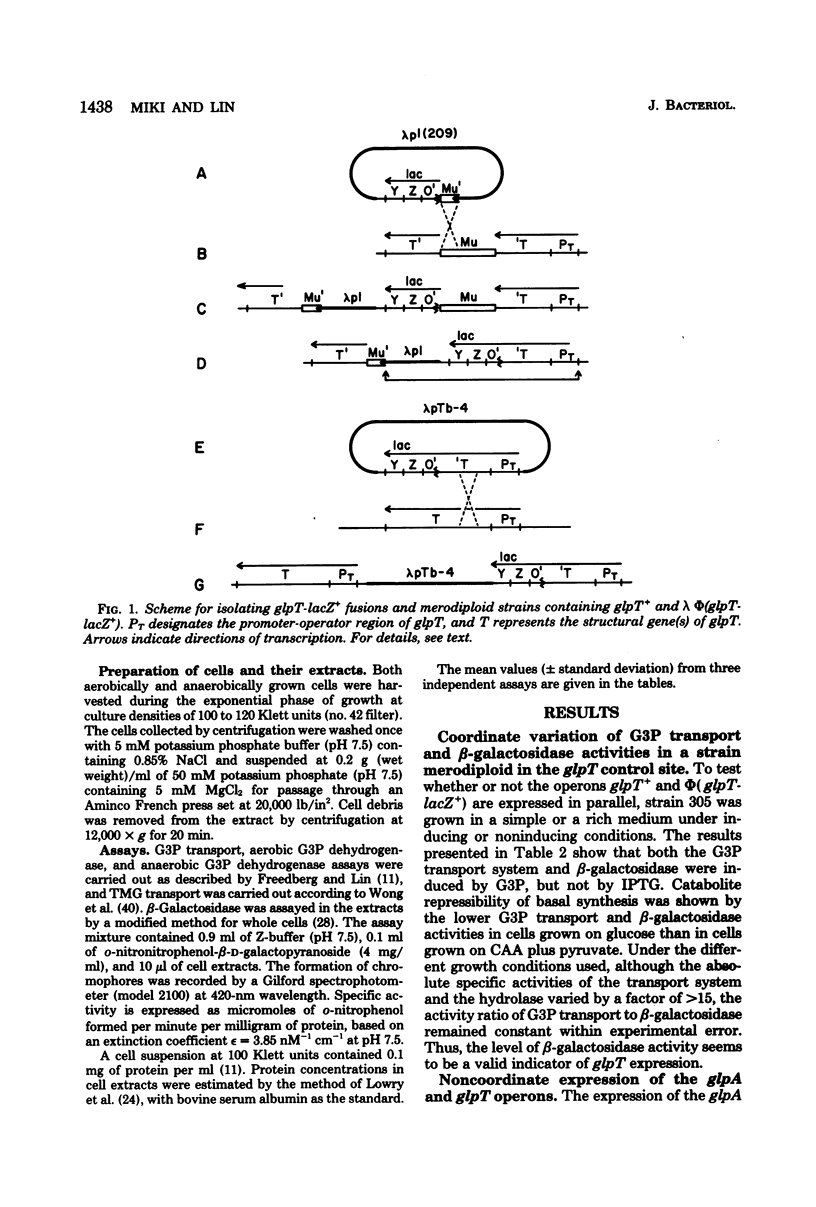
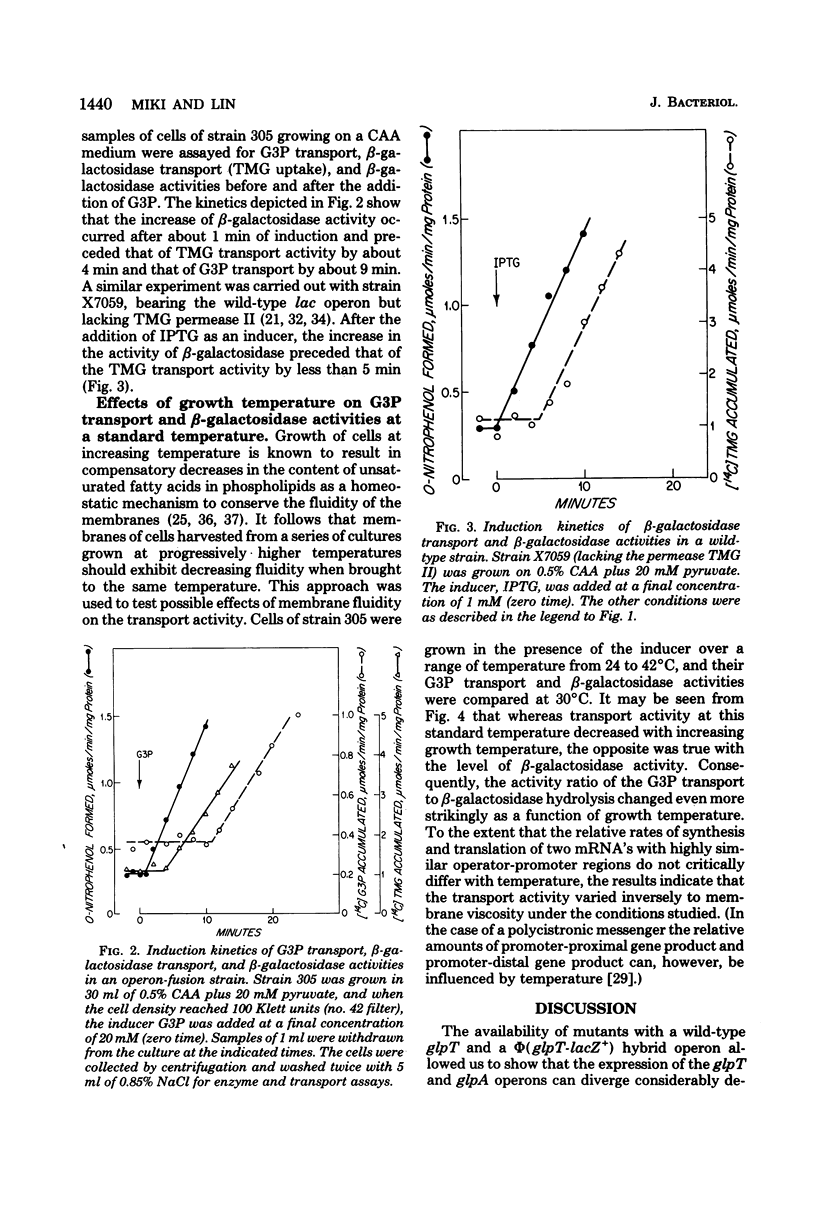
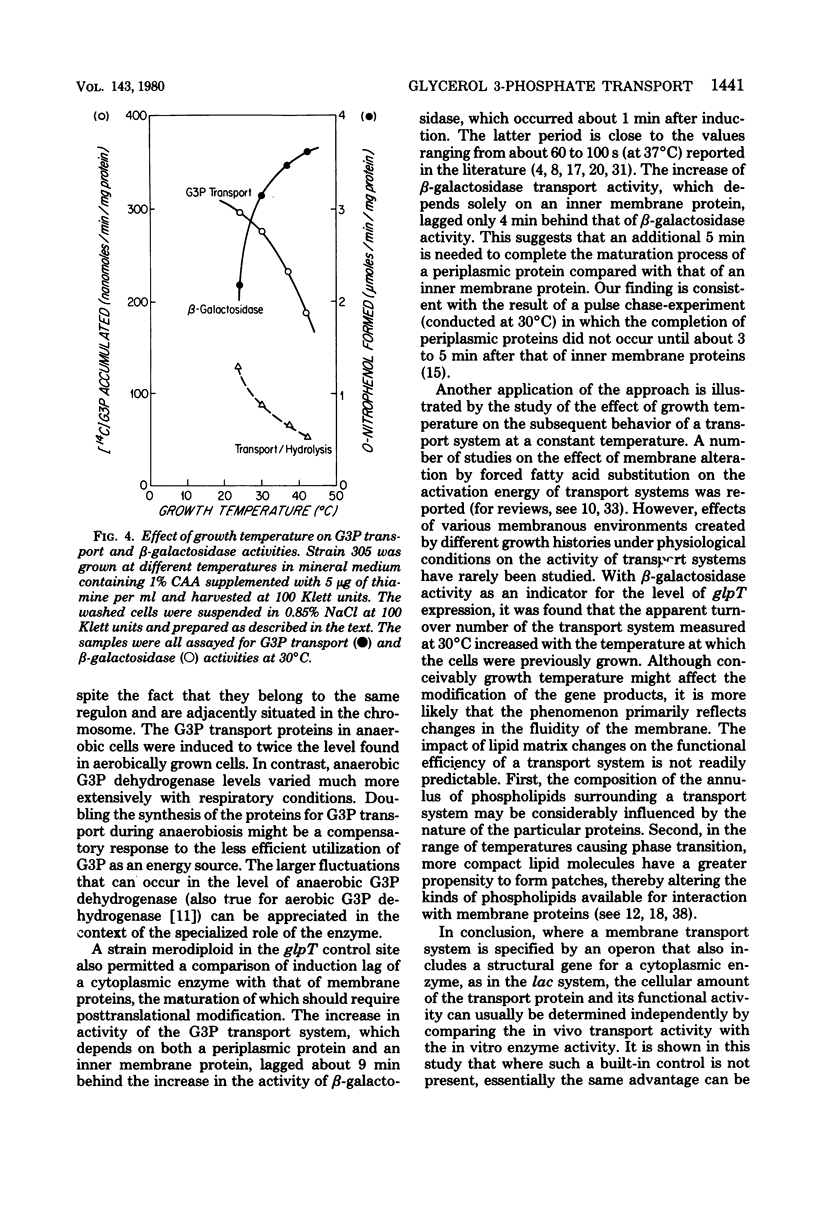
Abstract
Strains of Escherichia coli K-12 deleted in the native lac operon and bearing both a wild-type glpT operon encoding for sn-glycerol 3-phosphate (G3P) transport and a hybrid operon in which glpT operator and promoter regions are fused to the lacZ gene were constructed. In strains with such a hybrid operon, beta-galactosidase and beta-galactoside permease become inducible by G3P. In these mutants the function and maturation of the glpT-coded proteins should be distinguishable from the level of gene expression, since the beta-galactosidase activity can serve as an index of the latter. With the aid of such mutants, it was shown that: (i) the expressions of the two neighboring operons, glpT and glpA (encoding anaerobic G3P dehydrogenase), are not coordinate; (ii) upon induction, the appearance of the cytoplasmic beta-galactosidase activity preceded that of methyl-beta-D-thiogalactoside transport activity (requiring only a cytoplasmic membrane protein) by about 4 min and that of G3P transport activity (requiring both a cytoplasmic membrane protein and a periplasmic protein) by about 9 min; and (iii) when cells grown at several temperatures from 24 to 42 degrees C were measured for G3P transport activity at 30 degrees C, the activity increased with the growth temperature, indicating that, within the range studied, the rate of transport increases with the fluidity of membrane phospholipids.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argast M., Ludtke D., Silhavy T. J., Boos W. A second transport system for sn-glycerol-3-phosphate in Escherichia coli. J Bacteriol. 1978 Dec;136(3):1070–1083. doi: 10.1128/jb.136.3.1070-1083.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argast M., Schumacher G., Boos W. Characterization of a periplasmic protein related to sn-glycerol-3-phosphate transport in escherichia coli. J Supramol Struct. 1977;6(1):135–153. doi: 10.1002/jss.400060111. [DOI] [PubMed] [Google Scholar]
- BOEZI J. A., COWIE D. B. Kinetic studies of beta-galactosidase induction. Biophys J. 1961 Nov;1:639–647. doi: 10.1016/s0006-3495(61)86913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W., Hartig-Beecken I., Altendorf K. Purification and properties of a periplasmic protein related to sn-glycerol-3-phosphate transport in Escherichia coli. Eur J Biochem. 1977 Feb;72(3):571–581. doi: 10.1111/j.1432-1033.1977.tb11280.x. [DOI] [PubMed] [Google Scholar]
- Bukhari A. I. Bacteriophage mu as a transposition element. Annu Rev Genet. 1976;10:389–412. doi: 10.1146/annurev.ge.10.120176.002133. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Fusion of the Escherichia coli lac genes to the ara promoter: a general technique using bacteriophage Mu-1 insertions. Proc Natl Acad Sci U S A. 1975 Mar;72(3):809–813. doi: 10.1073/pnas.72.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Norris T. E., Koch A. L. Chain elongation rate of messenger and polypeptides in slowly growing Escherichia coli. J Mol Biol. 1971 Aug 28;60(1):1–19. doi: 10.1016/0022-2836(71)90442-6. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Freedberg W. B., Lin E. C. Genetic control of L-alpha-glycerophosphate system in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):371–387. doi: 10.1016/0022-2836(68)90415-4. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr Molecular biology of bacterial membrane lipids. Annu Rev Biochem. 1978;47:163–189. doi: 10.1146/annurev.bi.47.070178.001115. [DOI] [PubMed] [Google Scholar]
- Freedberg W. B., Lin E. C. Three kinds of controls affecting the expression of the glp regulon in Escherichia coli. J Bacteriol. 1973 Sep;115(3):816–823. doi: 10.1128/jb.115.3.816-823.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- Haest C. W., de Gier J., van Deenen L. L. Changes in the chemical and the barrier properties of the membrane lipids of E. coli by variation of the temperature of growth. Chem Phys Lipids. 1969 Dec;3(4):413–417. doi: 10.1016/0009-3084(69)90048-6. [DOI] [PubMed] [Google Scholar]
- Hendlin D., Stapley E. O., Jackson M., Wallick H., Miller A. K., Wolf F. J., Miller T. W., Chaiet L., Kahan F. M., Foltz E. L. Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science. 1969 Oct 3;166(3901):122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- Ito K., Sato T., Yura T. Synthesis and assembly of the membrane proteins in E. coli. Cell. 1977 Jul;11(3):551–559. doi: 10.1016/0092-8674(77)90073-3. [DOI] [PubMed] [Google Scholar]
- KEPES A. KINETICS OF INDUCED ENZYME SYNTHESIS. DETERMINATION OF THE MEAN LIFE OF GALACTOSIDASE-SPECIFIC MESSENGER RNA. Biochim Biophys Acta. 1963 Oct 15;76:293–309. [PubMed] [Google Scholar]
- Kahan F. M., Kahan J. S., Cassidy P. J., Kropp H. The mechanism of action of fosfomycin (phosphonomycin). Ann N Y Acad Sci. 1974 May 10;235(0):364–386. doi: 10.1111/j.1749-6632.1974.tb43277.x. [DOI] [PubMed] [Google Scholar]
- Kimura K., Izui K. Importance of membrane fluidity in the induction of alkaline phosphatase, a periplasmic enzyme, in Escherichia coli. Biochem Biophys Res Commun. 1976 Jun 7;70(3):900–906. doi: 10.1016/0006-291x(76)90676-8. [DOI] [PubMed] [Google Scholar]
- Kistler W. S., Lin E. C. Anaerobic L- -glycerophosphate dehydrogenase of Escherichia coli: its genetic locus and its physiological role. J Bacteriol. 1971 Dec;108(3):1224–1234. doi: 10.1128/jb.108.3.1224-1234.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C., KOCH J. P., CHUSED T. M., JORGENSEN S. E. Utilization of L-alpha-glycerophosphate by Escherichia coli without hydrolysis. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2145–2150. doi: 10.1073/pnas.48.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacroute F., Stent G. S. Peptide chain growth of -galactosidase in Escherichia coli. J Mol Biol. 1968 Jul 14;35(1):165–173. doi: 10.1016/s0022-2836(68)80044-0. [DOI] [PubMed] [Google Scholar]
- Lin E. C. Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol. 1976;30:535–578. doi: 10.1146/annurev.mi.30.100176.002535. [DOI] [PubMed] [Google Scholar]
- Lin E. C. The genetics of bacterial transport systems. Annu Rev Genet. 1970;4:225–262. doi: 10.1146/annurev.ge.04.120170.001301. [DOI] [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Lin E. C. Enzyme complex which couples glycerol-3-phosphate dehydrogenation to fumarate reduction in Escherichia coli. J Bacteriol. 1973 May;114(2):767–771. doi: 10.1128/jb.114.2.767-771.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Silhavy T. J., Andrews K. J. Resolution of glpA and glpT loci into separate operons in Escherichia coli K-12 strains. J Bacteriol. 1979 Apr;138(1):268–269. doi: 10.1128/jb.138.1.268-269.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki M. Correlation between metabolism of phosphatidylglycerol and membrane synthesis in Escherichia coli. J Mol Biol. 1972 Jul 21;68(2):249–264. doi: 10.1016/0022-2836(72)90212-4. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. The initial kinetics of enzyme induction. Biochim Biophys Acta. 1961 Apr 29;49:77–88. doi: 10.1016/0006-3002(61)90871-x. [DOI] [PubMed] [Google Scholar]
- PRESTIDGE L. S., PARDEE A. B. A SECOND PERMEASE FOR METHYL-THIO-BETA-D-GALACTOSIDE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1965 May 4;100:591–593. doi: 10.1016/0304-4165(65)90029-2. [DOI] [PubMed] [Google Scholar]
- Raetz C. R. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978 Sep;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt R. Analysis of melibiose mutants deficient in alpha-galactosidase and thiomethylgalactoside permease II in Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):462–471. doi: 10.1128/jb.96.2.462-471.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Hartig-Beecken I., Boos W. Periplasmic protein related to the sn-glycerol-3-phosphate transport system of Escherichia coli. J Bacteriol. 1976 May;126(2):951–958. doi: 10.1128/jb.126.2.951-958.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M. Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Feb;71(2):522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M. Temperature control of phospholipid biosynthesis in Escherichia coli. J Bacteriol. 1971 May;106(2):449–455. doi: 10.1128/jb.106.2.449-455.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilo L., Träuble H., Overath P. Mechanistic interpretation of the influence of lipid phase transitions on transport functions. Biochemistry. 1977 Apr 5;16(7):1283–1290. doi: 10.1021/bi00626a007. [DOI] [PubMed] [Google Scholar]
- Venkateswaran P. S., Wu H. C. Isolation and characterization of a phosphonomycin-resistant mutant of Escherichia coli K-12. J Bacteriol. 1972 Jun;110(3):935–944. doi: 10.1128/jb.110.3.935-944.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. T., Kashket E. R., Wilson T. H. Energy coupling in the lactose transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Jan;65(1):63–69. doi: 10.1073/pnas.65.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]


