Abstract
Background
Human embryonic stem cell (hESC)-derived cardiomyocytes potentially represent a powerful experimental model complementary to myocardium obtained from patients, relatively inaccessible for research purposes. We tested whether anesthetic-induced preconditioning (APC) with isoflurane elicits competent protective mechanisms in hESC-derived cardiomyocytes against oxidative stress to be used as a model of human cardiomyocytes for studying preconditioning.
Methods
H1 hESC cell line was differentiated into cardiomyocytes using growth factors activin A and bone morphogenetic protein-4. Living ventricular hESC-derived cardiomyocytes were identified using lentiviral vector expressing a reporter gene (enhanced green fluorescent protein) driven by a cardiac-specific human myosin light chain 2v promoter. Mitochondrial membrane potential, reactive oxygen species production, opening of mitochondrial permeability transition pore, and survival of hESC-derived cardiomyocytes were assessed using confocal microscopy. Oxygen consumption was measured in contracting cell clusters.
Results
Differentiation yielded a high percentage (∼85%) of cardiomyocytes in beating clusters that were positive for cardiac-specific markers and exhibited action potentials resembling mature cardiomyocytes. Isoflurane depolarized mitochondria, attenuated oxygen consumption, and stimulated generation of reactive oxygen species. APC protected these cells from oxidative stress-induced death and delayed mitochondrial permeability transition pore opening.
Conclusions
APC elicits competent protective mechanisms against oxidative stress in hESC-derived cardiomyocytes, suggesting the feasibility to use these cells as a model of human cardiomyocytes for studying APC and potentially other treatments/diseases. Our differentiation protocol is very efficient and yields a high percentage of cardiomyocytes. These results also suggest a promising ability of APC to protect and improve engraftment of hESC-derived cardiomyocytes into the ischemic heart.
Introduction
The mechanisms of drug action and pathophysiology of cardiac disease are mostly studied in animals and need to be validated in human models. However, research efforts are hampered by limited access to human myocardium. We investigated whether cardiomyocytes derived from human embryonic stem cells (hESCs) can be used as a complimentary experimental model of human cardiomyocytes to study anesthetic-induced preconditioning (APC). APC is a cardioprotective strategy that increases resistance to ischemia and reperfusion (I/R) by eliciting innate protective mechanisms.1,2
hESCs can be differentiated in vitro into various cell types, including cardiomyocytes, and potentially represent a powerful experimental model to screen drugs and study normal and pathological processes.3-5 These cardiomyocytes can phenotypically resemble functional human cardiomyocytes,6-9 and have been tested for cell replacement therapies in the treatment of heart disease in animals, with variable success.10,11 The ability of implanted hESC-derived cardiomyocytes to repair I/R-injured myocardium critically depends on their ability to survive the stressful environment within the host tissue, which can be improved by enhancing their resistance to activation of cell death pathways using a “prosurvival cocktail”.12 Interestingly, some components of the pro-survival cocktail have comparable effects to APC: inhibition of mitochondrial permeability transition pore (mPTP) opening,13 antiapoptotic pathway activation14 and opening of adenosine triphosphate-sensitive potassium channels.2
To identify the possibility that hESC-derived cardiomyocytes have a competent response to a preconditioning stimulus to be used as an experimental model for APC, we investigated whether preconditioning with the anesthetic isoflurane elicits distinct mediators of protection in these cells: reactive oxygen species (ROS) and opening of mitochondrial adenosine triphosphate-sensitive potassium (mitoKATP) channels as signal mediators, and a delay in mPTP opening as an endpoint of protection. The model was validated by comparing the obtained results to our previous work using adult human and adult animal cardiomyocytes. We achieved a high purity of differentiated cardiomyocytes (∼85% in beating areas). This study is the first to demonstrate that APC elicits characteristic endogenous cytoprotective mechanisms against oxidative stress in hESC-derived cardiomyocytes. Our results suggest that these cardiomyocytes could be used as an experimental model to study APC, and potentially other treatments/diseases in human cardiomyocytes. Our study implies that APC could be also used to protect hESC-derived cardiomyocytes and thereby increase their engraftment into the injured myocardium.
Materials and Methods
Human embryonic stem cell culture
H1 (WA01) hESC line from WiCell Research Institute Inc. (Madison, WI) was maintained on mouse embryonic fibroblasts in hypoxic conditions (4% O2/5% CO2). Feeder cells were treated with mitomycin C (Sigma-Aldrich, St. Louis, MO) to arrest mitosis and cultured in Dulbecco's modified Eagle's medium (Chemicon International, Temecula, CA) supplemented with 10% Fetal Bovine Serum (GIBCO, Carlsbad, CA) and 1% non-essential amino acids (Chemicon International). The hESCs were cultured in Dulbecco's modified Eagle's medium/F12 (GIBCO) supplemented with 20% knock-out serum (GIBCO), 1% nonessential amino acids, 1% penicillin-streptomycin, L-glutamine (Chemicon International), ß-mercaptoethanol (Sigma-Aldrich) and 4 ng/mL human recombinant basic fibroblast growth factor (Invitrogen, Carlsbad, CA). The colonies of hESCs were passaged every 5-7 days using a mechanical microdissection method. We used hESCs with passage numbers 39 to 43 for cell characterization (figs. 1 and 2), and 50 to 53 for APC testing (figs. 3-7).
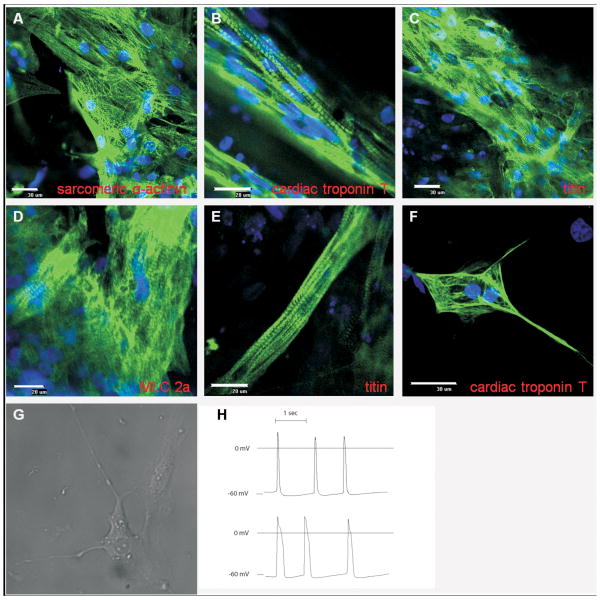
Figure 1. Immunolabeling and electrophysiological characterization of human embryonic stem cell-derived cardiomyocytes.
Confocal images of non-dissociated cells after 90 days from treatment with growth factors, and stained for cardiac sarcomeric proteins: (A) sarcomeric α-actinin, (B) cardiac-specific troponin T, (C) titin, and (D) cardiac-specific myosin light chain 2a (MLC 2a). A large percentage of positive cells are observed, including striated patterns indicating highly organized sarcomeres. (E) Occasional occurrence of cells with rod-shaped morphology that resemble adult cardiomyocytes. (F) Dissociated cell stained for cardiac-specific troponin T showing that cells maintain sarcomeric organization after dissociation. (G) A dissociated cell that is spontaneously and rhythmically contracting. (H) Representative recordings of action potentials: shorter, atrial-like (upper trace) and longer, ventricular-like (lower trace).
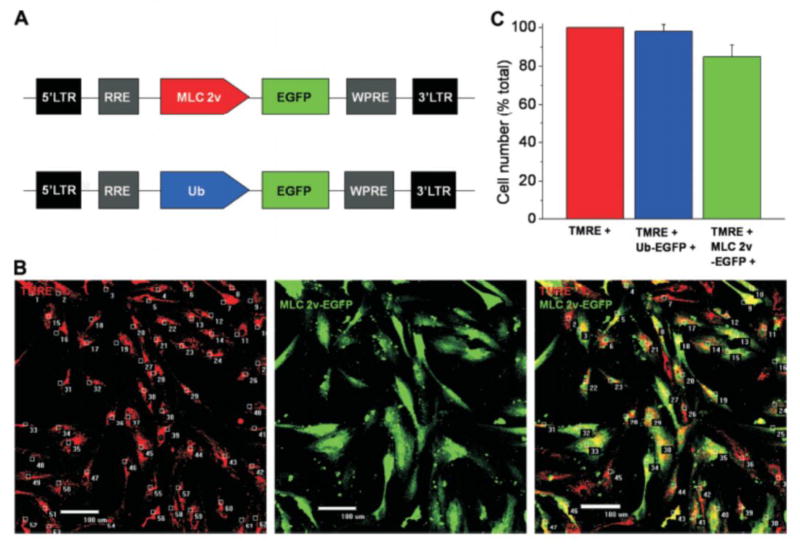
Figure 2. Labeling and counting of human embryonic stem cell (hESC)-derived cardiomyocytes using lentiviral vector.
(A) Schematic representation of lentiviral vectors, pHR(+)c.MLC 2v.EGFP.R(-)W(+) and pHR(+)c.Ub.EGFP.R(-)W(+) used for identifying cardiomyocytes and determining transduction efficiency, respectively. (B) To determine the total cell number in beating clustres, after dissociation cells were loaded with tetramethylrhodamine ethyl ester (TMRE; red) to visualize cell bodies. Myosin light chain 2v (MLC 2v)-enhanced green fluorescent protein (EGFP)-positive cells were counted by detecting green fluorescence, giving the number of ventricular myocytes. (C) Summarized data from five separate differentiation experiments show high percentage of hESC-derived cardiomyocytes. (Ub: ubiquitin).
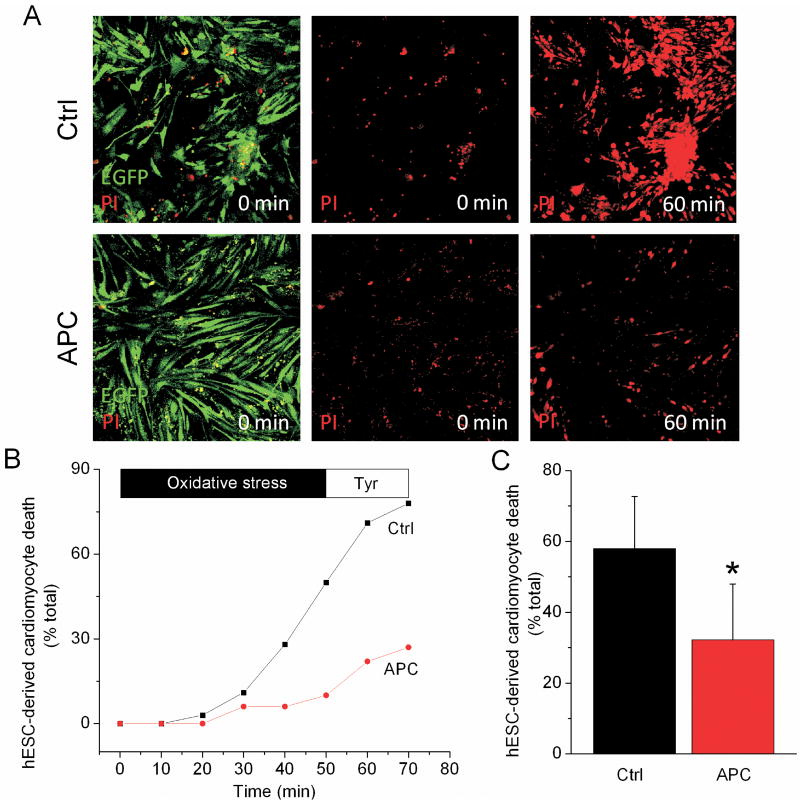
Figure 3. Human embryonic stem cell (hESC)-derived cardiomyocytes are protected from oxidative stress by anesthetic-induced preconditioning (APC).
(A) Myosin light chain 2v (MLC 2v)-enhanced green fluorescent protein-positive cells, i.e., hESC-derived cardiomyocytes, were identified by green fluorescence using confocal microscopy. Following exposure to oxidative stress and compared to control (Ctrl), APC decreased the number of hESC-derived cardiomyocytes that stained positive for red-fluorescent propidium iodide (PI), an indication of cell death. (B) The rate of increase in number of PI-positive cells, expressed as percent of total number of hESC-derived cardiomyocytes, is attenuated in APC group compared to Ctrl. (C) Summarized values after the application of H2O2, following 10 min of perfusion with Tyrode solution. * P < 0.05 versus Ctrl.
Figure 7. Preconditioning delays mPTP opening in human embryonic stem cell-derived cardiomyocytes.
(A) Mitochondrial permeability transition pore (mPTP) opening was induced by photoexcitation-generated oxidative stress and detected by rapid dissipation of tetramethylrhodamine ethyl ester (TMRE) fluorescence. (B) Representative signal traces from control (Ctrl), cyclosporine A (CsA)-treated cells, and anesthetic-induced preconditioning (APC). Arbitrary mPTP opening time was determined as the time when TMRE fluorescence intensity decreased by half between initial and residual fluorescence intensity. (C) mPTP blocker CsA and APC increased arbitrary mPTP opening time, which was blocked in presence of 5-hydroxydecanoate (APC + 5-HD). Preconditioning with 40 μM H2O2 (H2O2-PC) also delayed mPTP opening. * P < 0.05 versus Ctrl; # P < 0.05 versus APC + 5-HD.
Cardiac differentiation of hESCs
Colonies of hESCs were mechanically dissociated into small clumps, plated onto dishes pre-coated with Reduced Growth Factor Matrigel (BD-Biosciences, San Jose, CA), and cultured under hypoxic (4% O2) conditions. Cells were maintained pluripotent by daily feeding with medium conditioned by mouse embryonic fibroblasts supplemented with 4 ng/ml fibroblast growth factor-2 for the next seven days, followed by daily provision of RPMI/B27 medium (Invitrogen) supplemented with growth factors Activin A (50 ng/ml; R&D Systems, Minneapolis, MN) and bone morphogenetic protein-4 (10 ng/ml; R&D Systems) for the next five days. After that, cells were placed into normoxia and growth factors were withdrawn.
Microdissection and single cell dissociation
Ninety days after treatment with growth factors, the beating cell clusters were mechanically dissociated from the remaining cell aggregates under a dissecting microscope (SMZ1000, Nikon, Tokyo, Japan) and treated with 0.05 % trypsin-EDTA (Invitrogen) for 4 min to dissociate individual cells, which were plated onto Matrigel-coated coverslips.
Genetic marking of hESC-derived cardiomyocytes with a lentiviral vector
Dissociated cells were transduced with a lentiviral vector encoding human myosin light chain 2v (MLC 2v)-driven enhanced green fluorescent protein, (2.2 × 104 MOI). MLC 2v is a promoter specific for ventricular myocytes.15-17 A MLC 2v-enhanced green fluorescent protein cassette (kindly provided by Lior Gepstein, M.D., Ph.D., Associate Professor, The Bruce Rappaport Institute in the Medical Sciences, Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel5) was subcloned into lentiviral transfer plasmid pHR(+)c.Ub.MCSoligo.R(-)W(+). Lentiviral vector production and titering was performed as previously described.18,19
Laser-scanning confocal microscopy
Four days after lentiviral vector transduction, imaging was performed using a confocal microscope (Eclipse TE2000-U, Nikon) and data were analyzed with MetaMorph 6.1 software (Universal Imaging, West Chester, PA). Living hESC-derived cardiomyocytes were identified by detecting fluorescence of MLC 2v-driven enhanced green fluorescent protein-positive cells and experiments were conducted in Tyrode's solution (in mM: 132 NaCl, 10 HEPES, 10 glucose, 5 KCl, 1 CaCl2, 1.2 MgCl2, pH 7.4) at room temperature. The percentage of hESC-derived cardiomyocyte death was determined after exposing cells to oxidative stress induced by 10 mM H2O2 (Calbiochem, La Jolla, CA) applied for 50 min, followed by perfusion with Tyrode's solution for 10 min. The cells stained with red-fluorescent propidium iodide (2 μM, Sigma-Aldrich) were considered dead.20 hESCs-derived cardiomyocytes were preconditioned with 0.5 mM isoflurane (∼1 minimal alveolar concentration) applied for 15 min, followed by 5 min isoflurane washout (APC). After each experiment, a gas chromatography was used to test isoflurane concentrations, which varied ±10% of reported values. The tetramethylrhodamine ethyl ester (30 nM, Invitrogen) was used to detect ΔΨm in hESC-derived cardiomyocytes. Data are normalized to the first time point in baseline (100%). For the statistical analysis, the average value of time points after the treatment has reached the maximal effect (the last five frames) and the average baseline values were used. ROS production was monitored in cells loaded with dihydroethidium (10 μM, Invitrogen). For the statistical analysis, the rate of increase in ethidium fluorescence before or after isoflurane application and in the Time control was used. Opening of the mPTP was assessed as previously described in our laboratory,13 a method based on mPTP induction by photoexcitation-generated oxidative stress.21-24 The mPTP opening was detected by rapid dissipation of ΔΨm, observed as loss of tetramethylrhodamine ethyl ester fluorescence, which is sensitive to mPTP opening inhibition.13
Immunolabeling
After the fixation and permabilization, cells were treated with primary antibodies for: anti-α-actinin (Sigma, 1:100 dilution), anti-cardiac-specific troponin T (Thermo Scientific, Rockford, IL, 1:100), anti-myosin light chain 2a (MLC 2a, Synaptic Systems, Goettingen, Germany, 1:100), or anti-titin (Developmental Studies Hybridoma Bank, Iowa City, IA, 1:100), following treatment with corresponding secondary antibody, Alexa Fluor 488 (Invitrogen, 1:1000). Nuclei were stained with TOPRO-3 (Invitrogen, 1:1000).
Electrophysiology
Membrane potential (Em) was measured in microdissected beating clustures using 3M KCl-filled borosilicate glass microelectrodes (impedance 40-60 MΩ) in RPMI/B27 medium. Data were processed using a Grass RPS7C polygraph (Astro-Med/Grass Inc, West Warvick, RI) and Superscope II digital data acquisition system (GW Instruments, Somerville, MA).
Oxygen consumption
Spontaneously and rhythmically contracting cell clusters were separated by microdissection and respiration of cell clusters that predominantly contain cardiomyocytes was measured using an oxygen electrode (Hansatech Instruments, Norfolk, United Kingdom) at 37°C. Isoflurane was delivered at incremental steps and the rate of oxygen consumption after each isoflurane addition was normalized to baseline values.
Statistical analysis
Data are presented as mean ± SD. Each experimental group comprises hESC-derived cardiomyocytes from at least 3 different differentiations, where n indicates the number of independent experiments. For the statistical analyses SigmaStat 3.0 software (Aspire Software International, Ashburn, VA) was used. Statistical comparisons were performed using one-way analysis of variance or two-way repeated measures analysis of variance with Tukey or Dunnett's post hoc tests where appropriate. Unpaired t test was used for comparisons between two groups were appropriate. P values are from two-tailed tests. Differences at P < 0.05 were considered significant.
Results
Differentiation and characterization of hESC-derived cardiomyocytes
The presence of cardiomyocytes after cardiac differentiation of hESCs was observed as occurrence of spontaneously and rhythmically beating areas of contiguous cells in culture dishes, beginning approximately 10 days after the treatment with activin-A and bone morphogenetic protein-4 and lasting up to a one year (please see Supplemental Digital Content 1, which is a video showing beating areas that spanned almost the entire surface of the culture dish). As shown in figure 1, immunostaining revealed an abundance of cells positive for cardiac specific sarcomeric proteins, organized in characteristic striated pattern (fig. 1, A, B, C and D). Following the dissociation from culture dishes, cardiomyocytes retained their striated appearance (fig. 1F), with some continuing to exhibit spontaneous rhythmic contractions (please see Supplemental Digital Content 2, which is a video showing isolated contracting cells that is a characteristic of cardiomyocytes). Short (<130 ms) atrial-like action potentials, and long (>250 ms) ventricular-like action potentials, were recorded in dissociated, contracting cell clusters, indicating electrical activity and existence of functional sarcolemmal ion channels in these cardiomyocytes.
Genetic marking and labeling of live hESC-derived cardiomyocytes using a lentiviral vector
To identify living hESC-derived cardiomyocytes and determine differentiation efficiency, differentiated cells were genetically marked using a self-inactivating lentiviral vector containing a cardiac-specific promoter MLC 2v driving the expression of enhanced green fluorescent protein (fig. 2A). Lentiviral transduction efficiency, determined with ubiquitin-driven enhanced green fluorescent protein, was 98 ± 1% (fig. 2B). There was 85 ± 3% of cells that were MLC 2v-enhanced green fluorescent protein-positive, i.e., ventricular myocytes, indicating high efficiency of our differentiation protocol (fig. 2, B and C).
APC protects hESC-derived cardiomyocytes from oxidative stress
To test whether APC protects the hESC-derived cardiomyocytes from oxidative stress-induced cell death, the preconditioned and control cells were exposed to H2O2. The cardiomyocytes were identified as MLC 2v-enhanced green fluorescent protein-positive cells (green-fluorescent cells in fig. 3 A). APC attenuated the hESC-derived cardiomyocyte death compared to control, 32 ± 16% (n = 5) versus 58 ± 15% (n = 5), respectively (fig. 3, B and C). This correlates with our previous study which showed that APC protects adult human atrial cells from oxidative stress.25
Isoflurane depolarizes mitochondria in hESC-derived cardiomyocytes by opening mitoKATP channels
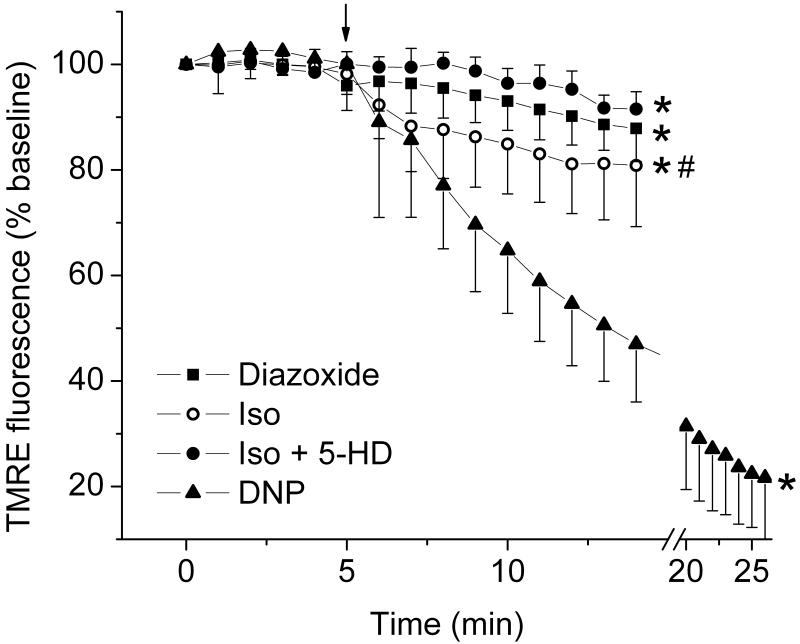
Using adult rat cardiomyocytes, we have previously shown that isoflurane induces opening of mitoKATP channels causing decrease in ΔΨm and thereby eliciting cardioprotection.26 In hESC-derived cardiomyocytes, diazoxide, an opener of mitoKATP channels,27 decreased tetramethylrhodamine ethyl ester fluorescence intensity from baseline value of 99.5 ± 1.1% (n = 12) to 90.2 ± 5.4% of baseline (n = 12), indicating opening of mitoKATP channels (fig. 4). Application of 0.5 mM isoflurane decreased tetramethylrhodamine ethyl ester fluorescence intensity from 99.6 ± 1.7% (n = 11) to 82.2 ± 9.8% of baseline (n = 11). This was attenuated in the presence of 5-hydroxydecanoate (5-HD), an inhibitor of mitoKATP channel opening,27 and the baseline fluorescence decreased from 99.7 ± 1.7% (n = 11) to 94.2 ± 2.4% of baseline (n = 11), indicating that mitochondrial depolarization by isoflurane is, in part, mediated by opening of mitoKATP channels.
Figure 4. Isoflurane opens mitoKATP channels and depolarizes mitochondria in human embryonic stem cell (hESC)-derived cardiomyocytes.
ΔΨm was monitored in dissociated hESC-derived cardiomyocytes using tetramethylrhodamine ethyl ester (TMRE) fluorescence. The time point when drugs were added is indicated by the arrow. Diazoxide (50 μM) decreased TMRE fluorescence intensity, indicating mitochondrial depolarization. Application of isoflurane (Iso) also decreased TMRE fluorescence intensity, an effect that was partly blocked with 200 μM of 5-hydroxydecanoate (5-HD). 2,4-dinitrophenol (DNP; 100 μM) completely depolarized mitochondria. In group Iso+5-HD, four observations in the first time point of baseline and three observations in the second time point of baseline are missing, and in group DNP, four observations in the first five time points of baseline are missing. * P < 0.05 versus baseline; # P < 0.05 versus Iso + 5-HD.
Oxygen consumption by cell clusters containing cardiomyocytes is inhibited by isoflurane
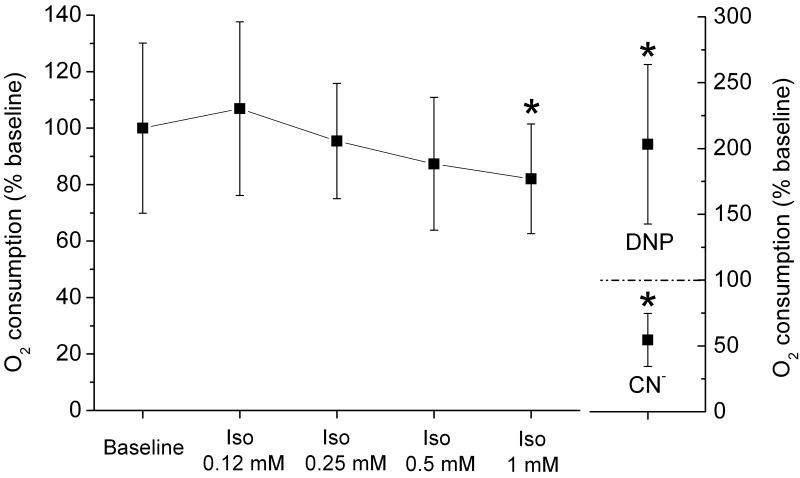
The rate of oxygen consumption of microdissected beating cell clusters that predominantly contained cardiomyocytes was monitored in the presence of incremental isoflurane concentration (fig. 5). At a concentration of 0.12 mM, isoflurane slightly, but not significantly increased baseline oxygen consumption from 100.0 ± 30.1% to 106.9 ± 30.8%. However, by increasing isoflurane concentration to 0.25, 0.5 and 1.0 mM, the oxygen consumption progressively decreased to 95.4 ± 20.4%, 87.3 ± 23.5%, and 82.0 ± 19.4% of baseline, respectively, (n = 7 in all groups). This indicates a suppression of respiration by isoflurane.
Figure 5. Isoflurane attenuates beating cell cluster oxygen consumption.
The rates of oxygen consumption in contracting cell clusters after the addition of incremental isoflurane (Iso) concentration isoflurane were normalized to baseline values. At the higher concentrations, isoflurane attenuated oxygen consumption. 2,4-dintrophenol (DNP;100 μM) and cyanide (CN-; 2 mM) were added to establish maximal and minimal rates of oxygen consumption. * P < 0.05 versus baseline.
Isoflurane enhances production of ROS by hESC-derived cardiomyocytes
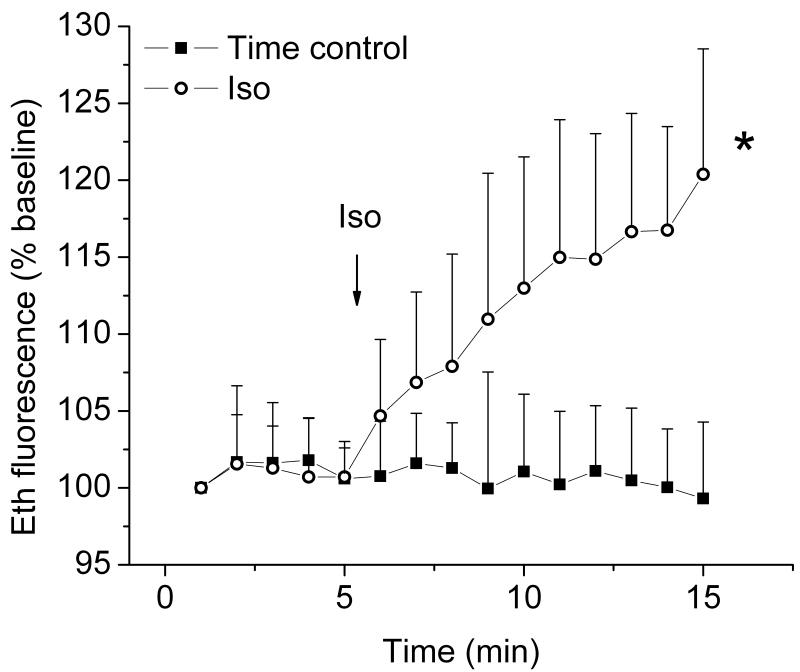
Production of ROS, important signaling molecules in preconditioning of adult cardiomyocytes,28,29 was monitored in hESCs-derived cardiomyocytes. As seen in figure 6, application of 0.5 mM isoflurane significantly increased the rate of change in Eth fluorescence intensity compared to its baseline or Time control (Isoflurane: 1.60 ± 0.59, n = 11; Isoflurane baseline: 0.01 ± 0.62, n = 11; and Time control: -0.15 ± 0.31 percentage points/min, n = 8). This indicated that isoflurane increases ROS production in hESC-derived cardiomyocytes.
Figure 6. Isoflurane enhances ROS production in human embryonic stem cell (hESC)-derived cardiomyocytes.
Reactive oxygen species (ROS) production in hESC-derived cardiomyocytes was measured by detecting fluorescence intensity of ethidium (Eth). Compared to the Time control, isoflurane (Iso) significantly increased Eth fluorescence intensity. In group Iso, three observations in the first two time points of baseline and five observations in the last time point of treatment are missing. * P < 0.05 versus Time control and baseline.
Preconditioning with isoflurane or hydrogen peroxide delays opening of mitochondrial permeability transition pore (mPTP)
Cardioprotective strategies, including APC delay opening of mPTP, a critical event in the transition towards cell death. mPTP opening-induced dissipation of ΔΨm was monitored in hESC-derived cardiomyocytes exposed to oxidative stress (fig. 7). Compared to control, mPTP inhibitor cyclosporine A (1 μM) increased the arbitrary mPTP opening time, 100.0 ± 24.8% (n = 11) versus 119.1 ± 14.6% of control (n = 10), respectively. Similarly to cyclosporine A, APC increased arbitrary mPTP opening time and application of 5-HD together with isoflurane abrogated this effect (control: 100.0 ± 18.2%, n = 12; APC: 139.4 ± 40.4%, n = 16; and APC+5-HD: 95.3 ± 11.6% of control, n = 16), confirming the role of mitoKATP channel opening in signal mediation of APC. Preconditioning with H2O2 increased arbitrary mPTP opening time to 130.5 ± 31.2% (n = 12) compared to control (100.0 ± 19.8%, n = 10).
Discussion
We have demonstrated an efficient method to differentiate cardiomyocytes from hESCs, indicated by genetic labeling using lentiviral vectors, showing that ∼85% of cells in beating clusters expressed cardiac-specific promoter MLC 2v. Efficient differentiation and a phenotype of a functional cardiomyocytes was also indicated by observing globally contracting cell clusters that widely expressed highly organized, cardiac-specific sarcomeric proteins and generated action potentials resembling those of mature heart cells. Moreover, we showed that preconditioning with isoflurane attenuates cell death and elicits competent mechanisms of protection in ventricular hESC-derived cardiomyocytes against oxidative stress. These included characteristic and important mediators of cardioprotection: opening of mitoKATP channels, ROS as signaling molecules, and a delay of oxidative stress-induced mPTP opening, an end-point of protection. Similar responses to APC between adult cardiomyocytes documented in our previous studies and hESC-derived cardiomyocytes demonstrated in this study indicate the feasibility of using hESC-derived cardiomyocytes as a model of human ventricular cardiac cells to study APC and potentially other treatments/diseases.
We showed that hESC-derived cardiomyocytes phenotypically resemble functional human cardiomyocytes by showing that these cells spontaneously and rhythmically contract, generate action potentials that, by shape and morphology resemble functional human cardiomyocytes. Moreover, these cardiomyocytes exhibit highly organized sarcomeric structures, indicated by immunostaining for cardiac-specific troponin T and MLC 2a and nonspecific sarcomeric α-actinin and titin. This is in accord with other laboratories that have described structural properties of hESC-derived cardiomyocytes.6,7,30 The presence of spontaneously and rhythmically beating cell clusters that extend throughout the culture dishes (see Supplemental Digital Content 1), indicate presence of pace-maker cells and cardiomyocytes that form a functional syncytium which exhibits synchronized action potential propagation.6,31 Other laboratories demonstrated electrophysiological and functional competence of hESC-derived cardiomyocytes by electrophysiological recordings, measurements of Ca2+ transients, as well as appropriate chronotropic responses to β- and muscarinic receptor stimulation.6-9,32-34 However, the extent of these cells' maturity requires further investigation.3 At minimum, studies describe hESC-derived cardiomyocytes as cells having characteristics of embryonic cardiomyocytes that may, with extended time in culture, differentiate into a mature cardiomyocyte phenotype.35
In this study, we achieved highly efficient differentiation of human cardiomyocytes from hESCs, obtaining unprecedented levels of ∼85% of cells positive for the cardiac-specific marker MLC 2v in beating cell clusters. An abundance of cardiomyocytes was corroborated by cardiac-specific immunostaining. Moreover, areas with spontaneously and rhythmically beating cells, a characteristic of cardiomyocytes, spanned the entire surface of the culture dish (see Supplemental Digital Content 1). We have applied bone morphogenetic protein-4, fibroblast growth factor and activin-A, all endoderm-secreted growth factors, to direct differentiation into cardiac lineage, based on findings that endoderm-induced cardiomyogenic signaling regulates heart development in the embryo.36 Previously published protocols utilizing same growth factors were modified in this study.12,37 Namely, pluripotent cells were exposed to activin-A at a relatively high level (50 ng/ml) for an extended period of time (five days). We speculate that this optimized the efficient production of mes-endoderm38 leading to progressive differentiation into the cardiomyogenic lineage. Our results compare highly favorably with other studies and approaches to differentiate cardiomyocytes, especially with a widely used method involving spontaneous differentiation termed embryoid bodies that yields low percentage of cardiomyocytes (≤ 10 %).6
Several studies have shown the functional heterogeneity of cardiomyocytes derived from hESCs and the presence of ventricular, atrial and nodal-like cells, with a ventricular phenotype being the most prevalent.7,9 In our experiments, the labeling of cells with the MLC 2v-enhanced green fluorescent protein revealed ∼85% of positive cells, indicating that at least 85% of cells are of ventricular cardiomyocyte phenotype, since MLC 2v is a ventricular myocyte-specific transcription factor. We also observed only a minor proportion of cells that stained positive for atrial myocyte-specific marker, MLC 2a in our immunohistochemistry experiments. Altogether, this indicates that, in agreement with the results from other laboratories, our differentiation protocol predominantly yields ventricular cardiomyocytes and only a small proportion of other cardiac cell types.
We have demonstrated molecular mechanisms by which preconditioning with isoflurane elicits competent defense against oxidative stress in hESC-derived cardiomyocytes. These protective mechanisms have been previously characterized in our and other laboratories using animal13,14,26,28 and, to lesser extent, human myocardium.25,29 However, it was unclear whether hESC-derived cardiomyocytes exhibit an adequate phenotype to resist oxidative stress, a hallmark of I/R injury.39 In this study, we showed that APC successfully protects hESC-derived cardiomyocytes from oxidative stress, which is in accordance with the results from our previous study using adult human atrial cardiomyocytes.25 To investigate the phenotypic similarity of the cardioprotective mechanisms by APC between adult cardiomyocytes and hESC-derived cardiomyocytes, we tested some of the most relevant mediators of protection. We showed here that isoflurane partly depolarizes mitochondria in a 5-HD-sensitive manner, suggesting opening of mitoKATP channels, as crucial mediators of cardioprotection.26,40 This effect was almost identical to isoflurane-induced opening of mitoKATP channels demonstrated with the similar approach in our previous study using adult rat cardiomyocytes,26 indicating a comparable response between adult and hESC-derived cardiomyocytes. We also showed here that isoflurane moderately enhances production of ROS, an important component of preconditioning signaling cascade.28,41 Our previous study demonstrated that desflurane and sevoflurane induce similar extent of ROS production in adult rat cardiomyocytes.28 The importance of ROS was confirmed here by showing that a low dose of H2O2 induces preconditioning and delays mPTP opening, which is in agreement with a study by Hanouz et al. that indicated critical importance of ROS signaling in APC using adult human atrial trabeculae.29 Anesthetic-induced increase in ROS production has been attributed to the opening of mitoKATP channels,41 but it can be also induced by partial obstruction of the electron transport chain,42 another effect of volatile anesthetics.43 In this study we showed that isoflurane suppresses respiration in beating cell clusters. This effect may correlate with the inhibition of the electron transport chain, but it could also reflect other effects of isoflurane, like alternation in metabolic pathways. The importance of mitoKATP channel opening for inducing cardioprotection by APC was verified here by showing that inhibition of channel opening by 5-HD abrogates the APC-induced delay in mPTP opening.
Our results indicate that preconditioning with isoflurane induces a delay in opening of the mPTP. This has a significant functional importance since the opening of mPTP has been recognized as the crucial event in the transition towards cell death during I/R injury.44 Cardioprotective strategies, including APC were found to induce a delay in mPTP opening and mPTP blockers may decrease infarct size by 30-50%.45 During I/R, opening of mPTP dissipates ΔΨm, preventing oxidative phosphorylation and initiates death pathways in the cell.44 Using an identical approach as in this study, we previously demonstrated that APC induces a similar delay in mPTP opening in adult rat cardiomyocytes,13 which further correlates with another study from our laboratory demonstrating that APC elicits cellular and mitochondrial protective mechanisms against oxidative stress in human adult cardiomyocytes.25 Taken altogether, all tested parameters indicate similar responses of adult cardiomyocytes and hESC-derived cardiomyocytes to APC.
Studies using hESC-derived cardiomyocytes to regenerate dysfunctional myocardium after I/R injury have had limited success due to factors including inefficient differentiation, poor engraftment and survival within injured myocardium.3 However, Laflamme et al. demonstrated that the use of a prosurvival cocktail during implantation of hESC-derived cardiomyocytes improved cell engraftment and functional recovery of the heart.12 This cocktail protected graft cells from the stressful environment of (post)ischemic myocardium by blocking cellular death pathways. Interestingly, APC involves inhibition of the same cellular death pathways as prosurvival cocktail.13,14,41 This suggests that APC could improve cardiomyocyte engraftment, with the added advantage that, unlike the components of the cocktail, volatile anesthetics are approved for the clinical application. In fact, the recent Guidelines by the American College of Cardiology/American Heart Association recommended the use of volatile anesthetics based on the findings of 15 randomized trials in patients undergoing coronary bypass surgery showing that volatile anesthetics decrease cardiac troponin release and improve ventricular function in comparison with intravenous anesthetics.46
A limitation of this study is the use of an in vitro generated cardiomyocytes that may lack some of the characteristics of adult cardiomyocytes, as discussed above. However, this study indicates competent responses of these cardiomyocytes to APC. Another limitation is the use of oxidative stress, which does not fully represent conditions during I/R injury, but it is a widely accepted model to study a reperfusion injury. Although APC exerts protection in isolated cardiomyocytes, this model does not take into account additional effects of anesthetics during APC in vivo, which includes the effects on other cell types, such as endothelial cells, or modulation of the immune response. A possible limitation is that we used hESC passages 39 to 43 to derive cardiomyocytes for cell characterization and passages 50 to 53 for APC testing.
In conclusion, our study shows for the first time that preconditioning with anesthetic isoflurane elicits competent defensive mechanisms against oxidative stress in ventricular hESC-derived cardiomyocytes. The similarity in responses to APC between adult cardiomyocytes documented in our previous work and hESC-derived cardiomyocytes demonstrated here implies that these cardiomyocytes can be used as a valuable experimental human model to study APC, and possibly other human diseases/treatments. As a complimentary model of human cardiomyocytes, hESC-derived cardiomyocytes offer new experimental advantages, overcoming previous limitations when using human myocardium from patient surgeries. Moreover, APC may be a promising tool for protecting hESC-derived cardiomyocytes during engraftment, thereby increasing regeneration of injured myocardium. Importantly, our study also demonstrates a simple and efficient modification of previously published protocols to differentiate human cardiomyocytes from hESCs.
Supplementary Material
Acknowledgments
We thank Anna Stadnicka, Ph.D. (Associate Professor, Department of Anesthesiology, Medical College of Wisconsin, Milwaukee, Wisconsin) and Wai-Meng Kwok, Ph.D. (Professor, Department of Anesthesiology, Medical College of Wisconsin, Milwaukee, Wisconsin) for helpful scientific discussions, Aniko Szabo, Ph.D. (Associate Professor, Department of Population Health, Medical College of Wisconsin, Milwaukee, Wisconsin) for the help with the statistical analyses, and Steve Contney, M.S. (Research Scientist, Department of Anesthesiology, Medical College of Wisconsin, Milwaukee, Wisconsin) and Sara Reszczynski (Technician, Department of Anesthesiology, Medical College of Wisconsin, Milwaukee, Wisconsin) for the technical assistance.
Sponsored by: National Institutes of Health (Bethesda, Maryland) grants to Zeljko J. Bosnjak (P01GM066730 and R01HL034708) and to Stephen A. Duncan (DK55743, DK087377, HL094857) and Advancing Healthier Wisconsin endowment (Milwaukee, Wisconsin) to Stephen A. Duncan and John Lough.
Footnotes
This work should be attributed to the Department of Anesthesiology, Medical College of Wisconsin, Milwaukee, WIsconsin.
This work has been presented at American Society of Anesthesiologists meeting, October 19, 2009, New Orleans, Louisiana, and at the Experimental Biology meeting, April 19, 2009, New Orleans, Louisiana
References
- 1.Zaugg M, Schaub MC. Signaling and cellular mechanisms in cardiac protection by ischemic and pharmacological preconditioning. J Muscle Res Cell Motil. 2003;24:219–49. doi: 10.1023/a:1026021430091. [DOI] [PubMed] [Google Scholar]
- 2.Stadnicka A, Marinovic J, Ljubkovic M, Bienengraeber MW, Bosnjak ZJ. Volatile anesthetic-induced cardiac preconditioning. J Anesth. 2007;21:212–9. doi: 10.1007/s00540-006-0486-6. [DOI] [PubMed] [Google Scholar]
- 3.van Laake LW, Hassink R, Doevendans PA, Mummery C. Heart repair and stem cells. J Physiol. 2006;577:467–78. doi: 10.1113/jphysiol.2006.115816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wobus AM, Boheler KR. Embryonic stem cells: Prospects for developmental biology and cell therapy. Physiol Rev. 2005;85:635–78. doi: 10.1152/physrev.00054.2003. [DOI] [PubMed] [Google Scholar]
- 5.Caspi O, Itzhaki I, Arbel G, Kehat I, Gepstein A, Huber I, Satin J, Gepstein L. In vitro electrophysiological drug testing using human embryonic stem cell derived cardiomyocytes. Stem Cells Dev. 2008;18:161–72. doi: 10.1089/scd.2007.0280. [DOI] [PubMed] [Google Scholar]
- 6.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–14. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van der Brink S, Hasink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, Passier R, Tertoolen L. Differentiation of human embryonic stem cells to cardiomyocytes: Role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–40. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 8.Dolnikov K, Shilkrut M, Zeevi-Levin N, Gerecht-Nir S, Amit M, Danon A, Istkovitz-Eldor J, Binah O. Functional properties of human embryonic stem cell-derived cardiomyocytes: Intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction. Stem Cells. 2006;24:236–45. doi: 10.1634/stemcells.2005-0036. [DOI] [PubMed] [Google Scholar]
- 9.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: Action potential characterization. Circ Res. 2003;93:32–9. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 10.Laflamme MA, Zbinden S, Epstein SE, Murry CE. Cell-based therapy for myocardial ischemia and infarction: Pathophysiological mechanisms. Annu Rev Pathol. 2007;2:307–39. doi: 10.1146/annurev.pathol.2.010506.092038. [DOI] [PubMed] [Google Scholar]
- 11.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–93. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 12.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassainpour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 13.Pravdic D, Sedlic F, Mio Y, Vladic N, Bienengraeber M, Bosnjak ZJ. Anesthetic-induced preconditioning delays opening of mitochondrial permeability transition pore via protein Kinase C-epsilon-mediated pathway. Anesthesiology. 2009;111:267–74. doi: 10.1097/ALN.0b013e3181a91957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raphael J, Abedat S, Rivo J, Meir K, Beeri R, Pugatsch T, Zou Z, Gozal Y. Volatile anesthetic preconditioning attenuates myocardial apoptosis in rabbits after regional ischemia and reperfusion via Akt signaling and modulation of Bcl-2 family proteins. J Pharmacol Exp Ther. 2006;318:186–94. doi: 10.1124/jpet.105.100537. [DOI] [PubMed] [Google Scholar]
- 15.Huber I, Itzhaki I, Caspi O, Arbel G, Tzurkeman M, Gepstein A, Habib M, Yankelson L, Kehat I, Gepstein L. Identification and selection of cardiomyocytes during human embryonic stem cell differentiation. FASEB J. 2007;21:2551–63. doi: 10.1096/fj.05-5711com. [DOI] [PubMed] [Google Scholar]
- 16.Henderson SA, Spencer M, Sen A, Kumar C, Siddiqui MA, Chien KR. Structure, organization, and expression of the rat cardiac myosin light chain-2 gene. Identification of a 250-base pair fragment which confers cardiac-specific expression. J Biol Chem. 1989;264:18142–8. [PubMed] [Google Scholar]
- 17.Zhu H, Garcia AV, Ross RS, Evans SM, Chien KR. A conserved 28-base-pair element (HF-1) in the rat cardiac myosin light-chain-2 gene confers cardiac-specific and alpha-adrenergic-inducible expression in cultured neonatal rat myocardial cells. Mol Cell Biol. 1991;11:2273–81. doi: 10.1128/mcb.11.4.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park F. Correction of bleeding diathesis without liver toxicity using arenaviral-pseudotyped HIV-1-based vectors in hemophilia A mice. Hum Gene Ther. 2003;14:1489–94. doi: 10.1089/104303403769211691. [DOI] [PubMed] [Google Scholar]
- 19.Park F, Sweeney WE, Jia G, Roman RJ, Avner ED. 20-HETE mediates proliferation of renal epithelial cells in polycystic kidney disease. J Am Soc Nephrol. 2008;19:1929–39. doi: 10.1681/ASN.2007070771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanden Hoek TL, Shao Z, Li C, Schumacker PT, Becker LB. Mitochondrial electron transport can become a significant source of oxidative injury in cardiomyocytes. J Mol Cell Cardiol. 1997;29:2441–50. doi: 10.1006/jmcc.1997.0481. [DOI] [PubMed] [Google Scholar]
- 21.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–14. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CI, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–49. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huser J, Rechenmacher CE, Blatter LA. Imaging the permeability pore transition in single mitochondria. Biophys J. 1998;74:2129–37. doi: 10.1016/S0006-3495(98)77920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huser J, Blatter LA. Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem J. 1999;343(Pt 2):311–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Mio Y, Bienengraeber MW, Marinovic J, Gutterman DD, Rakic M, Bosnjak ZJ, Stadnicka A. Age-related attenuation of isoflurane preconditioning in human atrial cardiomyocytes: Roles for mitochondrial respiration and sarcolemmal adenosine triphosphate-sensitive potassium channel activity. Anesthesiology. 2008;108:612–20. doi: 10.1097/ALN.0b013e318167af2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ljubkovic M, Mio Y, Marinovic J, Stadnicka A, Warltier DC, Bosnjak ZJ, Bienengraeber M. Isoflurane preconditioning uncouples mitochondria and protects against hypoxia-reoxygenation. Am J Physiol Cell Physiol. 2007;292:C1583–90. doi: 10.1152/ajpcell.00221.2006. [DOI] [PubMed] [Google Scholar]
- 27.Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD. Bioenergetic consequences of opening the ATP-sensitive K(+) channel of heart mitochondria. Am J Physiol Heart Circ Physiol. 2001;280:H649–57. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- 28.Sedlic F, Pravdic D, Ljubkovic M, Marinovic J, Stadnicka A, Bosnjak ZJ. Differences in production of reactive oxygen species and mitochondrial uncoupling as events in the preconditioning signaling cascade between desflurane and sevoflurane. Anesth Analg. 2009;109:405–11. doi: 10.1213/ane.0b013e3181a93ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanouz JL, Zhu L, Lemoine S, Durand C, Lepage O, Massetti M, Khayat A, Plaud B, Gerard JL. Reactive oxygen species mediate sevoflurane- and desflurane-induced preconditioning in isolated human right atria in vitro. Anesth Analg. 2007;105:1534–9. doi: 10.1213/01.ane.0000286170.22307.1a. [DOI] [PubMed] [Google Scholar]
- 30.Snir M, Kehat I, Gepstein A, Coleman R, Itskovitz-Eldor J, Livine E, Gepstein L. Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes. Am J Physiol Heart Circ Physiol. 2003;285:H2355–63. doi: 10.1152/ajpheart.00020.2003. [DOI] [PubMed] [Google Scholar]
- 31.Kehat I, Gepstein A, Spira A, Itskovitz-Eldor J, Gepstein L. High-resolution electrophysiological assessment of human embryonic stem cell-derived cardiomyocytes: A novel in vitro model for the study of conduction. Circ Res. 2002;91:659–61. doi: 10.1161/01.res.0000039084.30342.9b. [DOI] [PubMed] [Google Scholar]
- 32.Zhu WZ, Santana LF, Laflamme MA. Local control of excitation-contraction coupling in human embryonic stem cell-derived cardiomyocytes. PLoS One. 2009;4:e5407. doi: 10.1371/journal.pone.0005407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brito-Martins M, Harding SE, Ali NN. Beta(1)- and beta(2)-adrenoceptor responses in cardiomyocytes derived from human embryonic stem cells: Comparison with failing and non-failing adult human heart. Br J Pharmacol. 2008;153:751–9. doi: 10.1038/sj.bjp.0707619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J, Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–9. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 35.Sartiani L, Bettiol E, Stillitano F, Mugelli A, Cerbai E, Jaconi ME. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: A molecular and electrophysiological approach. Stem Cells. 2007;25:1136–44. doi: 10.1634/stemcells.2006-0466. [DOI] [PubMed] [Google Scholar]
- 36.Lough J, Sugi Y. Endoderm and heart development. Dev Dyn. 2000;217:327–42. doi: 10.1002/(SICI)1097-0177(200004)217:4<327::AID-DVDY1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 37.Yang L, Soonpaa MH, Adler ED, Roepke TK, Katmann SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–8. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 38.Liu H, Dalton S, Xu Y. Transcriptional profiling of definitive endoderm derived from human embryonic stem cells. Comput Syst Bioinformatics Conf. 2007;6:79–82. [PubMed] [Google Scholar]
- 39.Vanden Hoek TL, Qin Y, Wojcik K, Li CQ, Shao ZH, Anderson T, Becker LB, Hamann KJ. Reperfusion, not simulated ischemia, initiates intrinsic apoptosis injury in chick cardiomyocytes. Am J Physiol Heart Circ Physiol. 2003;284:H141–50. doi: 10.1152/ajpheart.00132.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marinovic J, Bosnjak ZJ, Stadnicka A. Distinct roles for sarcolemmal and mitochondrial adenosine triphosphate-sensitive potassium channels in isoflurane-induced protection against oxidative stress. Anesthesiology. 2006;105:98–104. doi: 10.1097/00000542-200607000-00018. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka K, Weihrauch D, Ludwig LM, Kersten JR, Pagel PS, Warltier DC. Mitochondrial adenosine triphosphate-regulated potassium channel opening acts as a trigger for isoflurane-induced preconditioning by generating reactive oxygen species. Anesthesiology. 2003;98:935–43. doi: 10.1097/00000542-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 42.Batandier C, Guigas B, Detaille D, El-Mir MJ, Fontaine E, Rigoulet M, Leverve XM. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr. 2006;38:33–42. doi: 10.1007/s10863-006-9003-8. [DOI] [PubMed] [Google Scholar]
- 43.Hanley PJ, Ray J, Brandt U, Daut J. Halothane, isoflurane and sevoflurane inhibit NADH:ubiquinone oxidoreductase (complex I) of cardiac mitochondria. J Physiol. 2002;544:687–93. doi: 10.1113/jphysiol.2002.025015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D, Ovize M. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol. 2005;38:367–74. doi: 10.1016/j.yjmcc.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JF, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Ornato JP, Page RL, Tarkington LG, Yancy CW. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116:e418–99. doi: 10.1161/CIRCULATIONAHA.107.185699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.