Abstract
Cytokine-mediated inflammation and abruption-induced thrombin generation are separately implicated in matrix metalloproteinase (MMP)-mediated weakening of fetal membranes (FM) leading to preterm premature rupture of the fetal membranes (PPROM). At term, FM of both labored vaginal and unlabored caesarian deliveries exhibit a weak zone overlying the cervix exhibiting ECM remodeling characterized by increased MMP9 protein and activity. We have reproduced these biochemical changes as well as FM weakening in vitro using tumor necrosis factor-alpha (TNF) and interleukin (IL)-1β, inflammatory cytokines implicated in PPROM. Additionally, we have reported that the antioxidant and NFκB inhibitor alpha-lipoic Acid (LA) blocks these TNF-induced effects. We now present the first direct evidence that thrombin also can induce FM weakening in vitro, and LA treatment inhibits this thrombin-induced weakening. Full thickness FM fragments from unlabored caesarian deliveries were incubated with increasing doses of thrombin (0–100 u/ml) for 48h. Fragments were then strength tested (breaking force and work to rupture) using our published methodology. MMP3 and 9 levels in tissue extracts were determined by Western blot and densitometry. To determine the effect of LA, FM fragments were incubated with control medium or 10 u/ml thrombin, with or without 0.25mM LA. Strength testing and MMP induction was determined. Thrombin induced a dose-dependent decrease in FM strength (42% baseline rupture force and 45% work to rupture) coupled with a dose-dependent increase in MMP3 and 9 expression (all p<.001). Treatment of FM with 0.25mM LA completely inhibited thrombin-induced FM weakening and MMP expression (all p<.001). Thrombin treatment of cultured FM induces mechanical weakening and increased MMP3 and 9. Treatment of FM with LA inhibits these thrombin-induced effects. We speculate LA may prove clinically useful in prevention of PPROM associated with abruption.
Introduction
Preterm premature rupture of the fetal membranes (PPROM) is the initiating event in approximately one third of preterm births [1–3]. The mechanisms by which fetal membranes (FM) weaken and rupture at term and preterm gestations have not been fully elucidated, but FM weaken and ultimately rupture concomitant with collagen remodeling and apoptosis [4, 5]. We have described a localized region of the FM overlying the cervix that is mechanically weak relative to other regions of the FM, and exhibits biochemical and histological characteristics suggestive of increased collagen remodeling. This paracervical weak zone is consistently present in FM of term infants delivered by Cesarean delivery with no labor [6]. It is also seen in the FM of term vaginally delivered infants [7]. Other investigators have also reported distinctive biochemical and histological features in the paracervical region of the FM suggestive of increased tissue remodeling [8–12]. Because the line of rupture generally traverses the remodeled, paracervical weak zone, we have presumed that FM rupture typically is initiated within this region [5]. Recently, we reported that FM weakening, concomitant with changes in biochemical markers of remodeling mimicking the natural weak zone, can be reproduced in FM incubated in vitro with either tumor necrosis factor-alpha (TNF) or interleukin-1beta (IL-1β) [13]. Using this as a model system for the investigation of the cytokine-induced FM weakening process, we have demonstrated that the antioxidant and NFκB inhibitor, alpha-lipoic acid (LA), inhibits cytokine-induced FM weakening [14].
Inflammation and collagen remodeling in PPROM is often assumed to be due to intra-amniotic infection which complicates more than 50% of such cases [1]. There is also strong evidence linking PPROM with decidual hemorrhage and placental abruption [15]. A high incidence of occult decidual hemorrhage (i.e., abruption) and retrochorionic hematoma formation reflected by decidual and extraplacental membrane hemosiderin deposition has been reported in patients with PPROM [16]. Decidual cells have abundant Tissue Factor [17, 18], the primary initiator of hemostasis. After decidual hemorrhage, decidual cell membrane bound Tissue Factor binds plasma-derived factor VIIa to initiate the extrinsic coagulation cascade and ultimately convert prothrombin to thrombin. Patients with PPROM have a higher median plasma concentration of Tissue Factor and a lower median plasma concentration of Tissue Factor pathway inhibitor (TFPI) than women with normal pregnancies [19]. A major function of thrombin is the conversion of fibrinogen to fibrin. Consistent with this mechanism, fibrinogen consumption is a standard method of assessing abruption severity. Moreover, elevated circulating thrombin-antithrombin complex levels predict the subsequent occurrence of preterm delivery due to preterm labor and/or PPROM [20–22].
Weakening of the FM involves extracellular matrix (ECM) remodeling by the matrix metalloproteinase family (MMPs). Most sources suggest involvement of the gelatinases MMP2 and MMP9 (23), although questions have been raised [24]. The MMP enzymes are secreted from cells as zymogens. Both gelatinases are more abundant and more active in the amniotic fluid and FM of patients with PPROM (25, 26). Thrombin can directly activate pro-MMP2 and pro-MMP9 to their active lower molecular weight forms, most efficiently in conjunction with one of the Membrane type (MT)-MMPs [27]. Thrombin also initiates decidual cell and FM induction of MMP1, MMP3, and MMP9 [28–30] by binding to and activating protease activated receptors, specifically, PAR1, PAR3, and PAR4. Induced MMP3 may have a key role in initiating the proteolytic cascade, as it has the ability to proteolytically activate the gelatinases (MMP2, MMP9) as well as act directly upon the ECM.
Although the evidence for thrombin associated PPROM is compelling, there has been no direct demonstration of thrombin’s ability to weaken the FM. The purpose of this study was to evaluate whether thrombin could directly weaken FM in vitro and then to determine whether LA could inhibit thrombin induced FM weakening as it does cytokine induced FM weakening.
Methods
Materials
All reagents were obtained from Sigma-Aldrich Chemical Company (St. Louis, MO) unless specified otherwise.
Biological Samples
The study protocol was approved by the Institutional Review Board of the MetroHealth Medical Center, Case Western Reserve University (Cleveland, OH). FM were collected from patients undergoing prelabor, repeat Cesarean section at term (37–40 weeks gestation). After delivery of the fetus, the FM overlying the cervix were marked by the obstetrician using a sterile swab with gentian violet. Membranes were discarded if they were meconium stained, if infection was suspected from clinical history, or if chorioamnionitis was detected in pathological review. Each set of FM was sectioned and cultured using our previously reported methodology [13, 14, 31]. Briefly, immediately following Cesarean delivery of the placenta and marking of membranes, FM were cut from the placental disc avoiding the gentian violet marked region and 1cm perimeters adjacent to the disc and adjacent to the tear line.
In order to minimize baseline variability inherent in placental studies the following steps were taken: 1. All FM were from repeat Cesarean sections with absolutely normal pregnancy histories; 2. The para-cervical weak zone was not utilized. This is a relatively smaller region in repeat Ceasaren section FM, but if utilized would be a clear source of variability. The weak zone was identified using our previously described methodology [6,7]. 3. FM which showed more than a minimal separation of the amnion from the choriodecidua were eliminated (32–35). FM are clearly weaker when these components are separated (32). Separation and decreased adherence of the FM components increases with gestation (34). Thus, in addition to directly decreasing the variability, this eliminated later gestation FM (39 wk +) in favor of 37–38 week FM. This last restriction eliminated 50% of otherwise acceptable FM. 5. Fragments for each of the experimental conditions were randomly allocated in sequentially cutting the FM. All of these steps likely result in more homogeneous starting material for these studies.
FM Explant Culture
Full thickness FM from a single patient placenta FM were used for each experiment. Fragments were cut from the remaining FM of each patient using microtome blades and cultured per previously described protocols [13, 14, 31]. Each experiment used a total of twelve intact FM fragments measuring 3cm x 3cm and each experiment was repeated three times using different patient FM. FM fragments were randomly separated into four treatment groups consisting of three fragments in each group and placed in 100mm2 culture dishes, amnion side down, containing 10ml of Earle’s minimum essential medium, alpha modification (MEM), antibiotic antimycotic solution, 50 mg/L gentamicin sulfate, and 0.2% lactalbumin hydrolysate (EMEML). Culture dishes were rocked gently in an atmosphere containing 5% CO2, air and 100% relative humidity at 37°C. After 24 h equilibration in EMEML, medium was removed and replaced without, or with the addition of thrombin for and additional 48h period of culture. In thrombin dose response experiments, four study conditions were used: 1. No thrombin (control); 2. 1 unit/ml; 3. 10 units/ml ; and, 4. 100 units/ml.
To determine whether LA modifies thrombin effects, cultures were first treated with 0.1% DMSO (vehicle) or 0.25 mM LA. After 6hr incubation, 10 u/ml thrombin was added to one-half of the control and one-half of the LA treated cultures. Incubations were carried out for an additional 48h. Following all incubations, FM samples were subjected to biomechanical testing as outlined below.
FM Biomechanical Testing
All mechanical testing was done on full thickness fragments. Treated FM fragments were removed from culture, washed twice in 20 ml of MEM, maintained in MEM, and kept moist for the entirety of biomechanical testing. FM physical properties were determined using our previously reported methodology [6, 7]. Briefly, biomechanical testing was performed using modified industrial rupture testing equipment (Com-Ten Industries, St Petersburg, FL) by the American Society for Testing and Materials standards. A mechanically driven, 1-cm diameter, rounded plunger was forced at a speed of 8.4 cm/min through membrane pieces supported on a fixture with a 2.5-cm diameter orifice. Force (applied to the FM) and resultant FM displacement data were collected continuously and analyzed by data reduction software. Rupture strength, stiffness and work to rupture were determined from generated force/displacement curves. After biomechanical testing, amnion was peeled from the choriodecidua of each fragment, snap frozen separately in liquid nitrogen, and stored at −70°C until processed later determination of MMP2, 3, and 9 by Western blotting as described below.
Sample Homogenization
Thawed amnion and choriodecidua samples were normalized to FM surface area prior to homogenization by combining each 3cm x 3cm sample with 2X RIPA Buffer (Santa Cruz Biotechnology, Santa Cruz, CA) to total volumes of 5ml (2X RIPA+amnion) and 20ml (2X RIPA+choriodecidua). All samples were maintained on ice throughout subsequent processing. Samples were homogenized twice for 30s (with a 30s rest on ice) using a Tekmar Tissuemizer (Cincinnati, OH) fitted with a 85mm x 10mm diameter probe at 100%. After homogenization, samples were allowed to extract on ice for 30m and then frozen at −70°C overnight. After thawing on ice, this homogenization protocol was again repeated. Thawed samples were strained through 2mm x 2mm stainless wire mesh and eluates were clarified by centrifugation at 12000 x g for 15m at 10°C. Supernatants were stored at −70°C prior to Western Blotting.
Western Blotting
Tissue lysates (20ul ) were combined with an equal volume of Laemmli Buffer(BIO-RAD, Hercules, CA) containing 5% β-Mercaptoethanol, and samples were incubated in a boiling water bath for 5 min. Denatured samples were electrophoresed on 4%–15% gels/Tris-HCl, and resolved proteins were electrophoretically transferred for 90m to polyvinylidene fluoride membrane (GE Healthcare, Piscataway, NJ) according to Bio-Rad protocols. Transfer efficiency (100%) was routinely monitored by Coomassie Blue (BIO-RAD, Hercules, CA) gel staining. Membranes were blocked in 5% nonfat dry milk/Tris-buffered saline, 0.5% Tween-20 (TBST) for 30 min and then incubated overnight at 10°C with MMP2(H-76), MMP3(1B4), or MMP9(GE-213) antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were washed three times in TBST, incubated with horseradish peroxidase-conjugated secondary antibody (1:10000) in 5% nonfat milk/TBST for 30 min, and then again washed three times in TBST. Chemiluminescent detection was performed using Lumigen-PS according to the manufacturer’s protocol (GE Healthcare), and blots were exposed against Super RX film (Fuji, Tokyo, Japan) for, either 5–15s for densitometry, or 20–45s for illustrative purposes. Active, human, recombinant, 66-kDa MMP2, 57-kDa MMP3, or 83-kDa MMP9 (90% purity by SDSPAGE; EMD Biosciences) were used as relative markers in the Western blots.
Quantitation and Statistical Analysis
Developed films and stained gels were scanned using an Epson Perfection 1200U scanner. Densitometry was performed using ImageJ software (National Institutes of Health, Bethesda, MD). The value for an equivalent scanned blank region within a ‘‘dye-only’’ lane of each blot was subtracted from the value for each lane containing protein. Values for each lane representing a treatment were then normalized (divided by) against the control (zero) lane value. Data points shown for cumulative Western blots represent the mean ±SD for three experiments using FM from three different patients. Data (biomechanical, biochemical) were analyzed by ANOVA followed by posthoc pairwise comparisons using Statview software. Data and results are termed significant when P < 0.05.
Results
Effect of Thrombin on FM Strength
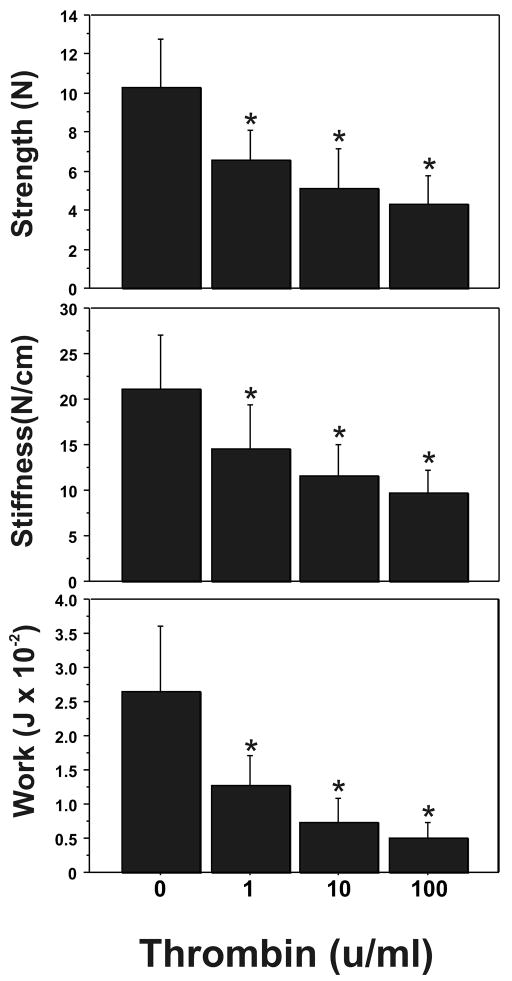
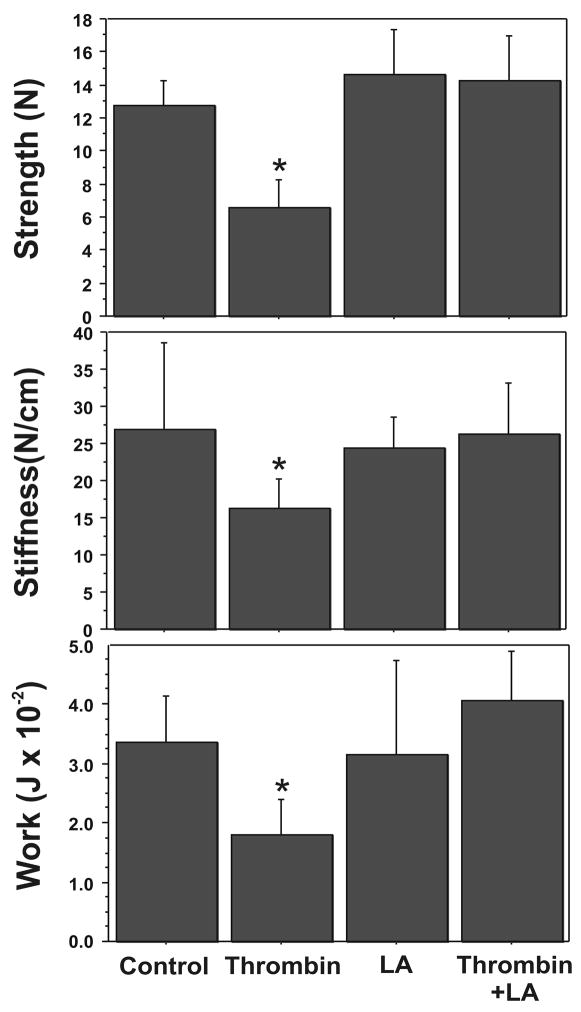
Thrombin induction of FM weakness occurs in a dose-dependent manner (Figure 1). Increasing doses (1–100 units/ml) of thrombin caused a stepwise decrease in the strength (breaking force), stiffness, and work required to rupture the full thickness FM. Thus, compared to controls, maximal induction of FM weakness, as evidenced by rupture strength (10.29 ± 2.49 N vs. 4.33 ± 1.45 N, P< 0.001), stiffness (21.17 ± 5.80 N/cm versus 9.63 ± 2.57 N/cm, P<0.001), and work to rupture (0.0264 ± 0.0096 J versus 0.0051 ± 0.0022 J; P< 0.001), was observed with the highest thrombin dose (100 units/ml) used (Figure 1). A significant weakening effect was seen in all parameters at the minimum dose of 1 unit/ml (P =.013). The maximal effect of thrombin treatment on FM strength occurred by 48 h; no effect of thrombin (100 units/ml) on FM strength was demonstrable at 24 h (data not shown).
Figure 1.
Effect of Increasing Thrombin Dose upon FM Mechanical Properties: Thrombin (0–100 u/ml) induced concentration-dependent decreases in FM rupture strength (upper panel), stiffness (middle panel) and work to rupture (bottom panel). All incubations were for 48 h. The data shown represent triplicate FM cultures repeated three times using three different placentas. (Data are presented mean ± SD, * p < 0.001 vs. no thrombin control)
Thrombin Induced Markers of Collagen Remodeling
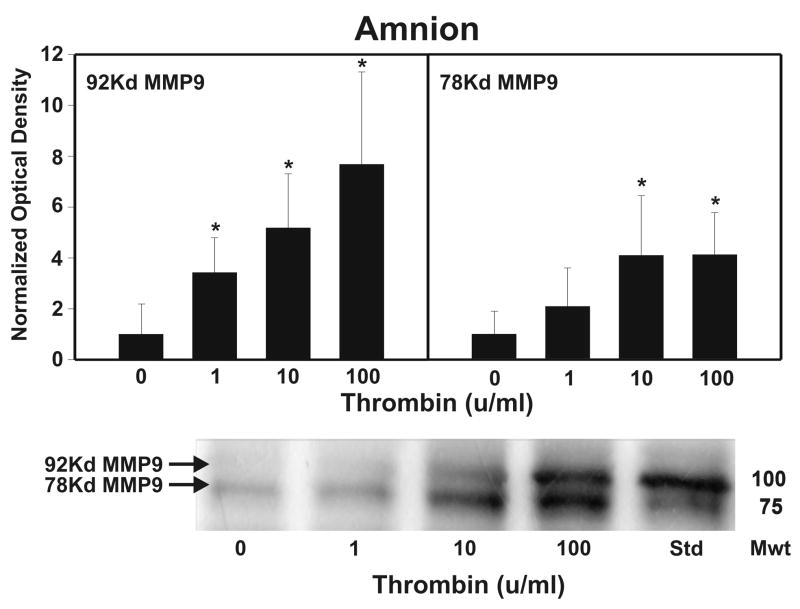
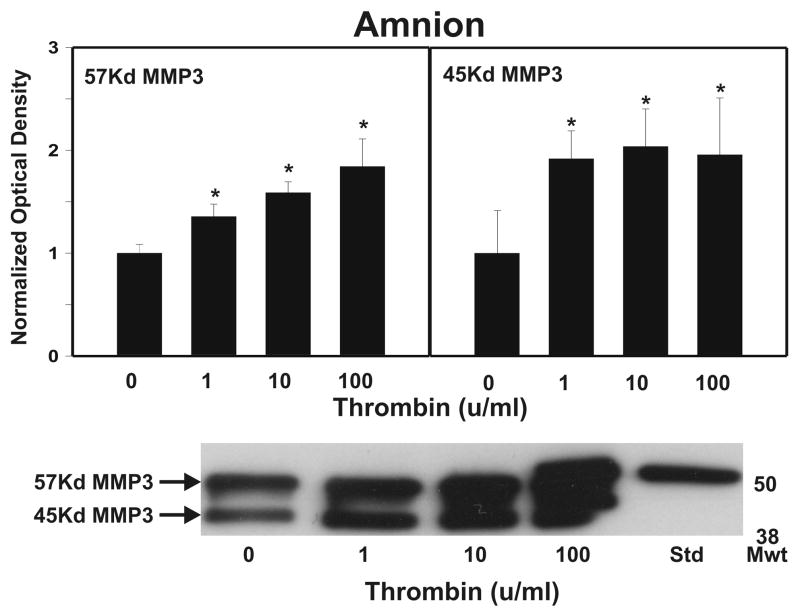
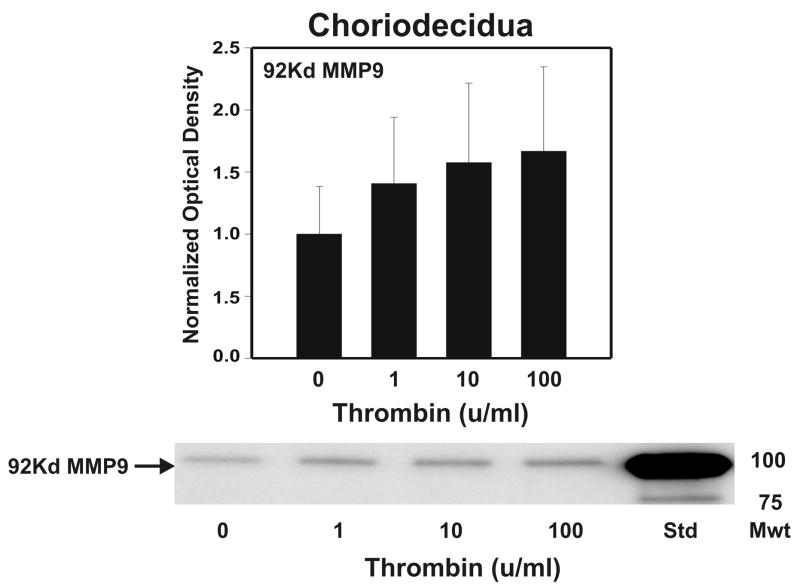
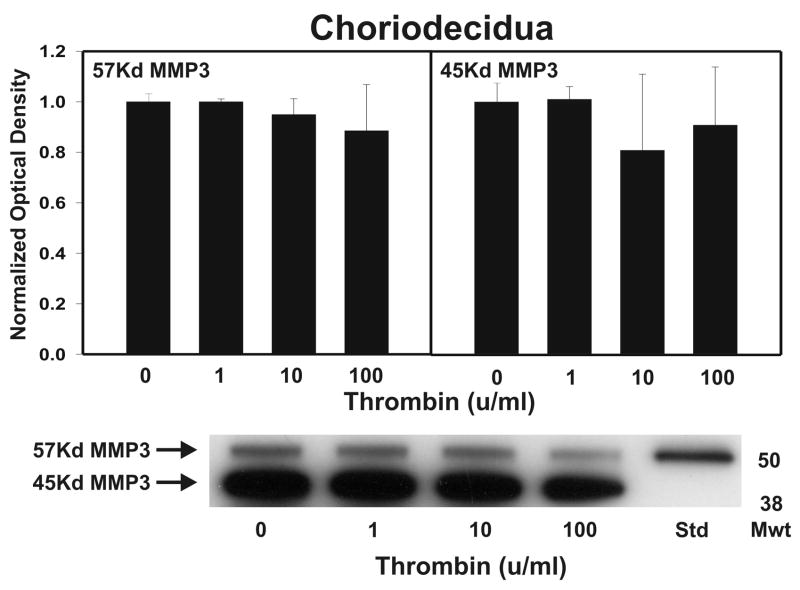
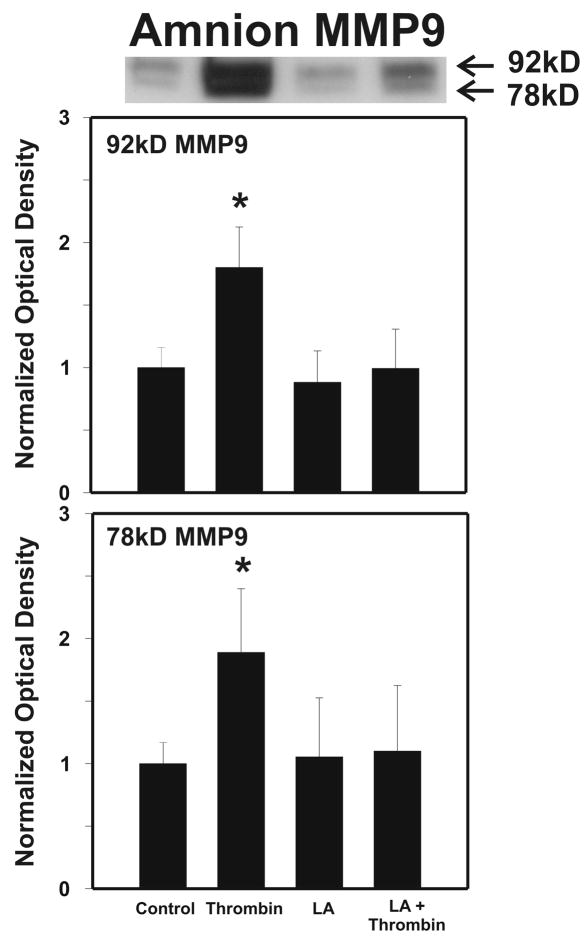
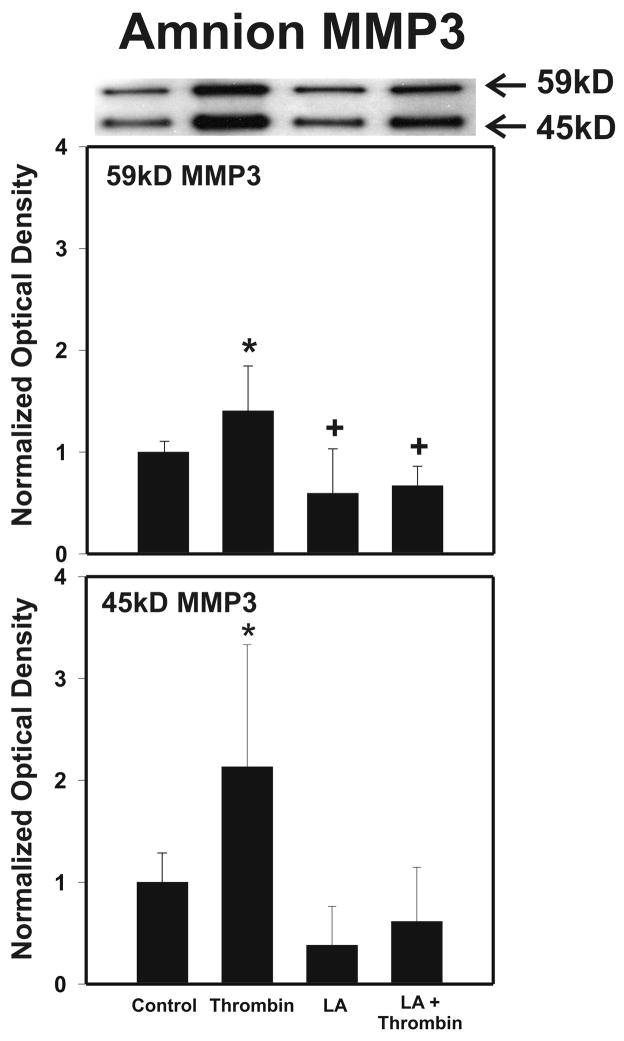
After strength testing the amnion and choriodecidua were separately tested for markers of collagen remodeling. Thrombin induced collagen degradation/remodeling in the amnion component of the FM, as evidenced by induction of MMP9 protein. Following 48 h incubation with thrombin (0–100 u/ml) in full thickness FM explants, the separated amnion component exhibited increases in both pro-MMP9 (92 kD) and active MMP9 (78 kD) proteins in a dose dependent manner (p < 0.01, Figure 2A). Levels of both the zymogen (57 kD) and active (45 kD) forms of MMP3, were also elevated in response to increasing thrombin doses in the amnion (p <0.01, Figure 2B). Amnionic MMP2 levels did not change with thrombin treatment (data not shown). No changes in MMPs were seen in the choriodecidua component of the FM as a result of thrombin incubation (Figure 3).
Figure 2.
Thrombin Induces MMP9 and MMP3 in Amnion: Thrombin (0–100 u/ml for 48 h) induced concentration-dependent increases in both the pro-MMP9 (92 kD) and the active MMP9 (78 kD) protein (Panel A); and in both the pro-MMP3 (57 kD) and the active MMP3 (45 kD) protein (Panel B); in the amnion component of treated FM explants. The Western blot shown is representative of three replicate experiments with three different placentas. To confirm the significance of qualitative results, triplicate blots were scanned and subjected to densitometric analysis. Each blot was normalized to the zero-thrombin controls as indicated in Methods. (Data are presented mean ± SD, * p < 0.01 vs. no thrombin control)
Figure 3.
Thrombin effect upon MMP9 and MMP3 in Choriodecidua: Thrombin (0–100 u/ml for 48 h) failed to show concentration-dependent increases in pro-MMP9 (92 kD) in the choriodecidua component of treated FM explants. The active MMP9 (78 kD) form was not detected (Panel A). Thrombin (0–100 u/ml for 48 h) failed to show concentration-dependent increases in either the pro-MMP3 (57 kD) or the active MMP3 (45 kD) protein in the choriodecidual component of treated FM explants (Panel B). The Western blot shown is representative of three replicate experiments with three different placentas. To confirm the significance of qualitative results, triplicate blots were scanned and subjected to densitometric analysis. Each blot was normalized to the zero-thrombin controls as indicated in Methods. (Data are presented mean ± SD, no significant differences were detected.)
α-Lipoic Acid Inhibits Thrombin Induced FM Weakness
Consistent with the dose response experiments, relative to controls, thrombin (10 u/ml) significantly decreased the force required to rupture FM fragments (12.78±1.51 N [control] versus 6.55±1.73 N [thrombin alone]; P < 0.001). Similarly, work to rupture (0.0334± 0.0079 J [control] versus 0.0179±0.0064 J [thrombin alone]; P < 0.001) and stiffness (27.0 ±11.5 N/cm [control] versus 16.18 ±4.08 N/cm [thrombin alone]; P< 0.001) also decreased significantly. However, incubation with 0.25 m LA completely inhibited thrombin-induced FM weakening (6.55±1.73 N [thrombin] versus 14.25±2.70 N [LA treatment followed by thrombin]; P < 0.001; Figure 4). The thrombin induced decreases in FM work to rupture and stiffness were also completely inhibited. Incubation with 0.25 mM LA without subsequent thrombin had no effect on the basal FM rupture strength or work to rupture.
Figure 4.
Lipoic Acid (LA) inhibits Thrombin induced-weakening: FM fragments were pre-incubated with or without LA (0.25 mM) for 6h, then incubated with or without the addition of Thrombin (10 u/ml) for 48h. Four groups are displayed: Control, LA pre-treatment alone, Thrombin alone, LA treatment + Thrombin. Rupture Strength (upper panel), Stiffness (middle panel) and Work to Rupture (lower panel) were determined as outlined in Methods. Data points represent pooled results of three experiments using three FM fragments per treatment group in each experiment. (Data are presented mean ± SD, * p < 0.001 vs. all other groups)
As in the dose response experiments, amnion was separated from choriodecidua and the amnion component was tested for biochemical markers of remodeling. LA (0.25 mM) alone had no effect upon the levels of either the pro-MMP9 or the active MMP9, but completely abolished the thrombin-induced increases (p < 0.01, Figure 5). In a similar fashion, MMP3 levels increased with thrombin, and this thrombin induced effect was again completely inhibited by LA (0.25 mM) treatment (p < 0.01, Figure 6). LA treatment also reduced 57kD zymogen form of MMP3 below the control level (p< 0.05, Figure 6). Because of the lack of effect of thrombin on MMPs in the choriodecidua in the dose response experiments, the effect of LA on the choriodecidua component of FM was not determined in these experiments.
Figure 5.
Lipoic Acid (LA) inhibits Thrombin induced increases in MMP9 in the amnion component of cultured FM. Intact FM fragments were pre-incubated with or without LA (0.25 mM) for 6h, then incubated with or without the addition of thrombin (10 u/ml) for 48h. After the incubations, the amnion was then separated from the choriodecidua and tested. Four groups are displayed: Control, LA pre-treatment, thrombin, LA treatment + thrombin. The Western blot shown is representative of three replicate experiments using three different placentas . Both the pro-MMP9 (92 kD) and the active MMP9 (78 kD) protein in the amnion component of treated FM explants are shown. To confirm the significance of qualitative results, blots were scanned and subjected to densitometric analysis. Each blot was normalized to the zero-thrombin controls as indicated in Methods (Data are presented mean ± SD, * p < 0.01 vs. all other groups).
Figure 6.
Lipoic Acid (LA) inhibits Thrombin induced increases in MMP3 in the amnion component of cultured FM. Intact FM fragments were pre-incubated with or without LA (0.25 mM) for 6h, then incubated with or without the addition of Thrombin (10 u/ml) for 48h. After the incubations, the amnion was then peeled from the choriodecidua and tested. Four groups are displayed: Control, LA pre-treatment, Thrombin, LA treatment + Thrombin. The Western blot shown is representative of three replicate experiments with three different placentas (N=9). Both the pro-MMP3 (57 kD) and the active MMP3 (45 kD) protein in the amnion component of treated FM explants are shown. To confirm the significance of qualitative results, blots were scanned and subjected to densitometric analysis. Each blot was normalized to the zero-thrombin controls as indicated in Methods (Data are presented mean ± SD, * p < 0.01 from all groups, + p < 0.05 from control).
Discussion
The major findings of these studies were: 1) Thrombin weakens FM in vitro in a dose dependent manner; 2) Thrombin increases both MMP3 and MMP9 in the amnion component of the FM in parallel with the weakening; 3) The antioxidant and NFκB inhibitor, LA, blocks both thrombin induced FM weakening as well as its effect upon MMP expression in the amnionic component of the FM. Thus thrombin produced following decidual hemorrhage/abruption is capable of weakening the FM which may result in term FM rupture or PPROM.
Preterm PROM is highly associated with decidual hemorrhage/abruption with resultant thrombin production [15, 20–22]. As thrombin is likely produced in the decidua and the main strength components of FM reside in the amnion, the weakening process is complex. Thrombin is produced by the extrinsic coagulation cascade after hemorrhage/abruption. Plasma Factor VII interacts with Tissue Factor presented by decidua cells and perhaps other cells of the choriodecidua [18] to initiate the extrinsic coagulation pathway culminating in the conversion of prothrombin to thrombin. Thrombin then may diffuse to the amnion and act directly on amnion ECM or upon amnion cells. Alternatively, thrombin may induce soluble factors in cells of the choriodecidua which in turn act upon the amnion cells or the products of amnion cells to cause ECM remodeling with resultant weakening. Thrombin may act directly upon pro-MMPs converting them to active forms capable of degrading collagen and microfibers in the ECM of either the amnion or the choriodecidua. Fang and colleagues recently reported thrombin activates pro-MMP1, 2, 3, and 9 in both human fetal lung fibroblasts (HFL-1 cells) and bronchial biopsy-derived primary cultures of lung fibroblasts [36]. However, a direct effect of thrombin is less likely in these studies as no effect was seen at 24h with high dose thrombin. Thrombin also may act by binding and activating PAR receptors. PAR1, 3 and 4 are known to bind thrombin. The PAR2 receptor does not bind thrombin but is activated by upstream coagulation components generated by thrombin (i.e., factor VIIa when bound to tissue factor) which may be produced in parallel with thrombin in vivo. The PAR1 receptor is present in endometrial stromal cells and decidua [37]. Stimulation of PARs by thrombin and by the PAR1 agonist Thrombin Receptor Activating Protein (TRAP) has been shown to induce MMP3 and MMP-1 in primary term decidual cell cultures [28, 30]. Similarly, thrombin induces MMP9 levels via PAR receptors in cultured amniochorion [29]. Thrombin may stimulate MMP9 induction in amnion cells in a similar fashion. As MMP3 and thrombin are both capable of activating other MMPs [38]; they are potentially able to move from the decidua or trophoblast cells in the choriodecidua to the amnion where induced MMP9 and the already produced MMP2 are present. Activation of these gelatinases could lead to ECM remodeling and degradation which could culminate in FM rupture.
The data demonstrate that LA inhibits the weakening and ECM remodeling effects of thrombin. LA (6,8-dithio-octanoic acid) is a naturally occurring antioxidant produced by the human liver and other tissues. It is also obtained from animal (heart, liver, kidneys) and plant (spinach, broccoli, tomatoes, garden peas and rice bran) sources in our diet [39]. LA is also available as a dietary supplement in its natural R-form and racemic R/S mixture. It is rapidly absorbed and reduced by NAD(P)H-dependent enzymes to the dithiol compound dihydrolipoic acid. We have previously reported that LA inhibits the FM weakening and remodeling effects of TNF and IL-1β [14]. As both cytokines and thrombin initiate development of reactive oxygen species, they should both be susceptible to the antioxidant effects of LA [40]. However, recent work, including our own, suggests that other antioxidants, Vitamin C and Vitamin E, have not been effective in preventing FM weakening in vitro, or the development of the normal physiological weak zone of the FM in vivo [31]. These antioxidants have also not been effective in preventing PPROM in two large clinical series [41, 42]. LA also inhibits NFκB which is activated by cytokines and thrombin as part of the induction of MMPs and other enzyme cascades which result in apoptosis and ECM remodeling. LA has been shown to inhibit TNF induced NFκB transcriptional activity and MMP9 expression in vascular smooth muscle cells, and attenuate LPS-induced NFkB:DNA-binding in monocytes by activating the PI3K/AKT pathway [43,44]. Indeed, NFκB has been hypothesized to have a central role in the onset of labor and FM rupture [10, 11] and inhibition of its transcriptional signaling would block the induction of many enzymes essential for FM weakening and rupture. The mechanism of inhibition of FM weakening by LA remains unknown at this time, however. Therefore, whether its antioxidant or NFκB inhibitory properties are involved remains speculative.
In summary, direct evidence is provided for the first time that thrombin can weaken FM in vitro and thus has the potential to contribute to FM rupture. These data provide a mechanism to account for the strong association between decidual hemorrhage/abruption and PPROM. LA, an antioxidant and NFκB inhibitor, is also shown to inhibit thrombin induced FM weakening and remodeling in vitro. This agent, which has previously been shown to also inhibit cytokine-induced FM weakening, warrants further study as a possible clinically useful agent for prevention of PPROM.
Footnotes
Location where study was performed: MetroHealth Medical Center, Cleveland, Ohio
Presentation: Presented at the Annual Meeting of the SGI, Orlando Fl, March 25, 2010
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mercer BM. Premature Rupture of the membranes. An expert’s view. Obstet Gynecol. 2003;101:178–193. doi: 10.1016/s0029-7844(02)02366-9. [DOI] [PubMed] [Google Scholar]
- 2.Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand. 2008;87:590–600. doi: 10.1080/00016340802005126. [DOI] [PubMed] [Google Scholar]
- 3.Parry S, Strauss JF. Premature rupture of the fetal membranes. N Engl J Med. 1998;338:663–670. doi: 10.1056/NEJM199803053381006. [DOI] [PubMed] [Google Scholar]
- 4.Menon R, Fortunato SJ. The role of matrix degrading enzymes and apoptosis in rupture of membranes. J Soc Gynecol Investig. 2004;11:427–437. doi: 10.1016/j.jsgi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Moore RM, Mansour JM, Redline RW, Mercer BM, Moore JJ. The physiology of fetal membrane rupture: insight gained from the determination of physical properties. Placenta. 2006;27:1037–1051. doi: 10.1016/j.placenta.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 6.El Khwad M, Stetzer B, Moore RM, Kumar D, Mercer B, Arikat S, et al. Term human fetal membranes have a weak zone overlying the lower uterine pole and cervix before onset of labor. Biol Reprod. 2005;72:720–6. doi: 10.1095/biolreprod.104.033647. [DOI] [PubMed] [Google Scholar]
- 7.El Khwad M, Pandey V, Stetzer B, Mercer BM, Kumar D, Moore RM, et al. Fetal membranes from term vaginal deliveries have a zone of weakness exhibiting characteristics of apoptosis and remodeling. J Soc Gynecol Investig. 2006;13:191–195. doi: 10.1016/j.jsgi.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 8.McLaren J, Malak TM, Bell SC. Structural characteristics of term human fetal membranes prior to labour: identification of an area of altered morphology overlying the cervix. Hum Reprod. 1999;14:237–241. doi: 10.1093/humrep/14.1.237. [DOI] [PubMed] [Google Scholar]
- 9.Reti NG, Lappas M, Riley C, et al. Why do membranes rupture at term? Evidence of increased cellular apoptosis in the supracervical fetal membranes. Am J Obstet Gynecol. 2007;196:484.e1–10. doi: 10.1016/j.ajog.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Lappas M, Odumetse TL, Riley C, et al. Pre-labour fetal membranes overlying the cervix display alterations in inflammation and NF-kappaB signalling pathways. Placenta. 2008;29:995–1002. doi: 10.1016/j.placenta.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Lappas M, Riley C, Rice GE, Permezel M. Increased expression of ac-FoxO1 protein in prelabor fetal membranes overlying the cervix: possible role in human fetal membrane rupture. Reprod Sci. 2009;16:635–41. doi: 10.1177/1933719109332831. [DOI] [PubMed] [Google Scholar]
- 12.Meinert M, Malmström A, Tufvesson E, et al. Labour induces increased concentrations of biglycan and hyaluronan in human fetal membranes. Placenta. 2007;28:482–486. doi: 10.1016/j.placenta.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Kumar D, Fung W, Moore RM, Pandey V, Fox J, Stetzer B, et al. Proinflammatory cytokines found in amniotic fluid induce collagen remodeling, apoptosis, and biophysical weakening of cultured human fetal membranes. Biol Reprod. 2006;74:29–34. doi: 10.1095/biolreprod.105.045328. [DOI] [PubMed] [Google Scholar]
- 14.Moore RM, Novak JB, Kumar D, Mansour JM, Mercer BM, Moore JJ. Alpha-lipoic acid inhibits tumor necrosis factor-induced remodeling and weakening of human fetal membranes. Biol Reprod. 2009;80:781–787. doi: 10.1095/biolreprod.108.073205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harger JH, Hsing AW, Tuomala RE, Gibbs RS, Mead PB, Eschenbach DA. Risk factors for preterm rupture of fetal membranes: a multicenter case-control study. Am J Obstet Gynecol. 1990;163:130–137. doi: 10.1016/s0002-9378(11)90686-3. [DOI] [PubMed] [Google Scholar]
- 16.Salafia CM, Lopez-Zeno JA, Sherer DM, Whittington SS, Minior VK, Vintzileos AM, et al. Histologic evidence of old intrauterine bleeding is more frequent in prematurity. Am J Obstet Gynecol. 1995;173:1065–1070. doi: 10.1016/0002-9378(95)91327-0. [DOI] [PubMed] [Google Scholar]
- 17.Lockwood CJ, Nemerson Y, Guller S, Krikun G, Alvarez M, Hausknecht V, et al. Progestational regulation of human endometrial stromal cell tissue factor expression during decidualization. J Clin Endocrinol Metab. 1993;76:231–236. doi: 10.1210/jcem.76.1.8421090. [DOI] [PubMed] [Google Scholar]
- 18.Lockwood CJ, Krikun G, Schatz F. Decidual cell-expressed tissue factor maintains hemostasis in human endometrium. Ann NY Acad Sci. 2001;943:77–88. doi: 10.1111/j.1749-6632.2001.tb03793.x. [DOI] [PubMed] [Google Scholar]
- 19.Erez O, Espinoza J, Chaiworapongsa T, Gotsch F, Kusanovic JP, Than NG, et al. A link between a hemostatic disorder and preterm PROM: a role for tissue factor and tissue factor pathway inhibitor. J Matern Fetal Neonatal Med. 2008;21:732–44. doi: 10.1080/14767050802361807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen T, Kuczynski E, O’Neill LM, Funai EF, Lockwood CJ. Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. J Matern Fetal Med. 2001;10:297–300. doi: 10.1080/714904361. [DOI] [PubMed] [Google Scholar]
- 21.Chaiworapongsa T, Espinoza J, Yoshimatsu J, Kim YM, Bujold E, Edwin S, Yoon BH, Romero R. Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002;11:368–373. doi: 10.1080/jmf.11.6.368.373. [DOI] [PubMed] [Google Scholar]
- 22.Elovitz MA, Baron J, Phillippe M. The role of thrombin in preterm parturition. Am J Obstet Gynecol. 2001;185:1059–1063. doi: 10.1067/mob.2001.117638. [DOI] [PubMed] [Google Scholar]
- 23.Romero R, Chaiworapongsa T, Espinoza J, Gomez R, Yoon BH, Edwin S, et al. Fetal plasma MMP9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol. 2002;187:1125–1130. doi: 10.1067/mob.2002.127312. [DOI] [PubMed] [Google Scholar]
- 24.Stuart EL, Evans GS, Powers HJ. The proteolytic profile of prelabour ruptured amnion at term: A case control study. Eur J Obstet Gynecol Reprod Biol. 2007;131:158–162. doi: 10.1016/j.ejogrb.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Tsatas D, Baker MS, Rice GE. Differential expression of proteases in human gestational tissues before, during and after spontaneous-onset labour at term. J Reprod Fertil. 1999;116:43–49. doi: 10.1530/jrf.0.1160043. [DOI] [PubMed] [Google Scholar]
- 26.Xu P, Alfaidy N, Challis JR. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in human placenta and fetal membranes in relation to preterm and term labor. J Clin Endocrinol Metab. 2002;87:1353–1361. doi: 10.1210/jcem.87.3.8320. [DOI] [PubMed] [Google Scholar]
- 27.Lafleur MA, Hollenberg MD, Atkinson SJ, Knauper V, Murphy G, Edwards DR. Activation of pro-(matrix metalloproteinase-2) (pro-MMP-2) by thrombin is membrane-type-MMP-dependent in human umbilical vein endothelial cells and generates a distinct 63 kDa active species. Biochem J. 2001;357:107–115. doi: 10.1042/0264-6021:3570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackenzie AP, Schatz F, Krikun G, Funai EF, Kadner S, Lockwood CJ. Mechanisms of abruption-induced premature rupture of the fetal membranes: Thrombin enhanced decidual matrix metalloproteinase-3 (stromelysin-1) expression. Am J Obstet Gynecol. 2004;191:1996–2001. doi: 10.1016/j.ajog.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Stephenson CD, Lockwood CJ, Ma Y, Guller S. Thrombin-dependent regulation of matrix metalloproteinase (MMP)-9 levels in human fetal membranes. J Matern Fetal Neonatal Med. 2005;18:17–22. doi: 10.1080/14767050500123632. [DOI] [PubMed] [Google Scholar]
- 30.Rosen T, Schatz F, Kuczynski E, Lam H, Koo AB, Lockwood CJ. Thrombin-enhanced matrix metalloproteinase-1 expression: a mechanism linking placental abruption with premature rupture of the membranes. J Matern Fetal Neonatal Med. 2002;11:11–17. doi: 10.1080/jmf.11.1.11.17. [DOI] [PubMed] [Google Scholar]
- 31.Mercer BM, Abdelrahim A, Moore RM, Novak J, Kumar D, Mansour JM, et al. The impact of Vitamin C supplementation in pregnancy and in-vitro upon fetal membrane strength and remodeling. Reprod Sci. 2010;17:685–695. doi: 10.1177/1933719110368870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arikat S, Novince RW, Mercer BM, Kumar D, Fox J, Mansour JM, Moore JJ. Separation of amnion from choriodecidua is an integral event to the rupture of normal fetal membranes and constitutes a significant component of the work required. Am J OB Gynecol. 2006;194:211–217. doi: 10.1016/j.ajog.2005.06.083. [DOI] [PubMed] [Google Scholar]
- 33.Kumar D, Novince R, Strohl A, Mercer BM, Mansour JM, Moore RM, et al. A new methodology to measure strength of adherence of the fetal membrane components, amnion and the choriodecidua. Placenta. 2009;30:560–563. doi: 10.1016/j.placenta.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strohl A, Kumar D, Novince R, Shaniuk P, Smith J, Bryant K, et al. Decreased Adherence and Spontaneous Separation of Fetal Membrane Layers--Amnion and Choriodecidua — a Possible Part of the Normal Weakening Process. Placenta. 2010;31:18–24. doi: 10.1016/j.placenta.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meinert M, Malmström A, Tufvesson E, Westergren-Thorsson G, Petersen AC, Laurent C, et al. Labour induces increased concentrations of biglycan and hyaluronan in human fetal membranes. Placenta. 2007;28:482–486. doi: 10.1016/j.placenta.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Fang Q, Liu X, Al-Mugotir M, Kobayashi T, Abe S, Kohyama T, et al. Thrombin and TNF-_/IL-1_ Synergistically Induce Fibroblast-Mediated Collagen Gel Degradation. Am J Respir Cell Mol Biol. 2006;35:714–721. doi: 10.1165/rcmb.2005-0026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hague S, Oehler MK, MacKenzie IZ, Bicknell R, Rees MC. Protease activated receptor-1 is down regulated by levonorgestrel in endometrial stromal cells. Angiogenesis. 2002;5:93–98. doi: 10.1023/a:1021510723157. [DOI] [PubMed] [Google Scholar]
- 38.Lockwood CJ, Murk W, Kayisli UA, Buchwalder LF, Huang ST, Funai EF, et al. Progestin and thrombin regulate tissue factor expression in human term decidual cells. J Clin Endocrinol Metab. 2009;94:2164–2170. doi: 10.1210/jc.2009-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh U, Jialal I. Alpha-lipoic acid supplementation and diabetes. Nutr Rev. 2008;66:646–57. doi: 10.1111/j.1753-4887.2008.00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Holland JA, Meyer JW, Chang M, O'Donnell RW, Johnson DK, Ziegler LM. Thrombin Stimulated Reactive Oxygen Species Production in Cultured Human Endothelial Cells Endothelium. 1998;6:113–121. doi: 10.3109/10623329809072198. [DOI] [PubMed] [Google Scholar]
- 41.Spinnato JA, Freire S, Pinto e Silva JL, et al. Antioxidant supplementation and premature rupture of the membranes: a planned secondary analysis. Am J Obstet Gynecol. 2008;199:433.e1–8. doi: 10.1016/j.ajog.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts JM, Myatt L, Spong CY, et al. Vitamin C and E do not reduce maternal and fetal/neonatal adverse outcomes related to pregnancy associated hypertension when administered to low risk nulliparous women from early gestation. N Engl J Med. 2010;362:1282–1291. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim H, Kim H, Park K, Kim Y, Kwon T, Park J, et al. α-Lipoic acid inhibits matrix metalloproteinase-9 expression by inhibiting NF-κB transcriptional activity. Exp Mol Med. 2007;1:106–113. doi: 10.1038/emm.2007.12. [DOI] [PubMed] [Google Scholar]
- 44.Zhang W, Wei H, Frei B. α-Lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. PNAS. 2007;104:4077–82. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]