Abstract
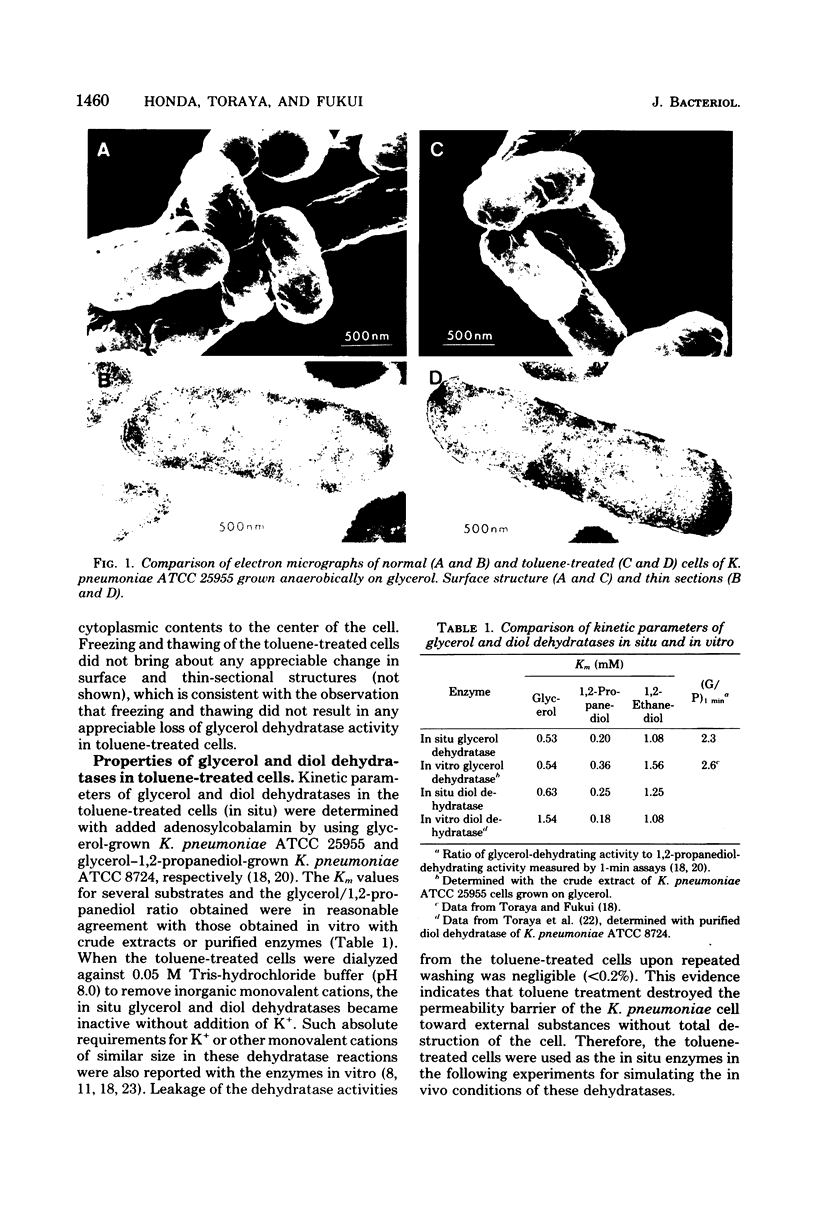
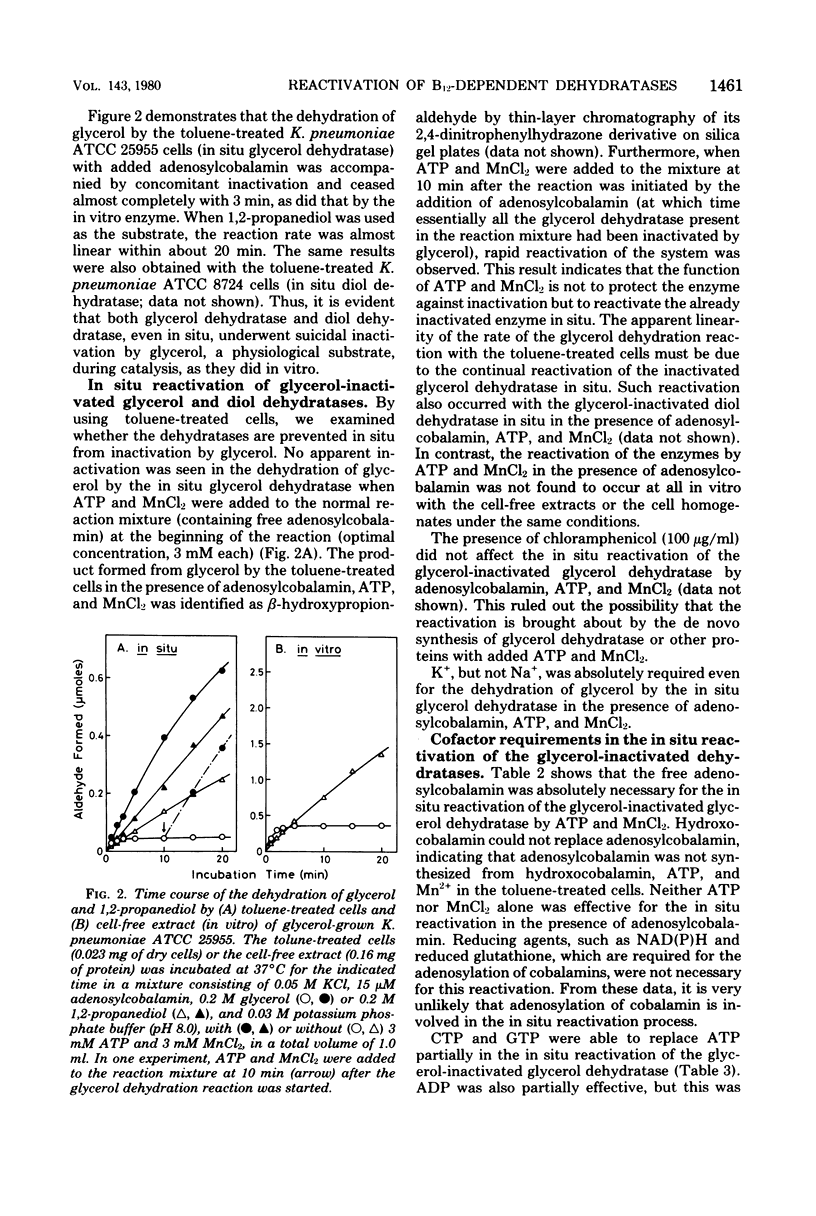
The catalytic properties of coenzyme B12-dependent glycerol dehydratase and diol dehydratase were studied in situ with Klebsiella pneumoniae cells permeabilized by toluene treatment, since the in situ enzymes approximate the in vivo conditions of the enzymes more closely than enzymes in cell-free extracts or cell homogenates. Both dehydratases in situ underwent rapid "suicidal" inactivation by glycerol during catalysis, as they do in vitro. The inactivated dehydratases in situ, however, were rapidly and continually reactivated by adenosine 5'-triphosphate (ATP) and Mn2+ in the presence of free adenosylcobalamin, although in cell-free extracts or in cell homogenates they could not be reactivated at all under the same reaction conditions. ATP was partially replaced by cytidine 5'-triphosphate or guanosine 5'-triphosphate but not by the beta, gamma-methylene analog of ATP in the in situ reactivation. Mn2+ was fully replaced by Mg2+ but only partially by Co2+. Hydroxocoblamin could not replace adenosylcobalamin in reactivation mixtures. The ability to reactivate the glycerol-inactivated dehydratases in situ was only seen in cells grown anaerobically in glycerol-containing media. This suggests that some factor(s) required for in situ reactivation is subject to induction by glycerol. Of the two possible mechanisms of in situ reactivation, i.e., the regeneration of adenosylcobalamin by Co-adenosylation of the bound inactivated coenzyme moiety (B12-adenosylation mechanism) and the displacement of the bound inactivated coenzyme moiety by free adenosyl-cobalamin (B12-exchange mechanism), the former seems very unlikely from the experimental results.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELES R. H., BROWNSTEIN A. M., RANDLES C. H. beta-Hydroxypropionaldehyde, an intermediate in the formation of 1,3-propanediol by Aerobacter aerogenes. Biochim Biophys Acta. 1960 Jul 15;41:530–531. doi: 10.1016/0006-3002(60)90054-8. [DOI] [PubMed] [Google Scholar]
- BRADY R. O., CASTANERA E. G., BARKER H. A. The enzymatic synthesis of cobamide coenzymes. J Biol Chem. 1962 Jul;237:2325–2332. [PubMed] [Google Scholar]
- Bachovchin W. W., Eagar R. G., Jr, Moore K. W., Richards J. H. Mechanism of action of adenosylcobalamin: glycerol and other substrate analogues as substrates and inactivators for propanediol dehydratase--kinetics, stereospecificity, and mechanism. Biochemistry. 1977 Mar 22;16(6):1082–1092. doi: 10.1021/bi00625a009. [DOI] [PubMed] [Google Scholar]
- Bachovchin W. W., Moore K. W., Richards J. H. Mechanism of action of adenosylcobalamin: hydrogen transfer in the inactivation of diol dehydratase by glycerol. Biochemistry. 1978 May 30;17(11):2218–2224. doi: 10.1021/bi00604a031. [DOI] [PubMed] [Google Scholar]
- Baker J. J., van der Drift C., Stadtman T. C. Purification and properties of -lysine mutase, a pyridoxal phosphate and B 12 coenzyme dependent enzyme. Biochemistry. 1973 Mar 13;12(6):1054–1063. doi: 10.1021/bi00730a006. [DOI] [PubMed] [Google Scholar]
- Forage R. G., Foster M. A. Resolution of the coenzyme B-12-dependent dehydratases of Klebsiella sp. and Citrobacter freundii. Biochim Biophys Acta. 1979 Aug 15;569(2):249–258. doi: 10.1016/0005-2744(79)90060-3. [DOI] [PubMed] [Google Scholar]
- Jackson R. W., DeMoss J. A. Effects of toluene on Escherichia coli. J Bacteriol. 1965 Nov;90(5):1420–1425. doi: 10.1128/jb.90.5.1420-1425.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE H. A., Jr, ABELES R. H. Purification and properties of dioldehydrase, and enzyme requiring a cobamide coenzyme. J Biol Chem. 1963 Jul;238:2367–2373. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morley C. G., Stadtman T. C. Studies on the fermentation of D-alpha-lysine. Purification and properties of an adenosine triphosphate regulated B 12-coenzyme-dependent D-alpha-lysine mutase complex from Clostridium sticklandii. Biochemistry. 1970 Dec 8;9(25):4890–4900. doi: 10.1021/bi00827a010. [DOI] [PubMed] [Google Scholar]
- PETERKOFSKY A., WEISSBACH H. Release of inorganic tripolyphosphate from adenosine triphosphate during vitamin B-12 coenzyme biosynthesis. J Biol Chem. 1963 Apr;238:1491–1497. [PubMed] [Google Scholar]
- Pawelkiewicz J., Zagalak B. Enzymic conversion of glycerol into beta-hydroxy-propionaldehyde in a cell-free extract from Aerobacter aerogenes. Acta Biochim Pol. 1965;12(3):207–218. [PubMed] [Google Scholar]
- Poznanskaya A. A., Yakusheva M. I., Yakovlev V. A. Study of the mechanism of action of adenosylcobalamindependent glycerol dehydratase from Aerobacter aerogenes. II. The inactivation kinetics of glycerol dehydratase complexes with adenosylobalamin and its analogs. Biochim Biophys Acta. 1977 Sep 15;484(1):236–243. doi: 10.1016/0005-2744(77)90128-0. [DOI] [PubMed] [Google Scholar]
- Reeves R. E., Sols A. Regulation of Escherichia coli phosphofructokinase in situ. Biochem Biophys Res Commun. 1973 Jan 23;50(2):459–466. doi: 10.1016/0006-291x(73)90862-0. [DOI] [PubMed] [Google Scholar]
- SMILEY K. L., SOBOLOV M. A cobamide-requiring glycerol dehydrase from an acrolein-forming Lactobacillus. Arch Biochem Biophys. 1962 Jun;97:538–543. doi: 10.1016/0003-9861(62)90118-2. [DOI] [PubMed] [Google Scholar]
- Schneider Z., Larsen E. G., Jacobson G., Johnson B. C., Pawelkiewicz J. Purification and properties of glycerol dehydrase. J Biol Chem. 1970 Jul 10;245(13):3388–3396. [PubMed] [Google Scholar]
- Schneider Z., Pawelkiewicz J. The properties of glycerol dehydratase isolated from Aerobacter aerogenes, and the properties of the apoenzyme subunits. Acta Biochim Pol. 1966;13(4):311–328. [PubMed] [Google Scholar]
- Toraya T., Fukui S. Immunochemical evidence for the difference between coenzyme-B12-dependent diol dehydratase and glycerol dehydratase. Eur J Biochem. 1977 Jun 1;76(1):285–289. doi: 10.1111/j.1432-1033.1977.tb11594.x. [DOI] [PubMed] [Google Scholar]
- Toraya T., Honda S., Fukui S. Fermentation of 1,2-propanediol with 1,2-ethanediol by some genera of Enterobacteriaceae, involving coenzyme B12-dependent diol dehydratase. J Bacteriol. 1979 Jul;139(1):39–47. doi: 10.1128/jb.139.1.39-47.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraya T., Honda S., Kuno S., Fukui S. Coenzyme B12-dependent diol dehydratase: regulation of apoenzyme synthesis in Klebsiella pneumoniae (Aerobacter aerogenes) ATCC 8724. J Bacteriol. 1978 Aug;135(2):726–729. doi: 10.1128/jb.135.2.726-729.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraya T., Kuno S., Fukui S. Distribution of coenzyme B12-dependent diol dehydratase and glycerol dehydratase in selected genera of Enterobacteriaceae and Propionibacteriaceae. J Bacteriol. 1980 Mar;141(3):1439–1442. doi: 10.1128/jb.141.3.1439-1442.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraya T., Shirakashi T., Kosuga T., Fukui S. Substrate specificity of coenzyme B12-dependent diol dehydrase: glycerol as both a good substrate and a potent inactivator. Biochem Biophys Res Commun. 1976 Mar 22;69(2):475–480. doi: 10.1016/0006-291x(76)90546-5. [DOI] [PubMed] [Google Scholar]
- Toraya T., Sugimoto Y., Tamao Y., Shimizu S., Fukui S. Propanediol dehydratase system. Role of monovalent cations in binding of vitamin B 12 coenzyme or its analogs to apoenzyme. Biochemistry. 1971 Aug 31;10(18):3475–3484. doi: 10.1021/bi00794a025. [DOI] [PubMed] [Google Scholar]
- Toraya T., Ushio K., Fukui S., Hogenkamp P. C. Studies on the mechanism of the adenosylcobalamin-dependent diol dehydrase reaction by the use of analogs of the coenzyme. J Biol Chem. 1977 Feb 10;252(3):963–970. [PubMed] [Google Scholar]
- Vitols E., Walker G. A., Huennekens F. M. Enzymatic conversion of vitamin B-12s to a cobamide coenzyme, alpha-(5,6-dimethylbenzimidazolyl)deoxyadenosylcobamide (adenosyl-B-12). J Biol Chem. 1966 Apr 10;241(7):1455–1461. [PubMed] [Google Scholar]
- Weitzman P. D., Hewson J. K. In situ regulation of yeast citrate synthase. Absence of ATP inhibition observed in vitro. FEBS Lett. 1973 Oct 15;36(2):227–231. doi: 10.1016/0014-5793(73)80374-6. [DOI] [PubMed] [Google Scholar]



