Abstract
The Programmed Cell Death 10 (PDCD10) (also known as Cerebral Cavernous Malformation-3; CCM3) gene encodes an evolutionarily conserved protein associated with cell apoptosis. Mutations in PDCD10 result in cerebral cavernous malformations, an important cause of cerebral hemorrhage. PDCD10 is associated with serine/threonine kinases and phosphatases and modulates the extracellular signal-regulated kinase (ERK) pathway suggesting a role of in the regulation of cellular growth. Here we provide evidence of a constitutive expression of PDCD10 in malignant T cells and cell lines from peripheral blood of cutaneous T cell lymphoma (Sezary syndrome) patients. PDCD10 is associated with Protein Phosphatase 2A (PP2A), a regulator of mitogenesis and apoptosis in malignant T cells. Inhibition of oncogenic signal pathways (Jak3, Notch1, and NF-κB partly inhibits the constitutive PDCD10 expression, while an activator of Jak3 and NFkB, interleukin-2 (IL-2), enhances PDCD10 expression. Functional data show that PDCD10 depletion by siRNA induces apoptosis and decreases proliferation of the sensitive cells. To our knowledge, these data provide the first functional link between PDCD10 and cancer.
Keywords: Cutaneous T-cell lymphoma, apoptosis, growth, PDCD10, CCM3
Introduction
Programmed Cell Death 10 (PDCD10)/Cerebral Cavernous Malformation-3(CCM3) is an evolutionarily conserved protein which has been associated with cell apoptosis. Inhibition of the nematode ortholog leads to embryoniclethality in 40% of the organisms (1) and PDCD10 is induced by apoptotic stimuli in a premyeloid cell line (2). Mutations in the PDCD10 gene cause cerebral cavernous malformations, which are vascular malformations that predispose to seizures and cerebral hemorrhages (3–7). Little is known about the function of PDCD10 in vivo but in a recent study, He et al. (8) obtained evidence that PDCD10 knock-out mice exhibited defects in angiogenesis and died at an early embryonic stage. Endothelial cell–specific deletion of PDCD10 reduced vascular endothelial growth factor receptor 2 (VEGFR2) signalling (8). PDCD10 was recruited to and stabilized VEGFR2 thereby facilitating ligand-mediated receptor signalling (8). Other studies have shown that PDCD10 stabilized GCKIII proteins to promote Golgi assembly and cell orientation (9), inhibited Rho kinases (10;11), and interacted with the serine/threonine protein kinases and phosphatases including the Fas-associated phosphatase and the Protein Phosphatase 2A (PP2A) (7;12). Likewise, PDCD10 was shown to modulate the activity of the extracellular signal-regulated kinase (ERK) pathway (13). Taken together, these and other data (13) indicate that PDCD10 plays a role in protein synthesis, migration, apoptosis and cellular proliferation raising the possibility that PDCD10 may also play a role in cancer.
Cutaneous T-cell lymphomas (CTCLs) are the most frequent primary lymphomas of the skin (14). They comprise a spectrum of lymphoproliferative disorders characterised by clonal proliferation and accumulation of neoplastic T lymphocytes in the epidermis. Mycosis fungoides (MF) and the leukemic variant Sezary syndrome (SS) are the two major clinical forms of CTCL (15). The etiology is unknown, but pro-oncogenic signalling pathways are typically constitutively activated in primary tumour cells and cell lines obtained from skin biopsies and peripheral blood of patients (16–20). Importantly, these signalling pathways including Jak3/STAT3, Notch-1, NFkB, BLK, and COX-2 drive malignant proliferation (16–20). Moreover, the Jak3/STAT3 and NFkB pathways partially protect malignant T cells from apoptosis. Malignant T cells may also display a deficient apoptotic response due to mutations or impaired expression (e.g. caused by epigenetic silencing) of molecules involved in the execution of apoptosis (18;21–25). However, these observations do not fully account for the malignant proliferation and resistance to apoptosis observed in CTCL. As our preliminary screening of apoptosis-resistance genes in CTCL patients revealed a constitutive mRNA expression of PDCD10 in the malignant T cells, the present study was undertaken to address the protein expression and function of PDCD10.
Materials and Methodology
Cell lines and cell cultures
The malignant T-cell lines SeAx and Sez-4 are established from the peripheral blood of patients diagnosed with SS (26–28), MyLa2000 is a malignant T-cell line established from a plaque biopsy of a patient with MF (26–28). MF1850 and MySi are two non-malignant T-cell lines derived from the skin of a patients with MF (26;28;29) (27). The malignant T-cell lines Jurkat (J-Tag) and JB6 have been described elsewhere (29,30) and Psor-2 is a T-cell line obtained from a punch biopsy of a patient with psoriasis vulgaris (31). The non-malignant T-cell lines from healthy donors have been described in detail elsewhere (32–35). MyLa, Jurkat and JB6 were cultured in conditional media (RPMI 1640, 2mM L-glutamine, 0.1 mg/ml penicillin and 0.1 mg/ml Strepotmycin all from Sigma-Aldrich, St Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS) (Life Technologies, Roskilde, Denmark). SeAx, Sez-4, Psor-2 and MySi were cultured in conditional media supplemented with 10% pooled human serum (HS) (Blood Bank, State University Hospital, Copenhagen, Denmark) and 103 U/ml IL-2 (Proleukin). MF1850 cells were cultured in the same media as SeAx with addition of 2.5 ng/mL IL-4 (Leinco, St Louis, MO, USA). The cell lines were tested regularly to be negative for Mycoplasma. Primary tumor cells were acquired from peripheral blood of three patients diagnosed with SS in accordance with the WHO-EORTC classification (36) and have been described elsewhere (27).
Reagents and antibodies
The antibodies against PDCD10 (CCM3), Malcavernin (CCM2), ERK, PP2A A α/β and PP2A C α/β were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antibody against STAT3 was from Cell Signaling Technology (Beverly, MA, USA) and the α-actin monoclonal antibody and 7-Amino-actinomycin D (7-AAD) were from Sigma-Aldrich. The Anti-rabbit and anti-mouse horseradish peroxidase (HRP) antibodies were from Dako Cytomation (Glostrup, Denmark). The anti-goat-HRP antibody was from Santa Cruz Biotechnology. Non-targeting SMARTpool small interfering RNA (siRNA) and STAT3 siRNA were from Dharmacon (Chicago, IL, USA) and PDCD10 siRNA was from Santa Cruz Biotechnology.
The Lck inhibitor (LckI), the Jak3 inhibitor I (Jak3I), Mg-132, PD98059 and γ-secretase inhibitor 1 (GSI 1) were from Calbiochem (San Diego, CA, USA). SP600125 (JNKI), Wortmannin (PI3KI), SB203580 (P38I) and Spironolactone were from Alexis (Laufelfigen, Switzerland), DMSO (dimethyl sulfoxide) from Sigma-Aldrich, and COX-2 inhibitor (Celebra) was from Pfizer (Ballerup, Denmark).
SiRNA transfection
SiRNA transfection were performed by using the Amaxa nucleofector system (Amaxa GmbH, Cologne, Germany), which is based on electroporation. In brief, 2×106 cells were collected and transfected using a final concentration of 5 nmol siRNA. The living transfected cells were counted and used for 3H-thymidine proliferation and 7-AAD apoptosis assays. The extent of protein knockdown was quantified by Western blotting of the remaining cells (37).
3H-thymidine incorporation proliferation assay
3H-thymidine incorporation proliferation assay was performed as described elsewhere (38,39). In brief, cells were grown in 96-well round-bottom tissue culture plates in a final volume of 150 µl for 48 hours. Sixteen hours before harvest, 1µCi 3H-thymidine was added to the cultures. The cells were harvested using a Filtermate harvester and 96-well microplates with bonded GF/C (PerkinElmer, USA). 3H-thymidine incorporation was measured in a TopCount scintillation counter (PerkinElmer). The proliferation was expressed as mean counts per minute (CPM) of a minimum of triplicated cultures, repeated in 8 independent experiments and shown as standard errors of means (SEM).
7-AAD apoptosis assay
For the quantification of apoptotic cells, 7-AAD labelling was used. In brief, cells were harvested, washed once in FACS buffer and, subsequently, in 200 µl PBS containing 0.05% saponin. Then, the samples were incubated with 400µl 7-AAD-Saponin-PBS for 30 minutes in the dark at room temperature and analyzed on a FACSCalibur using CellQuestPro software (Becton Dickinson, Brøndby, Denmark) as described previously (16).
RNA isolation and reverse transcriptase-PCR
Total RNA was isolated using RNeasy Mini Kit (Qiagen, Ballerup, Denmark) according to the manufacturer’s instructions and reverse transcriptase-PCR was performed as described elsewhere (37) (all reagents were from Invitrogen, Paisley, UK; Taq polymerase is from New England Biolabs, Danvers, MA, USA). Primers were designed with Primer3 v 0.4.0 software (Duke-NUS Graduate Medical School, Singapore) and synthesized by Eurofins MWG GmbH (Martinsried, Germany).
Protein extraction, immunoprecipitation, and Western Blotting
After treatment with or without inhibitors, the cells (2×106 cells/experiment for whole-cell lysates and 20×106 for immunoprecipitation) were rapidly pelleted, and lysed in ice-cold lysis buffer [1% NP-40, 20 mM Tris, HCl, pH 8.0, 137 mM NaCl 10% glycerol with the following inhibitors: 1 mM PMSF (in DMSO), 5 mM EDTA, 1 mM Na3VO4, 10 mg/ml aprotinin, 10 ml/ml indoleacetic acid, 10 mM NaF. Immunoblotting and immunoprecipitation were conducted as previously described (37). PP2A was immunoprecipitated from cell lysates with the use of a PP2A-Cα/β antibody Santa Cruz Biotechnology. Blots were evaluated by using enhanced chemiluminescence according to the manufacturer’s manual (Amersham Pharmacia, Ballerup, Denmark).
Statistics
3H-thymidine incorporation (performed as a minimum in triplicates) and 7-AAD staining assays were repeated 8 and 5 times, respectively. Data are reported as SEM and the differences were evaluated by the Wilcoxon Rank sum test for paired differences. A P-value <0.05 was considered to be statistically significant. Statistical analysis was performed by the use of GraphPad Prism Version 4.03 (GraphPad Software Inc., San Diego, CA) or Excel (Microsoft Corp., Redmond, WA).
Results
PDCD10 protein is expressed in T-cell lines and primary cells from SS patients
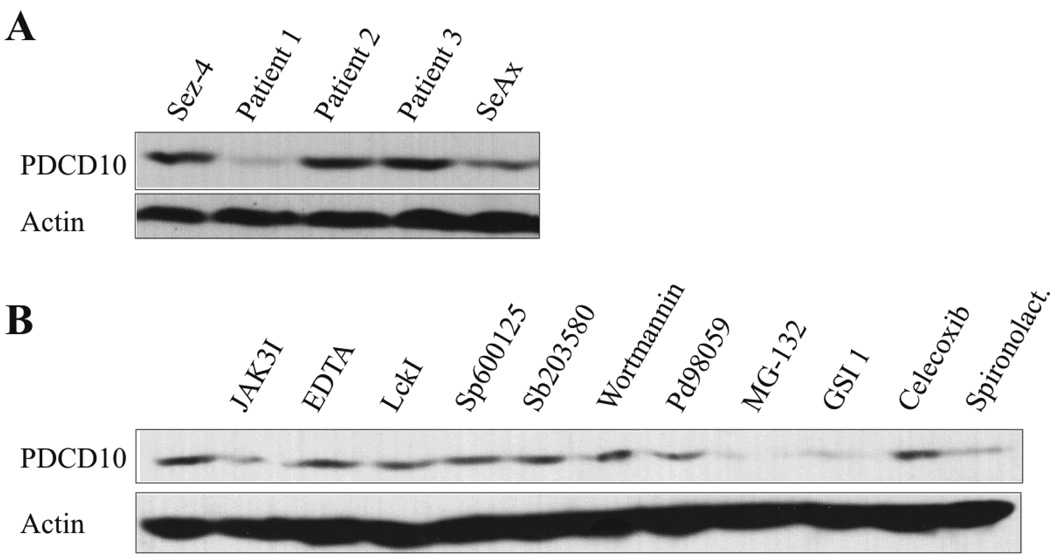
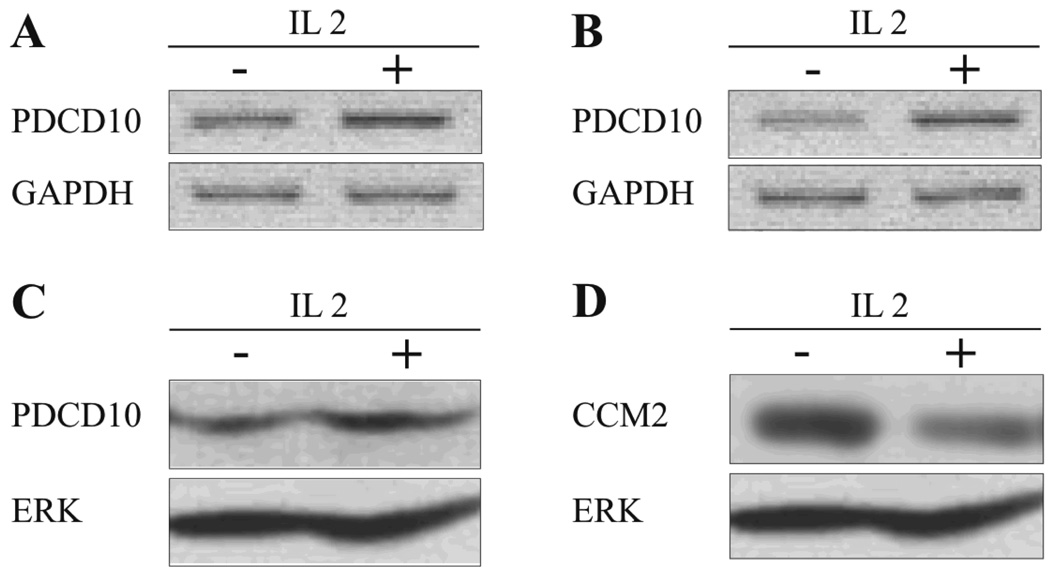
As shown in Fig. 1A, PDCD10 protein was expressed both in SS cell lines and in primary cells from the peripheral blood of two out of three SS patients. Likewise, PDCD10 protein was expressed in four malignant T-cell lines from MF patients and other T-cell malignancies (data not shown) In order to address whether oncogenic signalling pathways are involved in the constitutive PDCD10 expression, malignant T cells were treated with relevant inhibitors for 16 hours prior to Western Blot analysis. As shown in Fig. 1B, inhibitors of Notch (GSI 1) and the NFkB signalling pathway (MG-132 and spironolactone) partly inhibited PDCD10 expression (Fig. 1B, lane 9, 10 and 12). Likewise, an inhibitor of Jak3 (Jak3I), weakly inhibited PDCD10 expression whereas inhibitors of BLK, c-Jun N-terminal kinase, and p38 and Erk MAP kinases as well as a potent inhibitor of cyclooxyginase-2 (COX2) had no inhibitory effect (Fig. 1B) indicating that PDCD10 protein expression is partly regulated by Jak3, Notch1, and NFkB in these cells. As PDCD10 expression was not fully suppressed by these inhibitors (Fig. 1B, and data not shown), the expression appears to be regulated by other as yet unknown pathways. Jak3/STAT3 signalling is enhanced by IL-2 (40) and, as shown in Fig. 2A–C, IL-2 triggered a modest increase in PDCD10 mRNA and protein expression in responsive malignant- and non-malignant T cells. This effect was specific, as the expression of the closely related protein CCM2 was inhibited and not enhances by IL-2 (Fig. 2D). Interestingly, Jak3/STAT3, Notch1, and NFkB have been implicated in tumor growth and resistance to apoptosis in CTCL suggesting that PDCD10 might be involved in Jak3/Notch1/NFkB-mediated mitogenesis and anti-apoptosis.
Figure 1. Malignant SS cell lines and in primary leukemic Sézary cells constitutively express PDCD10.
(A)Western blot analysis of whole-cell lysates from SS cell lines established from peripheral blood of patients diagnosed with SS: Sez-4 (lane 1) and SeAx (lane 5), and from primary cells obtained from peripheral blood of three patients (patient 1–3) diagnosed with SS (Lane 2–4). The blots were probed with the antibodies against PDCD10 and visualized as bands at ~26kDa. (B) Malignant SS cells (SeAx) were treated with JAK3I (3 µM), EDTA (5 µM), LckI (10 µM), Sp600125 (5 µM), Sb203580 (10 µM), Wortmannin (5 µM), PD98059 (50 µM), MG-132 (10 µM), GSI 1 (5 µM), Celecoxib (50 µM), spironolactone (3 µM) or the vehicle (DMSO) for sixteen hours prior to Western blot analysis for the expression of PDCD10.
Figure 2. IL-2 triggers an increase in PDCD10 mRNA and protein expression malignant and non-malignant T cells.
PDCD10 mRNA expression in (A) non-malignant T cells (MF1850) and (B) malignant SS cells (SeAx) incubated with or without 1000 U/ml IL-2 for 24 hours (reverse transcriptase-PCR (RT-PCR)). (C) PDCD10 protein expression in malignant SS cells (SeAx) following incubation with or without 1000 U/ml IL-2 for 24 hours was determined by Western blot. (D) PDCD10 protein expression determined by Western blot of whole-cell lysates of malignant SS cells incubated with or without 1000 U/ml IL-2 for 24 hours.
PDCD10 protein is associated with Protein Phosphatase 2A (PP2A) in non-malignant and malignant T cells
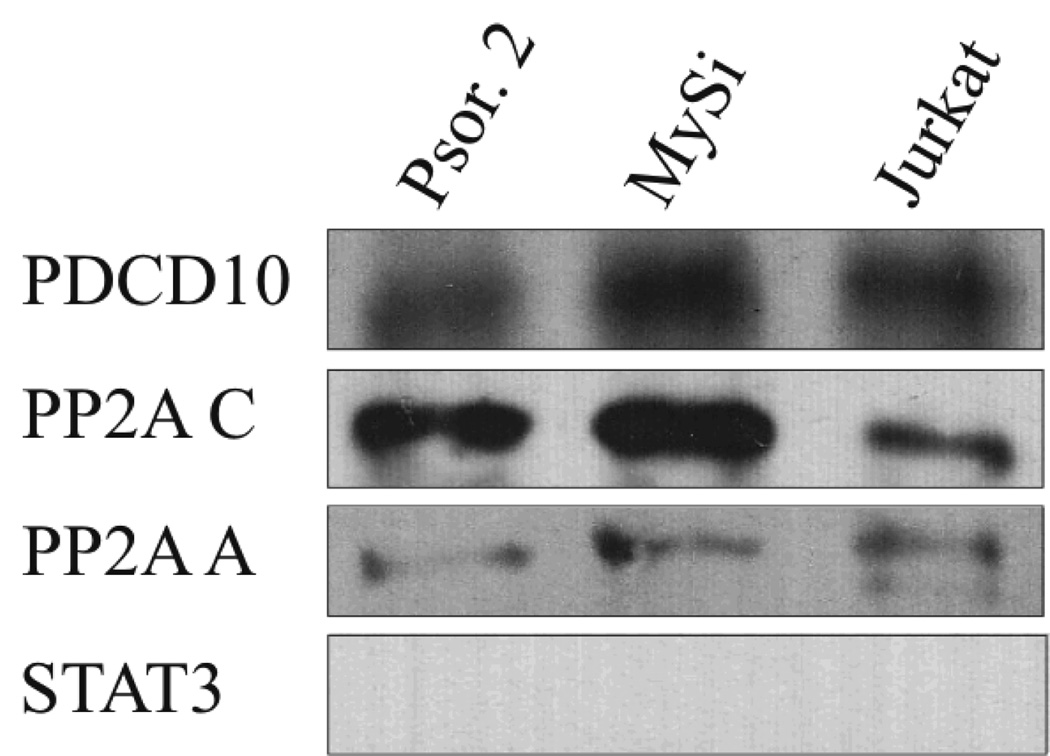
PDCD10 is associated with PP2A and ERK (4;8;13) and IL-2 modulates PP2A activity in non-malignant vascular cells (41;42). Therefore, we examined whether PDCD10 and PP2A were associated in non-malignant and malignant T cells and as shown in Fig. 3, PDCD10 co-precipitated with the PP2A catalytic (PP2Ac) subunit in malignant T cells (Fig. 3, lane 3, upper row) and control cells (Fig. 3, lanes 1 and 2). Re-blotting with an antibody against the scaffolding (PP2A A) α and β subunits showed that these subunits were also part of the complex (Fig. 3 third row) whereas re-blotting with an antibody against the PP2Ac subunit confirmed that PP2Ac had indeed been precipitated by the anti-PP2Ac antibody (Fig. 3, second row). These findings indicate that PDCD10 is constitutively associated with the PP2A complex in non-malignant and malignant T cells (Fig. 3, lane 3). Essentially similar findings were observed in non-malignant T cells (Fig. 3, lane 1 and 2), in general agreement with a recent study of the transfected epithelial HEK cells using MALDI-TOF mass spectrometry (12). STAT3 plays a key role in malignant proliferation and its activation is critically dependent on PP2A in malignant T cells (43) but STAT3 did not co-precipitate with PP2A suggesting that it is not a part of the PP2A/PDCD10 complex (Fig. 3, lane 4). Likewise, PDCD10 did not associated with the VEGF-R2 (data not shown) as malignant T cells do not express this receptor (17).
Figure 3. PP2A and PDCD10 are constitutively associated in malignant and non-malignant T cells.
Non-malignant (lane 1, Psor.2 and lane 2, MySi) and malignant (lane 3, Jurkat) T cells were immunoprecipitated with PP2A C. Subsequently, the expression of PDCD10, PP2A A, and PP2A C were determined by Western blot.
Specific down-regulation of PDCD10 inhibits proliferation and induces apoptosis
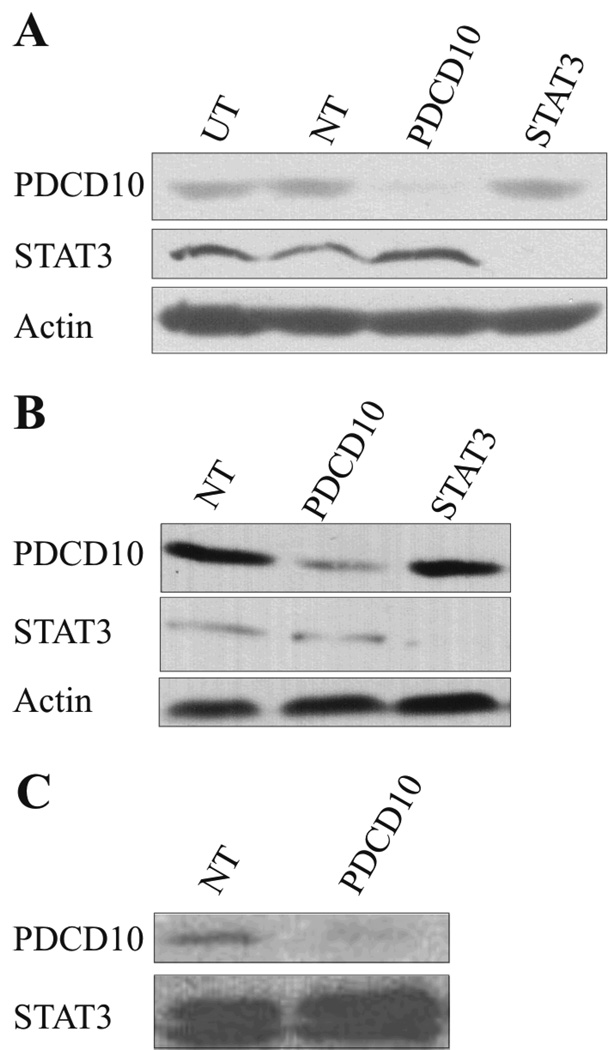
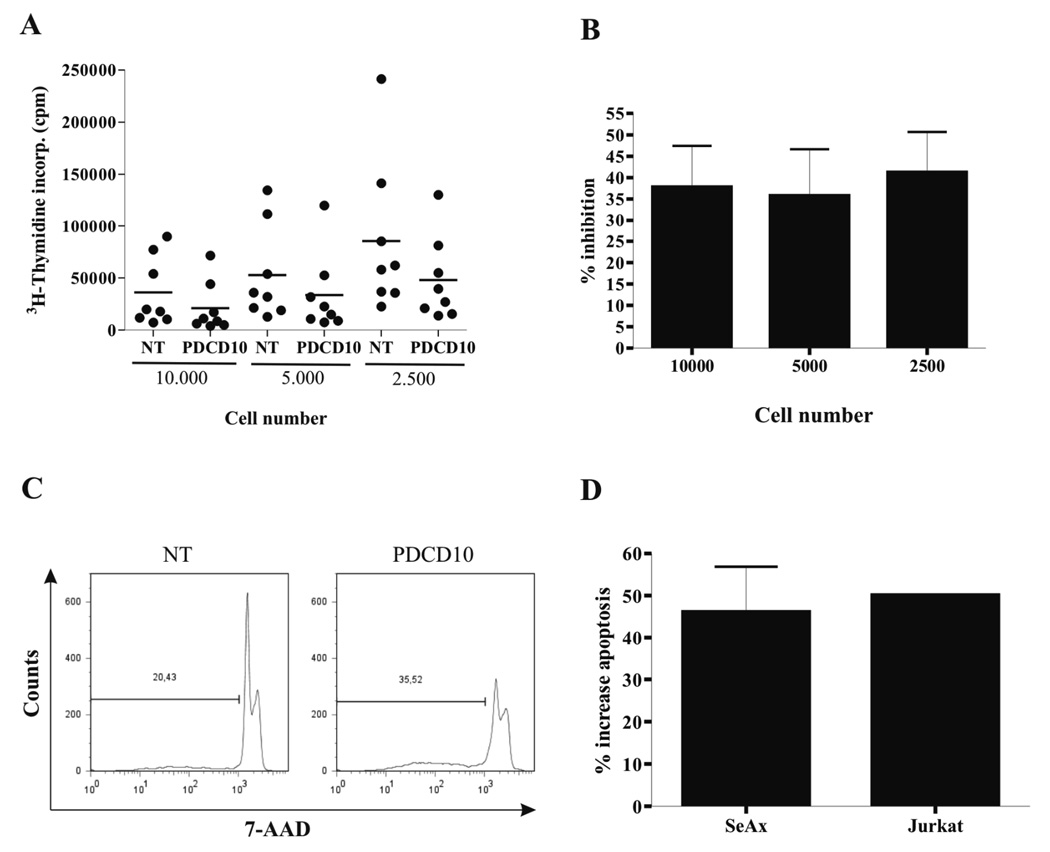
To investigate the function of PDCD10, malignant T cells were transfected with PDCD10 siRNA STAT3 siRNA, or non-targeting siRNA as a control, 48 hours prior to Western Blotting analysis for PDCD10 expression. As shown in Fig. 4A–C, PDCD10 siRNA down-regulated the expression of PDCD10 whereas the expression of STAT3 and actin was unaffected indicating that RNA interference was suitable for selective PDCD10 inhibition. Conversely, STAT3 siRNA blocked the expression of STAT3 whereas the expression of PDCD10 and actin was unaffected (Fig. 4A–B) suggesting that PDCD10 expression is regulated via a Jak3 dependent, STAT3- independent pathway (Fig. 1B versus Fig. 4A+B). Next, we examined whether PDCD10 knock-down influenced the spontaneous proliferation of the malignant T cells and as shown in Fig. 5 (A and B), PDCD10 siRNA triggered a statistically significant inhibition of 3H-thymidine-uptake in the malignant T cells when compared to cells treated with a non-targeting siRNA. Thus, the spontaneous proliferation was inhibited in SeAx cells by PDCD10 siRNA at all cell concentrations tested (38% inhibition, p < 0.001 at 10.000 cells per well, 36 % inhibition, p < 0.05 at 5.000 cells per well, 48 % inhibition, p < 0.001, at 2.500 cell per well, Wilcoxon Rank sum test for paired differences (Fig. 5B)). A similar level of inhibition was observed in other sensitive malignant T-cell lines including the Jurkat cell line (p < 0.0001), whereas other malignant T cell lines including the MyLa and JB6 cell lines were largely unaffected by the PDCD10 siRNA despite a clear-cut down-regulation of PDCD10 protein (Fig. 4B–C and data not shown). As PDCD10 has been implicated in apoptosis, we addressed whether PDCD10 siRNA influenced the degree of apoptosis. Accordingly, malignant T cells were transiently transfected with PDCD10 siRNA or a non-targeting control siRNA 48 hours prior to 7-AAD labelling and flow cytometric analysis. As shown in Fig. 5C, PDCD10 siRNA triggered a significant increase in apoptosis. Thus, 35% of apoptotic cells were observed in PDCD10 siRNA-treated cells compared to 20% in controls cultures (Fig. 5C). Essentially, similar results were obtained in five independent experiments (p < 0.05, Wilcoxon rank sum test for paired differences (Fig. 5D)) and in other sensitive cells including the Jurkat cell line (Fig. 5D). In contrast, no difference between PDCD10 siRNA and control siRNA treated cells was observed in the MyLa and JB6 cell lines (data not shown). As PP2A inhibition triggers serine 727 phosphosrylation of STAT3 and PP2A is associated with PDCD10, we assayed whether PDCD10 siRNAs modulated STAT3 expression and phosphorylation. However, PDCD10 knock-down had no effect on either the expression of STAT3 protein or the level of serine phosphorylation of STAT3 as judged from reactivity with a phospho-serine 727 specific antibody (Fig. 4A–C and data not shown).
Figure 4. Specific downregulation of PDCD10 in malignant T cell lines.
Three different malignant T-cell lines (A–C, SeAx, JB6 and MyLa respectively) were transiently transfected with non-targeting (NT), PDCD10- or STAT3-specific siRNA. Western blot of whole-cell extracts were prepared from the cells 48 hours after transfection and probed with antibodies against PDCD10, STAT3 and actin.
Figure 5. Specific downregulation of PDCD10 inhibits proliferation and induces apoptosis.
(A) Dot blot and (B) % inhibition of 3H-thymidine incorporation of malignant SS cells (SeAx) that were transiently transfected with non-targeting (NT) or PDCD-10-specific siRNA. 10.000, 5.000 or 2.500 transfected cells/well were cultured for 48 hours in a 96 round bottom culture plate. Sixteen hours before harvest, 3H-thymidine (1 µCi ~[0.037 MBq]/well) was added to the cultures and the results are expressed as mean counts per minute of eight independent experiments. (38% inhibition, p < 0.001 at 10.000 cells per well, 36 % inhibition, p < 0.05 at 5.000 cells per well, 48 % inhibition, p < 0.001, at 2.500 cell per well, Wilcoxon Rank sum test for paired differences). (C) SeAx cells were stained with 7-AAD and analyzed by flow cytometry. Data are representative of five independent experiments. (D) % increase in apoptosis of malignant SS cells (SeAx) and Jurkat cells 48 hours after transient transfection with non-targeting (NT) or PDCD10-specific siRNA. Error bars of SeAx represent SEM of five independent experiments while measurement of apoptosis in the Jurkat cell line following transfection only was performed once.
Discussion
In the present study, we provide the first evidence that PDCD10 is constitutively expressed in malignant T cells from SS and other T-cell malignancies. As judged from the effect of small molecular inhibitors, the expression of PDCD10 was partly dependent of the Jak3, Notch1, and NFkB signalling pathways which are constitutively active and involved in the spontaneous proliferation and survival of malignant T cells. This conclusion is in keeping with our findings that PDCD10 expression was enhanced by IL-2, a Jak3 dependent growth- and survival factor in T cells (43). However, a basic level of PDCD10 expression was not influenced by Jak3/Notch1/NFkB inhibition. Moreover, several cell lines did not respond to IL-2, and were resistant to Jak3/NFkB/Notch inhibition indicating that other transcription factors are also involved in PDCD10 expression. Inhibitors of the known oncogenic signalling pathways in malignant T cells (including COX-2, Src kinases, JNK(16;17)) had no effect and malignant T cells do not express the VEFG-R2 (17) which has previously been implicated in PDCD10 biology (8). Thus, it seems clear that as yet unidentified signalling molecules regulate the expression of PDCD10 in malignant and non-malignant T cells.
Inhibition of the nematode ortholog of PDCD10 gives rise to lethality in 40% of the embryos (1) suggesting a role for PDCD10 in the regulation of mitogenesis and apoptosis. Our findings of a highly significant effect of PDCD10 depletion by siRNA on the spontaneous proliferation of the sensitive, malignant T cells indicate that PDCD10 plays a regulatory role in malignant proliferation and/or anti-apoptosis. This conclusion was substantiated by the finding that PDCD10 siRNA in sensitive cells triggered an enhanced apoptosis when compared to a non-targeting control siRNA. Although PDCD10 is known to modulate ERK signalling (13), ERK is often inactive in malignant T cells (45) suggesting that ERK may not be involved in PDCD10-induced cell proliferation and survival. Therefore, we focused on STAT3 because STAT3 (i) is activated by Jak3 which is aberrantly activated in malignant T cells, (ii) regulates proliferation and survival in malignant T cells, (iii) depends on PP2A activity to stay functionally active, and (iv) can be a part of the multi-molecule complex involving PP2A (18;19;43;46). Our findings confirmed that PDCD10 is indeed associated with a complex of PP2A sub-units in normal and malignant T cells. However, STAT3 was not part of this multi-molecule complex as judged from our co-precipitation studies. Likewise, STAT3 expression and phosphorylation was not affected by PDCD10 depletion. As PP2A inhibition triggers a STAT3 hyper-phosphorylation on serine 727 (43), PDCD10 may not act via a modulation of PP2A enzymatic activity or substrate (STAT3) specificity. Moreover, STAT3 knock-down did not influence the expression of PDCD10. It is well established that Jak3 directly and indirectly activates several signalling molecules and transcription factors including STAT5 which is a key regulator of mitogenesis. Therefore, we speculate that PDCD10 expression is regulated via a Jak3- dependent, STAT3 independent pathway. As PDCD10 has recently been found to have a multitude of functions including Golgi-transport, cytoskeleton organization, and receptor stabilization and signalling (7–9), the effect of PDCD10 reported here could be due to a modulation of one or more of these processes.
It was puzzling that some cells were functionally sensitive to PDCD10 depletion whereas others were resistant despite similar level of the protein expression and despite similar degree of the protein knock-down by PDCD10 siRNA. It has become clear that PDCD10, in addition to its interaction with PP2A, can form complexes with other members of the CCM family including CCM2, a key mediator of receptor tyrosine kinases-dependent cell death in the neuroblastic tumors (47). Accordingly, we hypothesize that the balance between pro-apoptotic CCM2 and anti-apoptotic PDCD10 determines the net effect of the CCM2/PDCD10 complex on the apoptotic rate. In keeping with this hypothesis, IL-2 (a potent cell growth and survival factor) down-regulated the expression of CCM2 and boosted the expression of PDCD10. Interestingly, our preliminary findings suggest that the most sensitive cells expressed the highest levels of CCM2 (data not shown). Therefore, we speculate that PDCD10 knock-down in these cells shifted the CCM2/PDCD10 balance towards a pro-apoptotic dominance of CCM2. Studies are in progress to test this hypothesis.
In conclusion, our study shows that the majority of non-malignant and malignant T cells display constitutive expression of PDCD10, which in the sensitive cells protects against apoptosis and promotes cell proliferation.
Acknowledgments
This work was supported by grants from The University of Copenhagen, The Danish Cancer Society, The Danish Research Councils, The Novo Nordic Foundation, The Neye Foundation, The Lundbeck Foundation, The Beckett Foundation, and The National Cancer Institute. We wish to thank Keld Kaltoft (Århus University and CellCure Århus, Denmark) for the generous gift of the MyLa cell lines. The project part concerning establishment and study of CTCL cell lines by Dr. Keld Kaltoft has been approved by "Den videnskabsetiske Kommite i Århus Amt" (The science-ethical committee in Århus County).
Footnotes
Conflict-of-interest disclosure
The authors declare no competing financial interests.
Reference List
- 1.Kamath RS, Fraser AG, Dong Y, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Liu H, Zhang Y, Ma D. cDNA cloning and expression of an apoptosis-related gene, humanTFAR15 gene. Sci China C Life Sci. 1999;42:323–329. doi: 10.1007/BF03183610. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Tanriover G, Yano H, Friedlander R, Louvi A, Gunel M. Apoptotic functions of PDCD10/CCM3, the gene mutated in cerebral cavernous malformation 3. Stroke. 2009;40:1474–1481. doi: 10.1161/STROKEAHA.108.527135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen PY, Chang WS, Lai YK, Wu CW. c-Myc regulates the coordinated transcription of brain disease-related PDCD10-SERPINI1 bidirectional gene pair. Mol Cell Neurosci. 2009;42:23–32. doi: 10.1016/j.mcn.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Pagenstecher A, Stahl S, Sure U, Felbor U. A two-hit mechanism causes cerebral cavernous malformations: complete inactivation of CCM1, CCM2 or CCM3 in affected endothelial cells. Hum Mol Genet. 2009;18:911–918. doi: 10.1093/hmg/ddn420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stahl S, Gaetzner S, Voss K, et al. Novel CCM1, CCM2, and CCM3 mutations in patients with cerebral cavernous malformations: in-frame deletion in CCM2 prevents formation of a CCM1/CCM2/CCM3 protein complex. Hum Mutat. 2008;29:709–717. doi: 10.1002/humu.20712. [DOI] [PubMed] [Google Scholar]
- 7.Voss K, Stahl S, Schleider E, et al. CCM3 interacts with CCM2 indicating common pathogenesis for cerebral cavernous malformations. Neurogenetics. 2007;8:249–256. doi: 10.1007/s10048-007-0098-9. [DOI] [PubMed] [Google Scholar]
- 8.He Y, Zhang H, Yu L, et al. Stabilization of VEGFR2 signaling by cerebral cavernous malformation 3 is critical for vascular development. Sci Signal. 2010;3:ra26. doi: 10.1126/scisignal.2000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fidalgo M, Fraile M, Pires A, Force T, Pombo C, Zalvide J. CCM3/PDCD10 stabilizes GCKIII proteins to promote Golgi assembly and cell orientation. J Cell Sci. 2010;123:1274–1284. doi: 10.1242/jcs.061341. [DOI] [PubMed] [Google Scholar]
- 10.Stockton RA, Shenkar R, Awad IA, Ginsberg MH. Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. J Exp Med. 2010;207:881–896. doi: 10.1084/jem.20091258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borikova AL, Dibble CF, Sciaky N, et al. Rho kinase inhibition rescues the endothelial cell cerebral cavernous malformation phenotype. J Biol Chem. 2010;285:11760–11764. doi: 10.1074/jbc.C109.097220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goudreault M, D'ambrosio LM, Kean MJ, et al. A PP2A phosphatase high density interaction network identifies a novel striatin-interacting phosphatase and kinase complex linked to the cerebral cavernous malformation 3 (CCM3) protein. Mol Cell Proteomics. 2009;8:157–171. doi: 10.1074/mcp.M800266-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin JL, Chen HC, Fang HI, Robinson D, Kung HJ, Shih HM. MST4, a new Ste20-related kinase that mediates cell growth and transformation via modulating ERK pathway. Oncogene. 2001;20:6559–6569. doi: 10.1038/sj.onc.1204818. [DOI] [PubMed] [Google Scholar]
- 14.Bradford PT, Devesa SS, Anderson WF, Toro JR. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113:5064–5073. doi: 10.1182/blood-2008-10-184168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim EJ, Hess S, Richardson SK, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115:798–812. doi: 10.1172/JCI24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopp KL, Kauczok CS, Lauenborg B, et al. COX-2-dependent PGE(2) acts as a growth factor in mycosis fungoides (MF) Leukemia. 2010 doi: 10.1038/leu.2010.66. [DOI] [PubMed] [Google Scholar]
- 17.Krejsgaard T, Vetter-Kauczok CS, Woetmann A, et al. Jak3- and JNK-dependent vascular endothelial growth factor expression in cutaneous T-cell lymphoma. Leukemia. 2006;20:1759–1766. doi: 10.1038/sj.leu.2404350. [DOI] [PubMed] [Google Scholar]
- 18.Sommer VH, Clemmensen OJ, Nielsen O, et al. In vivo activation of STAT3 in cutaneous T-cell lymphoma. Evidence for an antiapoptotic function of STAT3. Leukemia. 2004;18:1288–1295. doi: 10.1038/sj.leu.2403385. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, Wang HY, Woetmann A, Raghunath PN, Odum N, Wasik MA. STAT3 induces transcription of the DNA methyltransferase 1 gene (DNMT1) in malignant T lymphocytes. Blood. 2006;108:1058–1064. doi: 10.1182/blood-2005-08-007377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamstrup MR, Gjerdrum LMR, Biskup E, Et Gniadecki R. Notch as a potential therapeutic target in cutaneous T-cell lymphoma. Blood. 2010 doi: 10.1182/blood-2009-12-260216. in press. [DOI] [PubMed] [Google Scholar]
- 21.Contassot E, French LE. Epigenetic causes of apoptosis resistance in cutaneous T-cell lymphomas. J Invest Dermatol. 2010;130:922–924. doi: 10.1038/jid.2009.427. [DOI] [PubMed] [Google Scholar]
- 22.Klemke CD, Brenner D, Weiss EM, et al. Lack of T-cell receptor-induced signaling is crucial for CD95 ligand up-regulation and protects cutaneous T-cell lymphoma cells from activation-induced cell death. Cancer Res. 2009;69:4175–4183. doi: 10.1158/0008-5472.CAN-08-4631. [DOI] [PubMed] [Google Scholar]
- 23.Ni X, Zhang C, Talpur R, Duvic M. Resistance to activation-induced cell death and bystander cytotoxicity via the Fas/Fas ligand pathway are implicated in the pathogenesis of cutaneous T cell lymphomas. J Invest Dermatol. 2005;124:741–750. doi: 10.1111/j.0022-202X.2005.23657.x. [DOI] [PubMed] [Google Scholar]
- 24.Wu J, Nihal M, Siddiqui J, Vonderheid EC, Wood GS. Low FAS/CD95 expression by CTCL correlates with reduced sensitivity to apoptosis that can be restored by FAS upregulation. J Invest Dermatol. 2009;129:1165–1173. doi: 10.1038/jid.2008.309. [DOI] [PubMed] [Google Scholar]
- 25.Zhang CL, Kamarashev J, Qin JZ, Burg G, Dummer R, Dobbeling U. Expression of apoptosis regulators in cutaneous T-cell lymphoma (CTCL) cells. J Pathol. 2003;200:249–254. doi: 10.1002/path.1341. [DOI] [PubMed] [Google Scholar]
- 26.Brender C, Lovato P, Sommer VH, et al. Constitutive SOCS-3 expression protects T-cell lymphoma against growth inhibition by IFNalpha. Leukemia. 2005;19:209–213. doi: 10.1038/sj.leu.2403610. [DOI] [PubMed] [Google Scholar]
- 27.Krejsgaard T, Gjerdrum LM, Ralfkiaer E, et al. Malignant Tregs express low molecular splice forms of FOXP3 in Sezary syndrome. Leukemia. 2008;22:2230–2239. doi: 10.1038/leu.2008.224. [DOI] [PubMed] [Google Scholar]
- 28.Woetmann A, Lovato P, Eriksen KW, et al. Nonmalignant T cells stimulate growth of T-cell lymphoma cells in the presence of bacterial toxins. Blood. 2007;109:3325–3332. doi: 10.1182/blood-2006-04-017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geisler C, Scholler J, Wahi MA, Rubin B, Weiss A. Association of the human CD3-zeta chain with the alpha beta-T cell receptor/CD3 complex. Clues from a T cell variant with a mutated T cell receptor-alpha chain. J Immunol. 1990;145:1761–1767. [PubMed] [Google Scholar]
- 30.Pasqualucci L, Wasik M, Teicher BA, et al. Antitumor activity of anti-CD30 immunotoxin (Ber-H2/saporin) in vitro and in severe combined immunodeficiency disease mice xenografted with human CD30+ anaplastic large-cell lymphoma. Blood. 1995;85:2139–2146. [PubMed] [Google Scholar]
- 31.Eriksen KW, Lovato P, Skov L, et al. Increased sensitivity to interferon-alpha in psoriatic T cells. J Invest Dermatol. 2005;125:936–944. doi: 10.1111/j.0022-202X.2005.23864.x. [DOI] [PubMed] [Google Scholar]
- 32.Amos CL, Woetmann A, Nielsen M, et al. The role of caspase 3 and BclxL in the action of interleukin 7 (IL-7): a survival factor in activated human T cells. Cytokine. 1998;10:662–668. doi: 10.1006/cyto.1998.0351. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann B, Odum N, Platz P, Ryder LP, Svejgaard A, Neilsen JO. Immunological studies in acquired immunodeficiency syndrome. Functional studies of lymphocyte subpopulations. Scand J Immunol. 1985;21:235–243. doi: 10.1111/j.1365-3083.1985.tb01426.x. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen M, Odum N, Bendtzen K, Ryder LP, Jakobsen BK, Svejgaard A. MHC class II molecules regulate growth in human T cells. Exp Clin Immunogenet. 1994;11:23–32. [PubMed] [Google Scholar]
- 35.Nielsen M, Svejgaard A, Skov S, et al. IL-2 induces beta2-integrin adhesion via a wortmannin/LY294002-sensitive, rapamycin-resistant pathway. Phosphorylation of a 125-kilodalton protein correlates with induction of adhesion, but not mitogenesis. J Immunol. 1996;157:5350–5358. [PubMed] [Google Scholar]
- 36.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 37.Krejsgaard T, Vetter-Kauczok CS, Woetmann A, et al. Ectopic expression of B-lymphoid kinase in cutaneous T-cell lymphoma. Blood. 2009;113:5896–5904. doi: 10.1182/blood-2008-09-181024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Odum N, Martin PJ, Schieven GL, et al. Signal transduction by HLA-DR is mediated by tyrosine kinase(s) and regulated by CD45 in activated T cells. Hum Immunol. 1991;32:85–94. doi: 10.1016/0198-8859(91)90104-h. [DOI] [PubMed] [Google Scholar]
- 39.Hofmann B, Ødum N, Platz P, et al. Immunological studies in AIDS; functional studies of lymphocyte subpopulations. Scand J Immunol. 1985;21:235–243. doi: 10.1111/j.1365-3083.1985.tb01426.x. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen M, Svejgaard A, Skov S, Odum N. Interleukin-2 induces tyrosine phosphorylation and nuclear translocation of stat3 in human T lymphocytes. Eur J Immunol. 1994;24:3082–3086. doi: 10.1002/eji.1830241225. [DOI] [PubMed] [Google Scholar]
- 41.Brockdorff J, Nielsen M, Dobson P, et al. Interleukin-2 induces a transient downregulation of protein phosphatase 1 and 2A activity in human T cells. Tissue Antigens. 1997;49:228–235. doi: 10.1111/j.1399-0039.1997.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 42.Brockdroff J, Nielsen M, Svejgaard A, et al. Interleukin-2 induces β2 integrin dependent adhesion through a protein phosphatase 2A dependent pathway in human T cells. Cytokine. 1997;9:333–339. doi: 10.1006/cyto.1996.0173. [DOI] [PubMed] [Google Scholar]
- 43.Woetmann A, Nielsen M, Christensen ST, et al. Inhibition of protein phosphatase 2A induces serine/threonine phosphorylation, subcellular redistribution, and functional inhibition of STAT3. Proc Natl Acad Sci U S A. 1999;96:10620–10625. doi: 10.1073/pnas.96.19.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malek TR, Yu A, Zhu L, Matsutani T, Adeegbe D, Bayer AL. IL-2 family of cytokines in T regulatory cell development and homeostasis. J Clin Immunol. 2008;28:635–639. doi: 10.1007/s10875-008-9235-y. [DOI] [PubMed] [Google Scholar]
- 45.Brockdorff J, Nielsen M, Kaltoft K, et al. Lck is involved in interleukin-2 induced proliferation but not cell survival in human T cells through a MAP kinase-independent pathway. Eur Cytokine Netw. 2000;11:225–231. [PubMed] [Google Scholar]
- 46.zhang Q, Raghunath PN, Xue L, et al. Multilevel Dysregulation of STAT3 activation in Anaplastic Lymphoma Kinase-positive T/Null-cell lymphoma. J Immunol. 2002;168:466. doi: 10.4049/jimmunol.168.1.466. [DOI] [PubMed] [Google Scholar]
- 47.Harel L, Costa B, Tcherpakov M, et al. CCM2 mediates death signaling by the TrkA receptor tyrosine kinase. Neuron. 2009;63:585–591. doi: 10.1016/j.neuron.2009.08.020. [DOI] [PubMed] [Google Scholar]