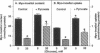Abstract
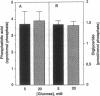
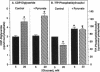
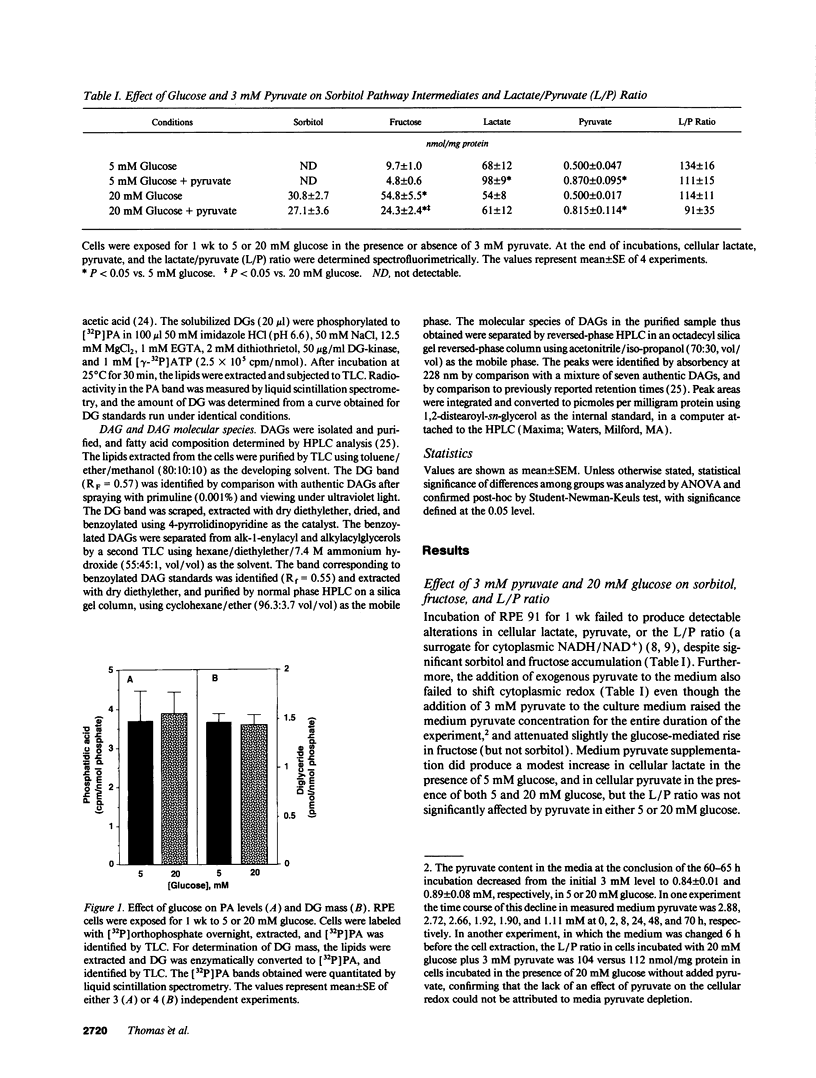
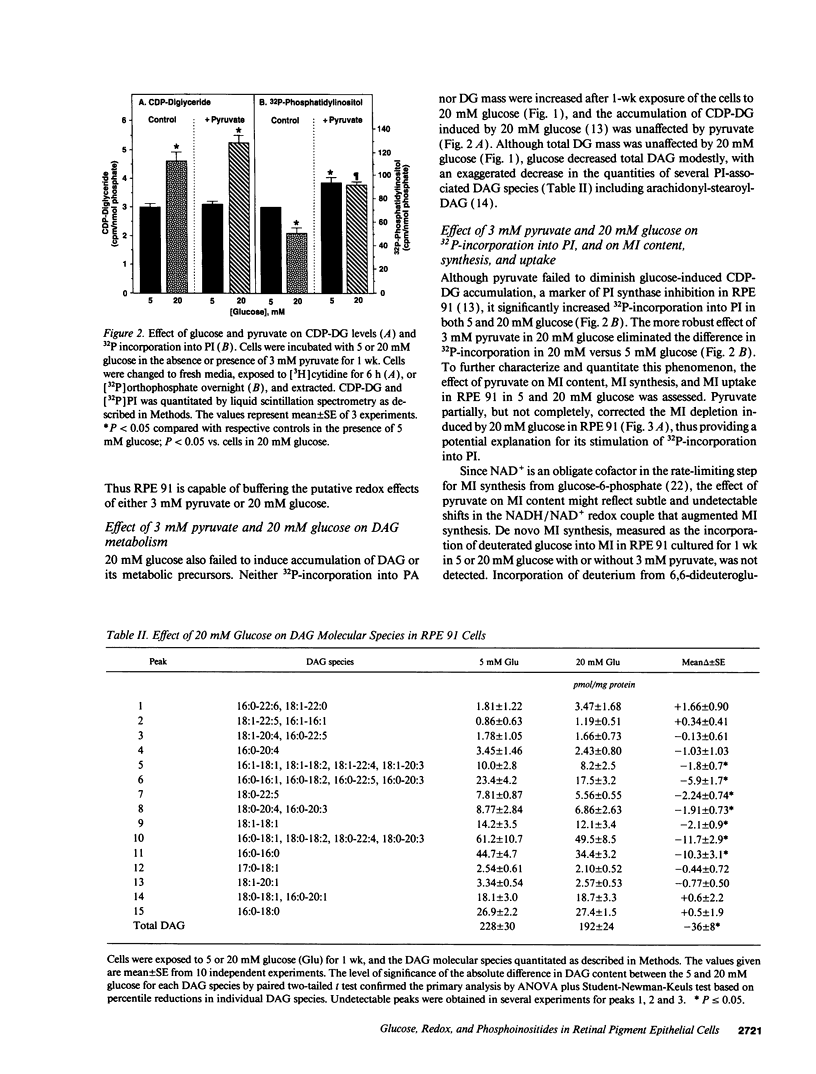
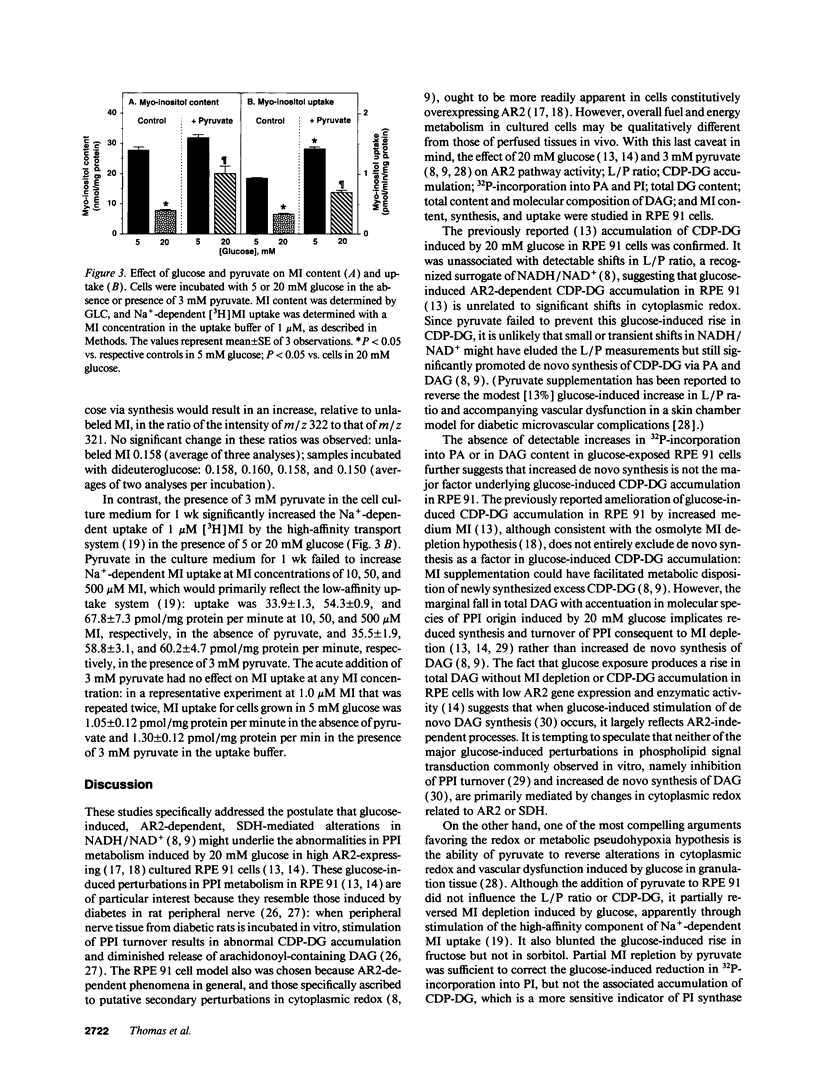
Sorbitol (aldose reductase) pathway flux in diabetes perturbs intracellular metabolism by two putative mechanisms: reciprocal osmoregulatory depletion of other organic osmolytes e.g., myo-inositol, and alterations in NADPH/NADP+ and/or NADH/NAD+. The "osmolyte" and "redox" hypotheses predict secondary elevations in CDP-diglyceride, the rate-limiting precursor for phosphatidylinositol synthesis, but through different mechanisms: the "osmolyte" hypothesis via depletion of intracellular myo-inositol (the cosubstrate for phosphatidylinositol-synthase) and the "redox" hypothesis through enhanced de novo synthesis from triose phosphates. The osmolyte hypothesis predicts diminished phosphoinositide-derived arachidonyl-diacylglycerol, while the redox hypothesis predicts increased total diacylglycerol and phosphatidic acid. In high aldose reductase expressing retinal pigment epithelial cells, glucose-induced, aldose reductase inhibitor-sensitive CDP-diglyceride accumulation and inhibition of 32P-incorporation into phosphatidylinositol paralleled myo-inositol depletion (but not cytoplasmic redox, that was unaffected by glucose) and depletion of arachidonyl-diacylglycerol. 3 mM pyruvate added to the culture medium left cellular redox unaltered, but stimulated Na(+)-dependent myo-inositol uptake, accumulation, and incorporation into phosphatidylinositol. These results favor myo-inositol depletion rather than altered redox as the primary cause of glucose-induced aldose reductase-related defects in phospholipid metabolism in cultured retinal pigment epithelial cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagnasco S. M., Murphy H. R., Bedford J. J., Burg M. B. Osmoregulation by slow changes in aldose reductase and rapid changes in sorbitol flux. Am J Physiol. 1988 Jun;254(6 Pt 1):C788–C792. doi: 10.1152/ajpcell.1988.254.6.C788. [DOI] [PubMed] [Google Scholar]
- Bagnasco S. M., Uchida S., Balaban R. S., Kador P. F., Burg M. B. Induction of aldose reductase and sorbitol in renal inner medullary cells by elevated extracellular NaCl. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1718–1720. doi: 10.1073/pnas.84.6.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M. B., Kador P. F. Sorbitol, osmoregulation, and the complications of diabetes. J Clin Invest. 1988 Mar;81(3):635–640. doi: 10.1172/JCI113366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N. E., Cotter M. A., Low P. A. Nerve blood flow in early experimental diabetes in rats: relation to conduction deficits. Am J Physiol. 1991 Jul;261(1 Pt 1):E1–E8. doi: 10.1152/ajpendo.1991.261.1.E1. [DOI] [PubMed] [Google Scholar]
- Carroll P. B., Thornton B. M., Greene D. A. Glutathione redox state is not the link between polyol pathway activity and myo-inositol-related Na+-K+-ATPase defect in experimental diabetic neuropathy. Diabetes. 1986 Nov;35(11):1282–1285. doi: 10.2337/diab.35.11.1282. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Davidson C. M., DeRubertis F. R. Increase in diacylglycerol mass in isolated glomeruli by glucose from de novo synthesis of glycerolipids. Diabetes. 1990 Jun;39(6):667–674. doi: 10.2337/diab.39.6.667. [DOI] [PubMed] [Google Scholar]
- Del Monte M. A., Maumenee I. H. New technique for in vitro culture of human retinal pigment epithelium. Birth Defects Orig Artic Ser. 1980;16(2):327–338. [PubMed] [Google Scholar]
- Del Monte M. A., Rabbani R., Diaz T. C., Lattimer S. A., Nakamura J., Brennan M. C., Greene D. A. Sorbitol, myo-inositol, and rod outer segment phagocytosis in cultured hRPE cells exposed to glucose. In vitro model of myo-inositol depletion hypothesis of diabetic complications. Diabetes. 1991 Oct;40(10):1335–1345. [PubMed] [Google Scholar]
- Dickman K. G., Mandel L. J. Glycolytic and oxidative metabolism in primary renal proximal tubule cultures. Am J Physiol. 1989 Aug;257(2 Pt 1):C333–C340. doi: 10.1152/ajpcell.1989.257.2.C333. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Lattimer-Greene S., Sima A. A. Pathogenesis of diabetic neuropathy: role of altered phosphoinositide metabolism. Crit Rev Neurobiol. 1989;5(2):143–219. [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A., Sima A. A. Are disturbances of sorbitol, phosphoinositide, and Na+-K+-ATPase regulation involved in pathogenesis of diabetic neuropathy? Diabetes. 1988 Jun;37(6):688–693. doi: 10.2337/diab.37.6.688. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Sima A. A., Stevens M. J., Feldman E. L., Lattimer S. A. Complications: neuropathy, pathogenetic considerations. Diabetes Care. 1992 Dec;15(12):1902–1925. doi: 10.2337/diacare.15.12.1902. [DOI] [PubMed] [Google Scholar]
- Henry D. N., Del Monte M., Greene D. A., Killen P. D. Altered aldose reductase gene regulation in cultured human retinal pigment epithelial cells. J Clin Invest. 1993 Aug;92(2):617–623. doi: 10.1172/JCI116629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita J. H. Mechanisms initiating cataract formation. Proctor Lecture. Invest Ophthalmol. 1974 Oct;13(10):713–724. [PubMed] [Google Scholar]
- Kwon H. M., Yamauchi A., Uchida S., Robey R. B., Garcia-Perez A., Burg M. B., Handler J. S. Renal Na-myo-inositol cotransporter mRNA expression in Xenopus oocytes: regulation by hypertonicity. Am J Physiol. 1991 Feb;260(2 Pt 2):F258–F263. doi: 10.1152/ajprenal.1991.260.2.F258. [DOI] [PubMed] [Google Scholar]
- Lee C., Fisher S. K., Agranoff B. W., Hajra A. K. Quantitative analysis of molecular species of diacylglycerol and phosphatidate formed upon muscarinic receptor activation of human SK-N-SH neuroblastoma cells. J Biol Chem. 1991 Dec 5;266(34):22837–22846. [PubMed] [Google Scholar]
- Lee T. S., MacGregor L. C., Fluharty S. J., King G. L. Differential regulation of protein kinase C and (Na,K)-adenosine triphosphatase activities by elevated glucose levels in retinal capillary endothelial cells. J Clin Invest. 1989 Jan;83(1):90–94. doi: 10.1172/JCI113889. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lee T. S., MacGregor L. C., Fluharty S. J., King G. L. Retraction. J Clin Invest. 1992 Feb;89(2):717–717. [PMC free article] [PubMed] [Google Scholar]
- Moriyama T., Garcia-Perez A., Burg M. B. Factors affecting the ratio of different organic osmolytes in renal medullary cells. Am J Physiol. 1990 Nov;259(5 Pt 2):F847–F858. doi: 10.1152/ajprenal.1990.259.5.F847. [DOI] [PubMed] [Google Scholar]
- Morrison A. D., Clements R. S., Jr, Travis S. B., Oski F., Winegrad A. I. Glucose utilization by the polyol pathway in human erythrocytes. Biochem Biophys Res Commun. 1970 Jul 13;40(1):199–205. doi: 10.1016/0006-291x(70)91066-1. [DOI] [PubMed] [Google Scholar]
- Nakamura J., Del Monte M. A., Shewach D., Lattimer S. A., Greene D. A. Inhibition of phosphatidylinositol synthase by glucose in human retinal pigment epithelial cells. Am J Physiol. 1992 Apr;262(4 Pt 1):E417–E426. doi: 10.1152/ajpendo.1992.262.4.E417. [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Turner R. J., Burg M. B. Osmoregulation of betaine transport in mammalian renal medullary cells. Am J Physiol. 1990 Apr;258(4 Pt 2):F1061–F1067. doi: 10.1152/ajprenal.1990.258.4.F1061. [DOI] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Pugliese G., Tilton R. G., Speedy A., Santarelli E., Eades D. M., Province M. A., Kilo C., Sherman W. R., Williamson J. R. Modulation of hemodynamic and vascular filtration changes in diabetic rats by dietary myo-inositol. Diabetes. 1990 Mar;39(3):312–322. doi: 10.2337/diab.39.3.312. [DOI] [PubMed] [Google Scholar]
- Pugliese G., Tilton R. G., Williamson J. R. Glucose-induced metabolic imbalances in the pathogenesis of diabetic vascular disease. Diabetes Metab Rev. 1991 Mar;7(1):35–59. doi: 10.1002/dmr.5610070106. [DOI] [PubMed] [Google Scholar]
- Sherman W. R., Stewart M. A., Zinbo M. Mass spectrometric study on the mechanism of D-glucose 6-phosphate-L-myo-inositol 1-phosphate cyclase. J Biol Chem. 1969 Oct 25;244(20):5703–5708. [PubMed] [Google Scholar]
- Stevens M. J., Henry D. N., Thomas T. P., Killen P. D., Greene D. A. Aldose reductase gene expression and osmotic dysregulation in cultured human retinal pigment epithelial cells. Am J Physiol. 1993 Sep;265(3 Pt 1):E428–E438. doi: 10.1152/ajpendo.1993.265.3.E428. [DOI] [PubMed] [Google Scholar]
- Thomas T. P., Feldman E. L., Nakamura J., Kato K., Lien M., Stevens M. J., Greene D. A. Ambient glucose and aldose reductase-induced myo-inositol depletion modulate basal and carbachol-stimulated inositol phospholipid metabolism and diacylglycerol accumulation in human retinal pigment epithelial cells in culture. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9712–9716. doi: 10.1073/pnas.90.20.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis S. F., Morrison A. D., Clements R. S., Jr, Winegrad A. I., Oski F. A. Metabolic alterations in the human erythrocyte produced by increases in glucose concentration. The role of the polyol pathway. J Clin Invest. 1971 Oct;50(10):2104–2112. doi: 10.1172/JCI106704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Chang K., Frangos M., Hasan K. S., Ido Y., Kawamura T., Nyengaard J. R., van den Enden M., Kilo C., Tilton R. G. Hyperglycemic pseudohypoxia and diabetic complications. Diabetes. 1993 Jun;42(6):801–813. doi: 10.2337/diab.42.6.801. [DOI] [PubMed] [Google Scholar]
- Winegrad A. I. Banting lecture 1986. Does a common mechanism induce the diverse complications of diabetes? Diabetes. 1987 Mar;36(3):396–406. doi: 10.2337/diab.36.3.396. [DOI] [PubMed] [Google Scholar]
- Yasuda H., Sonobe M., Yamashita M., Terada M., Hatanaka I., Huitian Z., Shigeta Y. Effect of prostaglandin E1 analogue TFC 612 on diabetic neuropathy in streptozocin-induced diabetic rats. Comparison with aldose reductase inhibitor ONO 2235. Diabetes. 1989 Jul;38(7):832–838. doi: 10.2337/diab.38.7.832. [DOI] [PubMed] [Google Scholar]
- Zhu X., Eichberg J. 1,2-diacylglycerol content and its arachidonyl-containing molecular species are reduced in sciatic nerve from streptozotocin-induced diabetic rats. J Neurochem. 1990 Sep;55(3):1087–1090. doi: 10.1111/j.1471-4159.1990.tb04604.x. [DOI] [PubMed] [Google Scholar]
- Zhu X., Eichberg J. A myo-inositol pool utilized for phosphatidylinositol synthesis is depleted in sciatic nerve from rats with streptozotocin-induced diabetes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9818–9822. doi: 10.1073/pnas.87.24.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]