Abstract
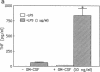
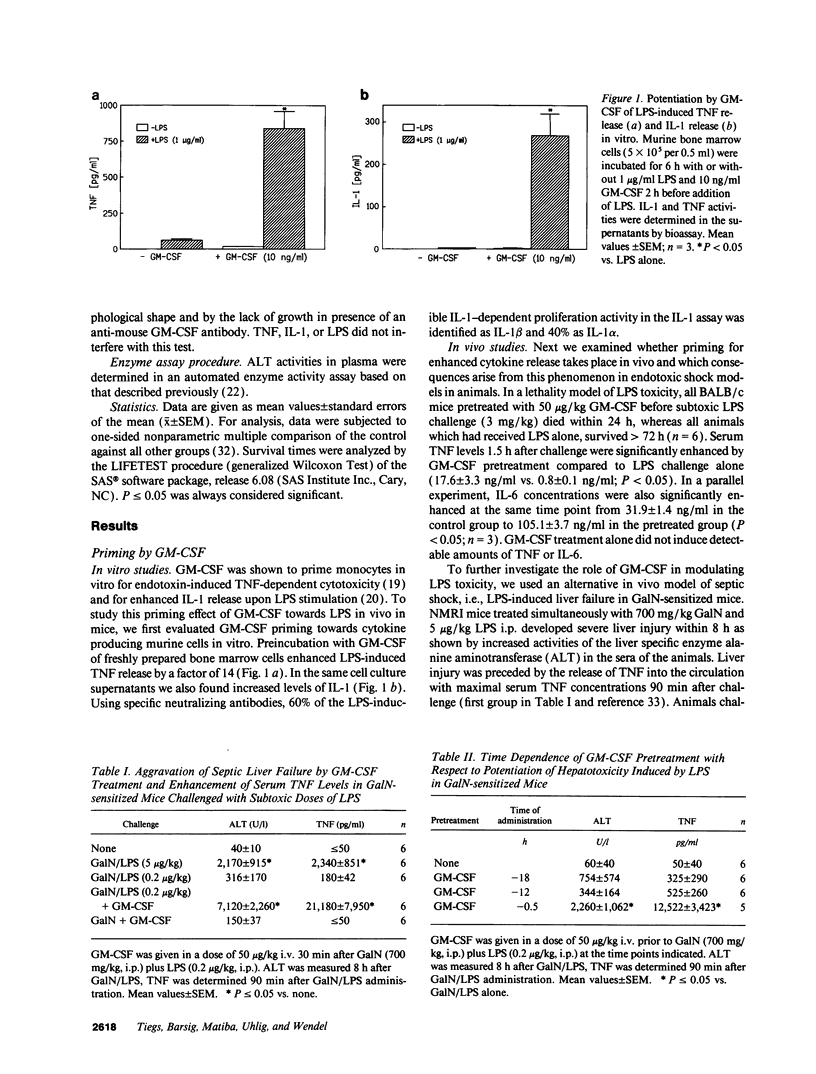
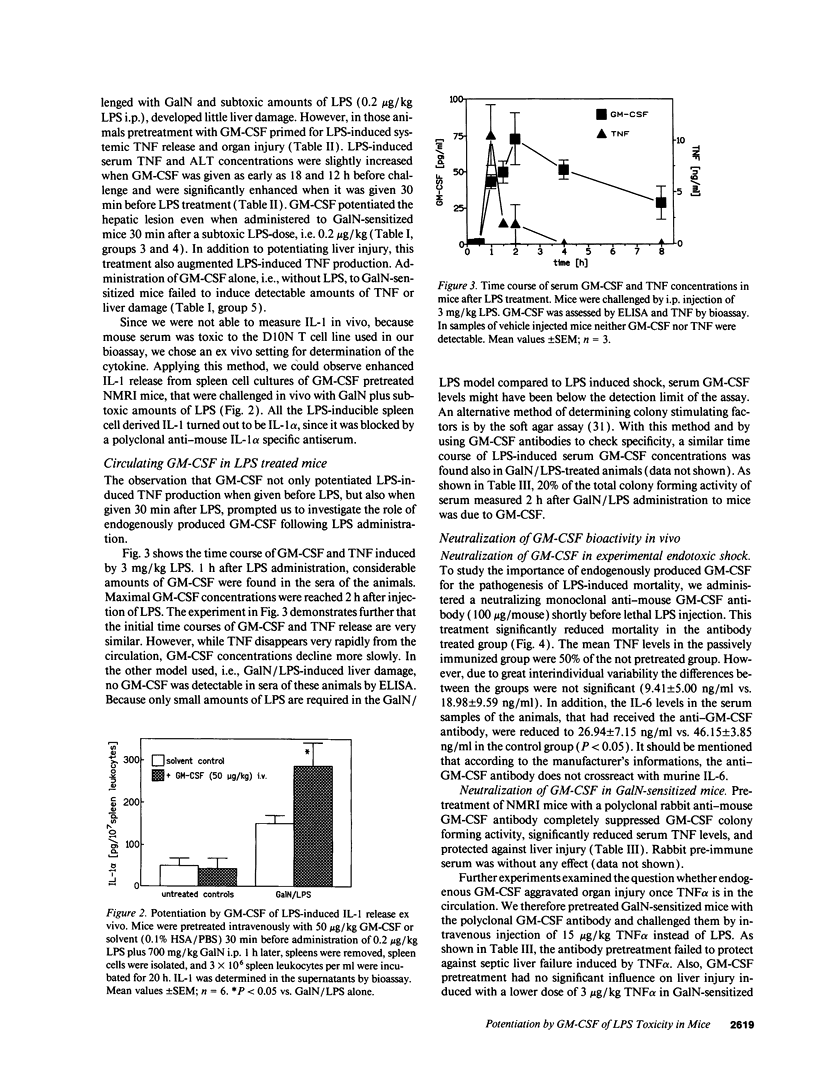
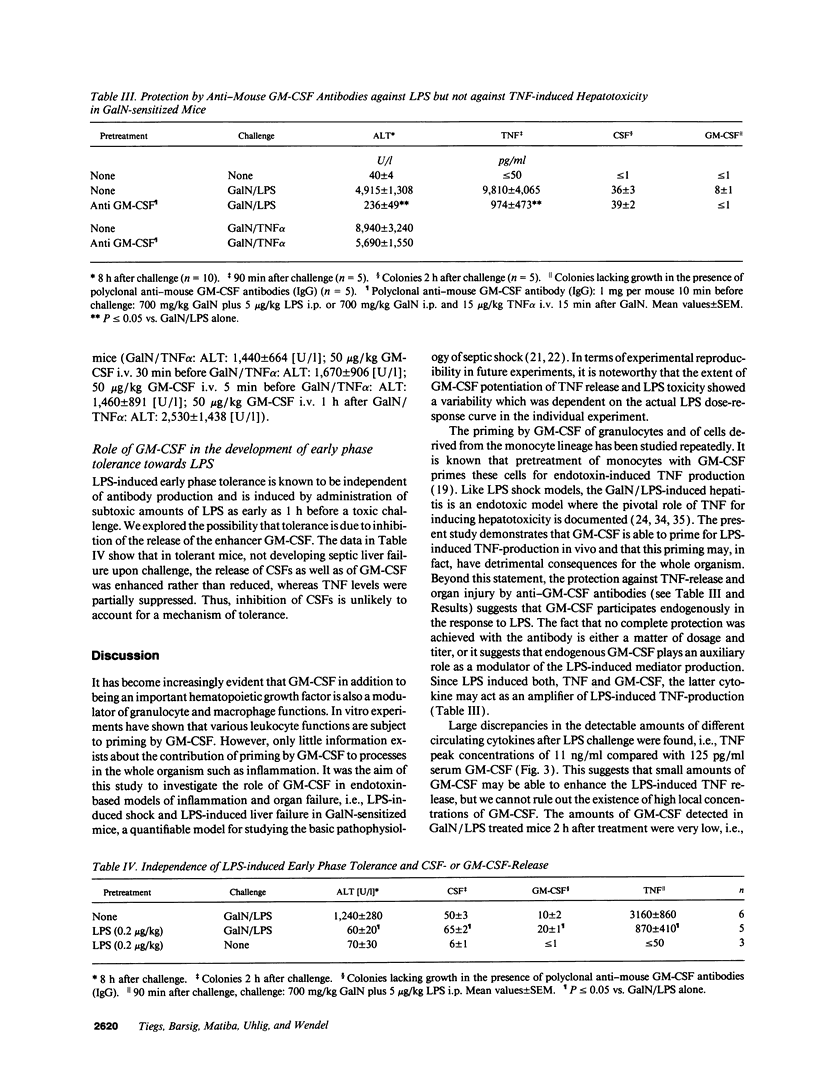
GM-CSF is known to prime leukocytes for inflammatory stimuli in vitro. The objective of this study was to investigate the role of GM-CSF in vivo in a systemic inflammatory reaction syndrome. The results demonstrate a potentiation of LPS toxicity by GM-CSF in a mortality model as well as in a septic liver failure model in mice. Pretreatment of animals with 50 micrograms/kg GM-CSF induced lethality within 24 h in mice challenged with a subtoxic dose of LPS while controls survived > 72 h. A monoclonal anti-GM-CSF antibody significantly protected against a lethal LPS dose. Serum GM-CSF was inducible by LPS and peaked at 2 h. GM-CSF pretreatment dramatically potentiated systemic TNF release and hepatotoxicity induced by a subtoxic dose of LPS in galactosamine-sensitized mice. Potentiation of LPS hepatotoxicity was possible until 30 min after LPS challenge. Polyclonal anti-GM-CSF IgG protected against septic liver failure in this model and attenuated serum TNF concentrations. In vitro an ex vivo experiments revealed that after GM-CSF pretreatment LPS-induced IL-1 release from bone marrow or spleen cells was also enhanced. These findings suggest that GM-CSF represents an endogenous enhancer of LPS-induced organ injury, possibly by potentiating the release of proinflammatory cytokines such as TNF and IL-1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander H. R., Doherty G. M., Buresh C. M., Venzon D. J., Norton J. A. A recombinant human receptor antagonist to interleukin 1 improves survival after lethal endotoxemia in mice. J Exp Med. 1991 Apr 1;173(4):1029–1032. doi: 10.1084/jem.173.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout M. A., Wang E. A., Clark S. C., Sieff C. A. Human recombinant granulocyte-macrophage colony-stimulating factor increases cell-to-cell adhesion and surface expression of adhesion-promoting surface glycoproteins on mature granulocytes. J Clin Invest. 1986 Aug;78(2):597–601. doi: 10.1172/JCI112615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M. H., Aronson F. R. Recombinant human GM-CSF in myelosuppression of chemotherapy (continued) N Engl J Med. 1989 Apr 6;320(14):939–940. doi: 10.1056/NEJM198904063201416. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Bone R. C. The pathogenesis of sepsis. Ann Intern Med. 1991 Sep 15;115(6):457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- Burgess A. W., Metcalf D. The nature and action of granulocyte-macrophage colony stimulating factors. Blood. 1980 Dec;56(6):947–958. [PubMed] [Google Scholar]
- Cannistra S. A., Vellenga E., Groshek P., Rambaldi A., Griffin J. D. Human granulocyte-monocyte colony-stimulating factor and interleukin 3 stimulate monocyte cytotoxicity through a tumor necrosis factor-dependent mechanism. Blood. 1988 Mar;71(3):672–676. [PubMed] [Google Scholar]
- Cavaillon J. M., Munoz C., Fitting C., Misset B., Carlet J. Circulating cytokines: the tip of the iceberg? Circ Shock. 1992 Oct;38(2):145–152. [PubMed] [Google Scholar]
- Chensue S. W., Terebuh P. D., Remick D. G., Scales W. E., Kunkel S. L. In vivo biologic and immunohistochemical analysis of interleukin-1 alpha, beta and tumor necrosis factor during experimental endotoxemia. Kinetics, Kupffer cell expression, and glucocorticoid effects. Am J Pathol. 1991 Feb;138(2):395–402. [PMC free article] [PubMed] [Google Scholar]
- Cicco N. A., Lindemann A., Content J., Vandenbussche P., Lübbert M., Gauss J., Mertelsmann R., Herrmann F. Inducible production of interleukin-6 by human polymorphonuclear neutrophils: role of granulocyte-macrophage colony-stimulating factor and tumor necrosis factor-alpha. Blood. 1990 May 15;75(10):2049–2052. [PubMed] [Google Scholar]
- Cohen L., David B., Cavaillon J. M. Interleukin-3 enhances cytokine production by LPS-stimulated macrophages. Immunol Lett. 1991 May;28(2):121–126. doi: 10.1016/0165-2478(91)90109-n. [DOI] [PubMed] [Google Scholar]
- Damas P., Ledoux D., Nys M., Vrindts Y., De Groote D., Franchimont P., Lamy M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992 Apr;215(4):356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker K., Keppler D., Rudigier J., Domschke W. Cell damage by trapping of biosynthetic intermediates. The role of uracil nucleotides in experimental hepatitis. Hoppe Seylers Z Physiol Chem. 1971 Mar;352(3):412–418. doi: 10.1515/bchm2.1971.352.1.412. [DOI] [PubMed] [Google Scholar]
- Deitch E. A. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992 Aug;216(2):117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio J. F., Billing P., Williams R., Gasson J. C. Human granulocyte-macrophage colony-stimulating factor and other cytokines prime human neutrophils for enhanced arachidonic acid release and leukotriene B4 synthesis. J Immunol. 1988 Jun 15;140(12):4315–4322. [PubMed] [Google Scholar]
- Dinarello C. A. Role of interleukin-1 in infectious diseases. Immunol Rev. 1992 Jun;127:119–146. doi: 10.1111/j.1600-065x.1992.tb01411.x. [DOI] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Fischer H. G., Frosch S., Reske K., Reske-Kunz A. B. Granulocyte-macrophage colony-stimulating factor activates macrophages derived from bone marrow cultures to synthesis of MHC class II molecules and to augmented antigen presentation function. J Immunol. 1988 Dec 1;141(11):3882–3888. [PubMed] [Google Scholar]
- Freudenberg M. A., Kumazawa Y., Meding S., Langhorne J., Galanos C. Gamma interferon production in endotoxin-responder and -nonresponder mice during infection. Infect Immun. 1991 Oct;59(10):3484–3491. doi: 10.1128/iai.59.10.3484-3491.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granowitz E. V., Porat R., Orencole S. F., Callahan M. V., Lynch E. A., Wolff S. M., Dinarello C. A. Granulocyte-macrophage colony-stimulating factor synthesis during experimental endotoxemia in humans. J Infect Dis. 1992 Nov;166(5):1204–1205. doi: 10.1093/infdis/166.5.1204. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Spertini O., Ernst T. J., Belvin M. P., Levine H. B., Kanakura Y., Tedder T. F. Granulocyte-macrophage colony-stimulating factor and other cytokines regulate surface expression of the leukocyte adhesion molecule-1 on human neutrophils, monocytes, and their precursors. J Immunol. 1990 Jul 15;145(2):576–584. [PubMed] [Google Scholar]
- Görgen I., Hartung T., Leist M., Niehörster M., Tiegs G., Uhlig S., Weitzel F., Wendel A. Granulocyte colony-stimulating factor treatment protects rodents against lipopolysaccharide-induced toxicity via suppression of systemic tumor necrosis factor-alpha. J Immunol. 1992 Aug 1;149(3):918–924. [PubMed] [Google Scholar]
- Hopkins S. J., Humphreys M. Simple, sensitive and specific bioassay of interleukin-1. J Immunol Methods. 1989 Jun 21;120(2):271–276. doi: 10.1016/0022-1759(89)90252-4. [DOI] [PubMed] [Google Scholar]
- Katschinski T., Galanos C., Coumbos A., Freudenberg M. A. Gamma interferon mediates Propionibacterium acnes-induced hypersensitivity to lipopolysaccharide in mice. Infect Immun. 1992 May;60(5):1994–2001. doi: 10.1128/iai.60.5.1994-2001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly N. M., Cross A. S. Interleukin-6 is a better marker of lethality than tumor necrosis factor in endotoxin treated mice. FEMS Microbiol Immunol. 1992 Aug;4(6):317–322. doi: 10.1111/j.1574-6968.1992.tb05011.x. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Gasson J., Tobler A. Transcriptional and posttranscriptional modulation of myeloid colony-stimulating factor expression by tumor necrosis factor and other agents. Mol Cell Biol. 1988 Aug;8(8):3432–3438. doi: 10.1128/mcb.8.8.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann V., Freudenberg M. A., Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. J Exp Med. 1987 Mar 1;165(3):657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke G. J., Burgess A. W. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (1). N Engl J Med. 1992 Jul 2;327(1):28–35. doi: 10.1056/NEJM199207023270106. [DOI] [PubMed] [Google Scholar]
- Lieschke G. J., Burgess A. W. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (2). N Engl J Med. 1992 Jul 9;327(2):99–106. doi: 10.1056/NEJM199207093270207. [DOI] [PubMed] [Google Scholar]
- Lindemann A., Riedel D., Oster W., Meuer S. C., Blohm D., Mertelsmann R. H., Herrmann F. Granulocyte/macrophage colony-stimulating factor induces interleukin 1 production by human polymorphonuclear neutrophils. J Immunol. 1988 Feb 1;140(3):837–839. [PubMed] [Google Scholar]
- Lindemann A., Riedel D., Oster W., Ziegler-Heitbrock H. W., Mertelsmann R., Herrmann F. Granulocyte-macrophage colony-stimulating factor induces cytokine secretion by human polymorphonuclear leukocytes. J Clin Invest. 1989 Apr;83(4):1308–1312. doi: 10.1172/JCI114016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Metcalf D. Acute antigen-induced elevation of serum colony stimulating factor (CFS) levels. Immunology. 1971 Sep;21(3):427–436. [PMC free article] [PubMed] [Google Scholar]
- Moore M. A. The clinical use of colony stimulating factors. Annu Rev Immunol. 1991;9:159–191. doi: 10.1146/annurev.iy.09.040191.001111. [DOI] [PubMed] [Google Scholar]
- Morstyn G., Lieschke G. J., Sheridan W., Layton J., Cebon J. Pharmacology of the colony-stimulating factors. Trends Pharmacol Sci. 1989 Apr;10(4):154–159. doi: 10.1016/0165-6147(89)90168-5. [DOI] [PubMed] [Google Scholar]
- Nolan J. P. Intestinal endotoxins as mediators of hepatic injury--an idea whose time has come again. Hepatology. 1989 Nov;10(5):887–891. doi: 10.1002/hep.1840100523. [DOI] [PubMed] [Google Scholar]
- Oster W., Lindemann A., Mertelsmann R., Herrmann F. Granulocyte-macrophage colony-stimulating factor (CSF) and multilineage CSF recruit human monocytes to express granulocyte CSF. Blood. 1989 Jan;73(1):64–67. [PubMed] [Google Scholar]
- Piquet-Pellorce C., Dy M. Effect of lipopolysaccharides on histamine synthesis by hematopoietic cells. Cell Immunol. 1991 Jul;135(2):360–371. doi: 10.1016/0008-8749(91)90281-f. [DOI] [PubMed] [Google Scholar]
- Rapoport A. P., Abboud C. N., DiPersio J. F. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF): receptor biology, signal transduction, and neutrophil activation. Blood Rev. 1992 Mar;6(1):43–57. doi: 10.1016/0268-960x(92)90007-d. [DOI] [PubMed] [Google Scholar]
- Seelentag W. K., Mermod J. J., Montesano R., Vassalli P. Additive effects of interleukin 1 and tumour necrosis factor-alpha on the accumulation of the three granulocyte and macrophage colony-stimulating factor mRNAs in human endothelial cells. EMBO J. 1987 Aug;6(8):2261–2265. doi: 10.1002/j.1460-2075.1987.tb02499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socinski M. A., Cannistra S. A., Sullivan R., Elias A., Antman K., Schnipper L., Griffin J. D. Granulocyte-macrophage colony-stimulating factor induces the expression of the CD11b surface adhesion molecule on human granulocytes in vivo. Blood. 1988 Aug;72(2):691–697. [PubMed] [Google Scholar]
- Stehle B., Weiss C., Ho A. D., Hunstein W. Serum levels of tumor necrosis factor alpha in patients treated with granulocyte-macrophage colony-stimulating factor. Blood. 1990 May 1;75(9):1895–1896. [PubMed] [Google Scholar]
- Sullivan R., Griffin J. D., Simons E. R., Schafer A. I., Meshulam T., Fredette J. P., Maas A. K., Gadenne A. S., Leavitt J. L., Melnick D. A. Effects of recombinant human granulocyte and macrophage colony-stimulating factors on signal transduction pathways in human granulocytes. J Immunol. 1987 Nov 15;139(10):3422–3430. [PubMed] [Google Scholar]
- Tiegs G., Niehörster M., Wendel A. Leukocyte alterations do not account for hepatitis induced by endotoxin or TNF alpha in galactosamine-sensitized mice. Biochem Pharmacol. 1990 Sep 15;40(6):1317–1322. doi: 10.1016/0006-2952(90)90398-5. [DOI] [PubMed] [Google Scholar]
- Tiegs G., Wolter M., Wendel A. Tumor necrosis factor is a terminal mediator in galactosamine/endotoxin-induced hepatitis in mice. Biochem Pharmacol. 1989 Feb 15;38(4):627–631. doi: 10.1016/0006-2952(89)90208-6. [DOI] [PubMed] [Google Scholar]
- Wakabayashi G., Gelfand J. A., Burke J. F., Thompson R. C., Dinarello C. A. A specific receptor antagonist for interleukin 1 prevents Escherichia coli-induced shock in rabbits. FASEB J. 1991 Mar 1;5(3):338–343. doi: 10.1096/fasebj.5.3.1825816. [DOI] [PubMed] [Google Scholar]
- Wendel A. Biochemical pharmacology of inflammatory liver injury in mice. Methods Enzymol. 1990;186:675–680. doi: 10.1016/0076-6879(90)86166-s. [DOI] [PubMed] [Google Scholar]
- Whetton A. D. The biology and clinical potential of growth factors that regulate myeloid cell production. Trends Pharmacol Sci. 1990 Jul;11(7):285–289. doi: 10.1016/0165-6147(90)90010-6. [DOI] [PubMed] [Google Scholar]
- Zlotnik A., Moore K. W. Interleukin 10. Cytokine. 1991 Sep;3(5):366–371. doi: 10.1016/1043-4666(91)90039-g. [DOI] [PubMed] [Google Scholar]