. [DOI] [PubMed] [Google Scholar]
- 14.Movassaghi M, Schmidt MA. Org. Lett. 2005;7:2453–2456. doi: 10.1021/ol050773y. [DOI] [PubMed] [Google Scholar]
-
15.The deuterium labeling study below supports that the free carbene derived from IMes is a competent Brønsted base.
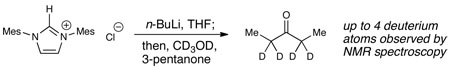
- 16.Deuterium labeling studies with alcohols to determine any potential kinetic isotope effects have not been productive, presumably since the active protons of the products can rapidly exchange with the alcohol proton under the reaction conditions, see ref. 15.
-
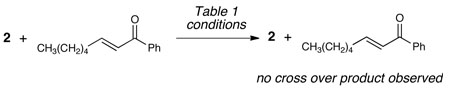
17.The crossover experiment with 2 and (E)-1-phenyloct-2-en-1-one shown below supports that the conjugate addition is not reversible under NHC conditions. However, at temperatures beginning at 70 °C, the products begin to revert back to starting materials, indicated reversibility.
- 18.For examples of these unwanted reactions, see: Büchi G, Hansen JH, Knutson D, Koller E. J. Am. Chem. Soc. 1958;80:5517–5524. Kabas G, Rutz HC. Tetrahedron. 1966;22:1219–1226.
- 19.He M, Struble JR, Bode JW. J. Am. Chem. Soc. 2006;128:8418–8420. doi: 10.1021/ja062707c. [DOI] [PubMed] [Google Scholar]
- 20.The enantiomeric excess does not change as a function of time during the reaction. This reaction was done in triplicate with ee values between 10 and 11% ee. See supporting information for details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.