Abstract
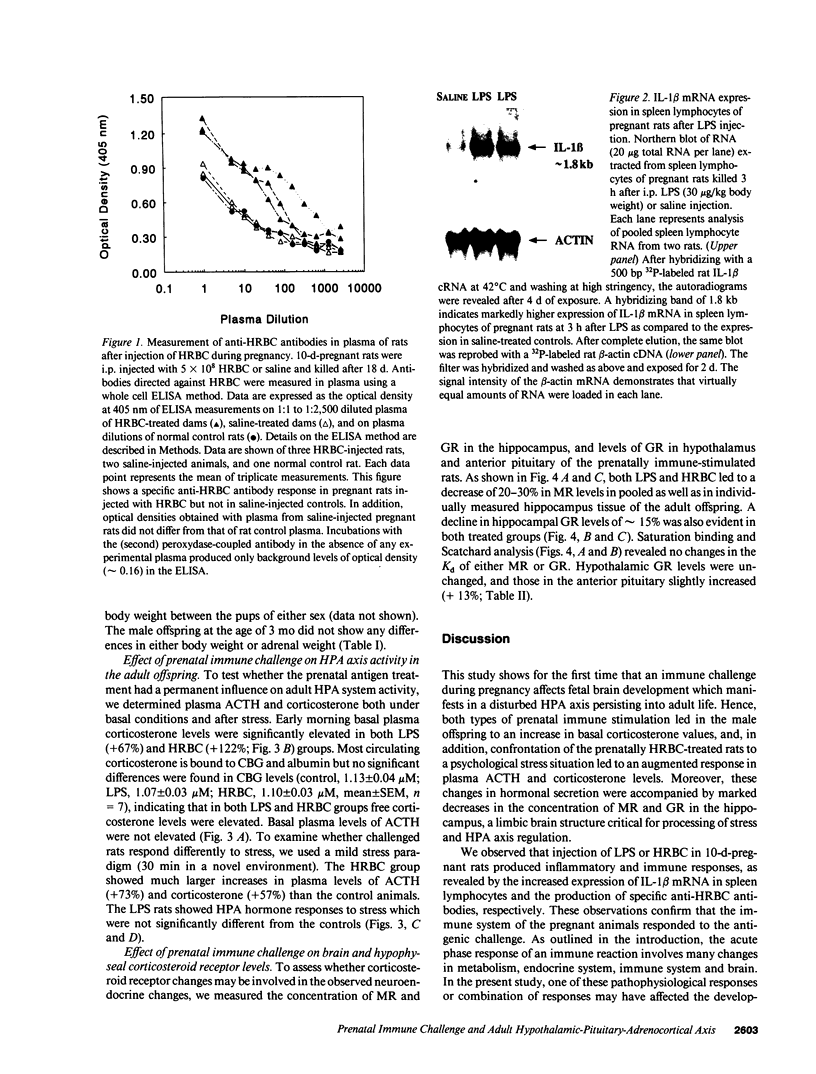
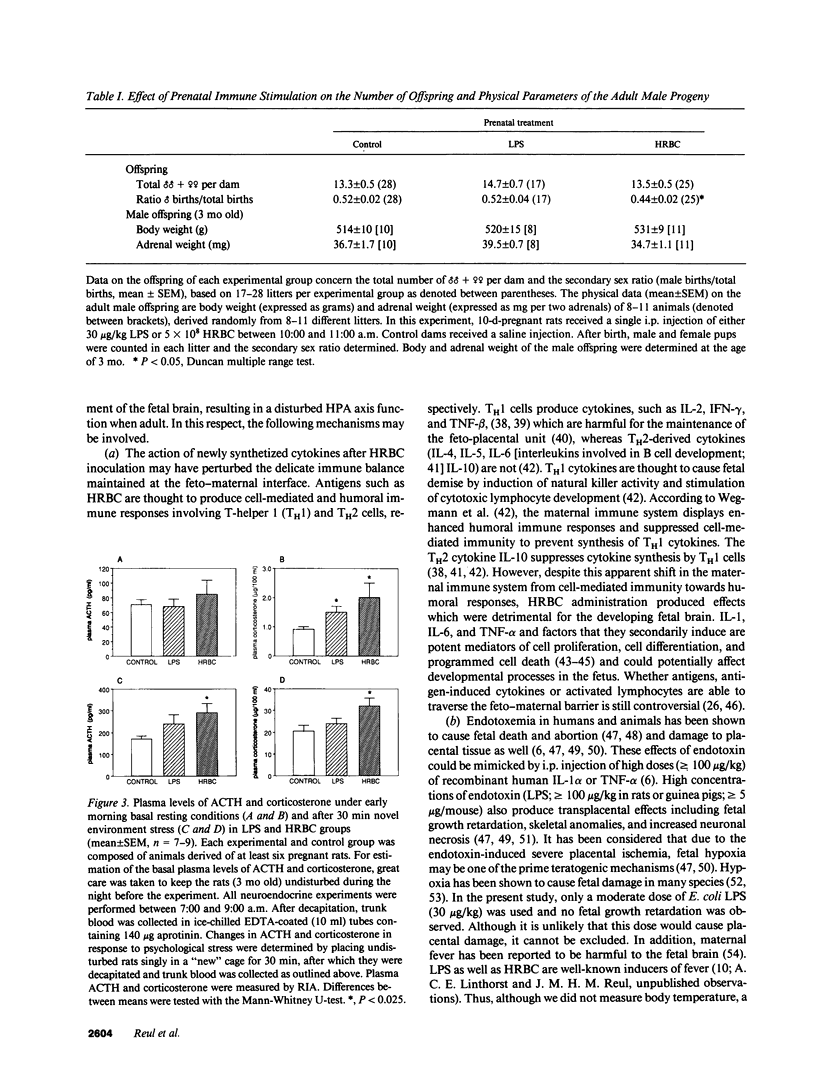
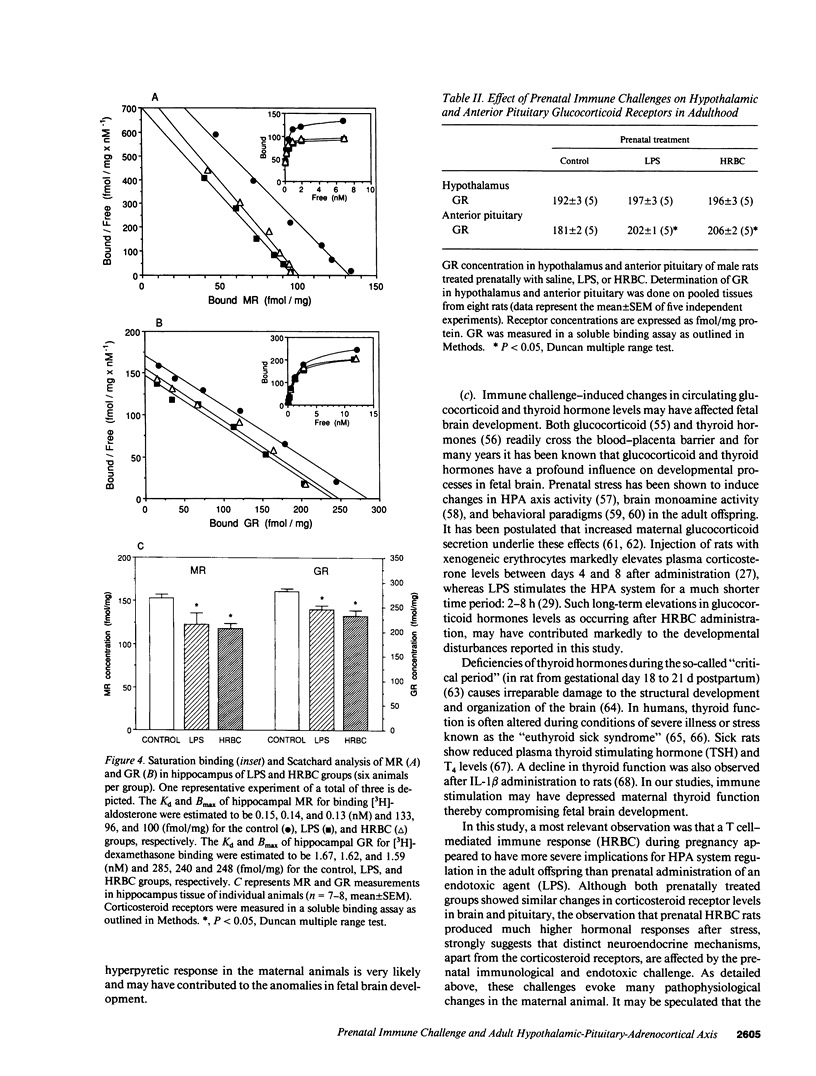
We investigated whether non-abortive maternal infections would compromise fetal brain development and alter hypothalamic-pituitary-adrenocortical (HPA) axis functioning when adult. To study putative teratogenic effects of a T cell-mediated immune response versus an endotoxic challenge, 10-d-pregnant rats received a single intraperitoneal injection of 5 x 10(8) human red blood cells (HRBC) or gram-negative bacterial endotoxin (Escherichia coli LPS: 30 micrograms/kg). The adult male progeny (3 mo old) of both experimental groups showed increased basal plasma corticosterone levels. In addition, after novelty stress the HRBC group, but not the LPS group, showed increased ACTH and corticosterone levels. Both groups showed substantial decreases in mineralocorticoid (MR) and glucocorticoid receptor (GR) levels in the hippocampus, a limbic brain structure critical for HPA axis regulation, whereas GR concentrations in the hypothalamus were unchanged and in anterior pituitary were slightly increased. HRBC and LPS indeed stimulated the maternal immune system as revealed by specific anti-HRBC antibody production and enhanced IL-1 beta mRNA expression in splenocytes, respectively. This study demonstrates that a T cell-mediated immune response as well as an endotoxic challenge during pregnancy can induce anomalies in HPA axis function in adulthood. Clinically, it may be postulated that disturbed fetal brain development due to prenatal immune challenge increases the vulnerability to develop mental illness involving inadequate responses to stress.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADER R., CONKLIN P. M. HANDLING OF PREGNANT RATS: EFFECTS ON EMOTIONALITY OF THEIR OFFSPRING. Science. 1963 Oct 18;142(3590):411–412. doi: 10.1126/science.142.3590.411. [DOI] [PubMed] [Google Scholar]
- Altshuler G. Some placental considerations related to neurodevelopmental and other disorders. J Child Neurol. 1993 Jan;8(1):78–94. doi: 10.1177/088307389300800111. [DOI] [PubMed] [Google Scholar]
- Balkwill F. R., Burke F. The cytokine network. Immunol Today. 1989 Sep;10(9):299–304. doi: 10.1016/0167-5699(89)90085-6. [DOI] [PubMed] [Google Scholar]
- Bateman A., Singh A., Kral T., Solomon S. The immune-hypothalamic-pituitary-adrenal axis. Endocr Rev. 1989 Feb;10(1):92–112. doi: 10.1210/edrv-10-1-92. [DOI] [PubMed] [Google Scholar]
- Beckmann I., Meisel-Mikołajczyk F., Leszczynski P., Brooijmans M., Wallenburg H. C. Endotoxin-induced fetal growth retardation in the pregnant guinea pig. Am J Obstet Gynecol. 1993 Feb;168(2):714–718. doi: 10.1016/0002-9378(93)90521-j. [DOI] [PubMed] [Google Scholar]
- Besedovsky H. O., del Rey A. Immune-neuroendocrine circuits: integrative role of cytokines. Front Neuroendocrinol. 1992 Jan;13(1):61–94. [PubMed] [Google Scholar]
- Besedovsky H., Sorkin E., Keller M., Müller J. Changes in blood hormone levels during the immune response. Proc Soc Exp Biol Med. 1975 Nov;150(2):466–470. doi: 10.3181/00379727-150-39057. [DOI] [PubMed] [Google Scholar]
- Besedovsky H., del Rey A., Sorkin E., Dinarello C. A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986 Aug 8;233(4764):652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- Burger A. G., Hughes J. N., Saville E. Starvation and thyroid function: effects on thermogenesis and serum thyrotropin. Life Sci. 1981 Apr 13;28(15-16):1737–1744. doi: 10.1016/0024-3205(81)90345-3. [DOI] [PubMed] [Google Scholar]
- Chaouat G., Menu E., Clark D. A., Dy M., Minkowski M., Wegmann T. G. Control of fetal survival in CBA x DBA/2 mice by lymphokine therapy. J Reprod Fertil. 1990 Jul;89(2):447–458. doi: 10.1530/jrf.0.0890447. [DOI] [PubMed] [Google Scholar]
- Checkley S. Neuroendocrine mechanisms and the precipitation of depression by life events. Br J Psychiatry Suppl. 1992 Feb;(15):7–17. [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coid C. R., Sandison H., Slavin S., Altman D. G. Escherichia coli infection in mice and impaired fetal development. Br J Exp Pathol. 1978 Jun;59(3):292–297. [PMC free article] [PubMed] [Google Scholar]
- Dahl G. M., Telemo E., Weström B. R., Jakobsson I., Lindberg T., Karlsson B. W. The passage of orally fed proteins from mother to foetus in the rat. Comp Biochem Physiol A Comp Physiol. 1984;77(2):199–201. doi: 10.1016/0300-9629(84)90046-x. [DOI] [PubMed] [Google Scholar]
- De Kloet E. R., Reul J. M. Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology. 1987;12(2):83–105. doi: 10.1016/0306-4530(87)90040-0. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Dubuis J. M., Dayer J. M., Siegrist-Kaiser C. A., Burger A. G. Human recombinant interleukin-1 beta decreases plasma thyroid hormone and thyroid stimulating hormone levels in rats. Endocrinology. 1988 Nov;123(5):2175–2181. doi: 10.1210/endo-123-5-2175. [DOI] [PubMed] [Google Scholar]
- Dunn A. J. Endotoxin-induced activation of cerebral catecholamine and serotonin metabolism: comparison with interleukin-1. J Pharmacol Exp Ther. 1992 Jun;261(3):964–969. [PubMed] [Google Scholar]
- Dussault J. H., Ruel J. Thyroid hormones and brain development. Annu Rev Physiol. 1987;49:321–334. doi: 10.1146/annurev.ph.49.030187.001541. [DOI] [PubMed] [Google Scholar]
- Edwards M. J., Penny R. H., Zevnik I. A brain cell deficit in newborn guinea-pigs following prenatal hyperthermia. Brain Res. 1971 May 7;28(2):341–345. doi: 10.1016/0006-8993(71)90666-4. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greden J. F., Gardner R., King D., Grunhaus L., Carroll B. J., Kronfol Z. Dexamethasone suppression tests in antidepressant treatment of melancholia. The process of normalization and test-retest reproducibility. Arch Gen Psychiatry. 1983 May;40(5):493–500. doi: 10.1001/archpsyc.1983.01790050019002. [DOI] [PubMed] [Google Scholar]
- Guilbert L., Robertson S. A., Wegmann T. G. The trophoblast as an integral component of a macrophage-cytokine network. Immunol Cell Biol. 1993 Feb;71(Pt 1):49–57. doi: 10.1038/icb.1993.5. [DOI] [PubMed] [Google Scholar]
- Haesaert B., Ornoy A. Transplacental effects of endotoxemia on fetal mouse brain, bone, and placental tissue. Pediatr Pathol. 1986;5(2):167–181. doi: 10.3109/15513818609041199. [DOI] [PubMed] [Google Scholar]
- Hall G. A., Jones P. W. An experimental study of Salmonella dublin abortion in cattle. Br Vet J. 1976 Jan-Feb;132(1):60–65. doi: 10.1016/s0007-1935(17)34788-7. [DOI] [PubMed] [Google Scholar]
- Hart B. L. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988 Summer;12(2):123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Holsboer-Trachsler E., Stohler R., Hatzinger M. Repeated administration of the combined dexamethasone-human corticotropin releasing hormone stimulation test during treatment of depression. Psychiatry Res. 1991 Aug;38(2):163–171. doi: 10.1016/0165-1781(91)90041-m. [DOI] [PubMed] [Google Scholar]
- Holsboer F., Liebl R., Hofschuster E. Repeated dexamethasone suppression test during depressive illness. Normalisation of test result compared with clinical improvement. J Affect Disord. 1982 Jun;4(2):93–101. doi: 10.1016/0165-0327(82)90039-8. [DOI] [PubMed] [Google Scholar]
- Kent S., Bluthe R. M., Dantzer R., Hardwick A. J., Kelley K. W., Rothwell N. J., Vannice J. L. Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9117–9120. doi: 10.1073/pnas.89.19.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger M. J. Fever: role of pyrogens and cryogens. Physiol Rev. 1991 Jan;71(1):93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Moyer J. A., Herrenkohl L. R., Jacobowitz D. M. Stress during pregnancy: effect on catecholamines in discrete brain regions of offspring as adults. Brain Res. 1978 Apr 7;144(1):173–178. doi: 10.1016/0006-8993(78)90446-8. [DOI] [PubMed] [Google Scholar]
- Munck A., Guyre P. M., Holbrook N. J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984 Winter;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- O'Callaghan E., Sham P., Takei N., Glover G., Murray R. M. Schizophrenia after prenatal exposure to 1957 A2 influenza epidemic. Lancet. 1991 May 25;337(8752):1248–1250. doi: 10.1016/0140-6736(91)92919-s. [DOI] [PubMed] [Google Scholar]
- Okusawa S., Gelfand J. A., Ikejima T., Connolly R. J., Dinarello C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988 Apr;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornoy A., Altshuler G. Maternal endotoxemia, fetal anomalies, and central nervous system damage: a rat model of a human problem. Am J Obstet Gynecol. 1976 Jan 15;124(2):196–204. doi: 10.1016/s0002-9378(16)33298-7. [DOI] [PubMed] [Google Scholar]
- Patrick M. J. Influence of maternal renal infection on the foetus and infant. Arch Dis Child. 1967 Apr;42(222):208–213. doi: 10.1136/adc.42.222.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield S. P., Hendrich C. E. The role of thyroid hormones in prenatal and neonatal neurological development--current perspectives. Endocr Rev. 1993 Feb;14(1):94–106. doi: 10.1210/edrv-14-1-94. [DOI] [PubMed] [Google Scholar]
- Reul J. M., Stec I., Söder M., Holsboer F. Chronic treatment of rats with the antidepressant amitriptyline attenuates the activity of the hypothalamic-pituitary-adrenocortical system. Endocrinology. 1993 Jul;133(1):312–320. doi: 10.1210/endo.133.1.8391426. [DOI] [PubMed] [Google Scholar]
- Reul J. M., de Kloet E. R. Anatomical resolution of two types of corticosterone receptor sites in rat brain with in vitro autoradiography and computerized image analysis. J Steroid Biochem. 1986 Jan;24(1):269–272. doi: 10.1016/0022-4731(86)90063-4. [DOI] [PubMed] [Google Scholar]
- Reul J. M., de Kloet E. R. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985 Dec;117(6):2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Reul J. M., de Kloet E. R., van Sluijs F. J., Rijnberk A., Rothuizen J. Binding characteristics of mineralocorticoid and glucocorticoid receptors in dog brain and pituitary. Endocrinology. 1990 Aug;127(2):907–915. doi: 10.1210/endo-127-2-907. [DOI] [PubMed] [Google Scholar]
- Rivier C., Vale W. In the rat, interleukin-1 alpha acts at the level of the brain and the gonads to interfere with gonadotropin and sex steroid secretion. Endocrinology. 1989 May;124(5):2105–2109. doi: 10.1210/endo-124-5-2105. [DOI] [PubMed] [Google Scholar]
- Rothuizen J., Reul J. M., van Sluijs F. J., Mol J. A., Rijnberk A., de Kloet E. R. Increased neuroendocrine reactivity and decreased brain mineralocorticoid receptor-binding capacity in aged dogs. Endocrinology. 1993 Jan;132(1):161–168. doi: 10.1210/endo.132.1.8380372. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W., Steinbusch H. W., Verhofstad A. A. The distribution and cells of origin of serotonergic inputs to the paraventricular and supraoptic nuclei of the rat. Brain Res. 1983 Oct 31;277(2):355–360. doi: 10.1016/0006-8993(83)90945-9. [DOI] [PubMed] [Google Scholar]
- Silen M. L., Firpo A., Morgello S., Lowry S. F., Francus T. Interleukin-1 alpha and tumor necrosis factor alpha cause placental injury in the rat. Am J Pathol. 1989 Aug;135(2):239–244. [PMC free article] [PubMed] [Google Scholar]
- Spencer R. L., McEwen B. S. Adaptation of the hypothalamic-pituitary-adrenal axis to chronic ethanol stress. Neuroendocrinology. 1990 Nov;52(5):481–489. doi: 10.1159/000125632. [DOI] [PubMed] [Google Scholar]
- Spencer R. L., Young E. A., Choo P. H., McEwen B. S. Adrenal steroid type I and type II receptor binding: estimates of in vivo receptor number, occupancy, and activation with varying level of steroid. Brain Res. 1990 Apr 23;514(1):37–48. doi: 10.1016/0006-8993(90)90433-c. [DOI] [PubMed] [Google Scholar]
- TEDESCHI C. G., INGALLS T. H. Vascular anomalies of mouse fetuses exposed to anoxia during pregnancy. Am J Obstet Gynecol. 1956 Jan;71(1):16–28. doi: 10.1016/0002-9378(56)90673-1. [DOI] [PubMed] [Google Scholar]
- Takahashi L. K., Kalin N. H., Barksdale C. M., Vanden Burgt J. A., Brownfield M. S. Stressor controllability during pregnancy influences pituitary-adrenal hormone concentrations and analgesic responsiveness in offspring. Physiol Behav. 1988;42(4):323–329. doi: 10.1016/0031-9384(88)90273-9. [DOI] [PubMed] [Google Scholar]
- Tuomisto J., Männistö P. Neurotransmitter regulation of anterior pituitary hormones. Pharmacol Rev. 1985 Sep;37(3):249–332. [PubMed] [Google Scholar]
- Van Eekelen J. A., Jiang W., De Kloet E. R., Bohn M. C. Distribution of the mineralocorticoid and the glucocorticoid receptor mRNAs in the rat hippocampus. J Neurosci Res. 1988 Sep;21(1):88–94. doi: 10.1002/jnr.490210113. [DOI] [PubMed] [Google Scholar]
- Ward I. L. Prenatal stress feminizes and demasculinizes the behavior of males. Science. 1972 Jan 7;175(4017):82–84. doi: 10.1126/science.175.4017.82. [DOI] [PubMed] [Google Scholar]
- Wartofsky L., Burman K. D. Alterations in thyroid function in patients with systemic illness: the "euthyroid sick syndrome". Endocr Rev. 1982 Spring;3(2):164–217. doi: 10.1210/edrv-3-2-164. [DOI] [PubMed] [Google Scholar]
- Wegmann T. G., Lin H., Guilbert L., Mosmann T. R. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993 Jul;14(7):353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- Wilke D. L., Tseu S. R., Rhees R. W., Fleming D. E. Effects of environmental stress or ACTH treatment during pregnancy on maternal and fetal plasma androstenedione in the rat. Horm Behav. 1982 Sep;16(3):293–303. doi: 10.1016/0018-506x(82)90028-9. [DOI] [PubMed] [Google Scholar]
- Yasuda N., Greer M. A. Evidence that the hypothalamus mediates endotoxin stimulation of adrenocorticotropic hormone secretion. Endocrinology. 1978 Mar;102(3):947–953. doi: 10.1210/endo-102-3-947. [DOI] [PubMed] [Google Scholar]
- Zarrow M. X., Philpott J. E., Denenberg V. H. Passage of 14C-4-corticosterone from the rat mother to the foetus and neonate. Nature. 1970 Jun 13;226(5250):1058–1059. doi: 10.1038/2261058a0. [DOI] [PubMed] [Google Scholar]
- di Giovine F. S., Duff G. W. Interleukin 1: the first interleukin. Immunol Today. 1990 Jan;11(1):13–20. doi: 10.1016/0167-5699(90)90005-t. [DOI] [PubMed] [Google Scholar]








