Abstract
Extracellular matrix (ECM) remodeling regulates multiple cellular functions required for normal development and tissue repair. Matrix metalloproteinases (MMPs) are key mediators of this process and membrane targeted MMPs (MT-MMPs) in particular have been shown to be important in normal development of specific organs. In this study we investigated the role of MT1-MMP in kidney development. We demonstrate that loss of MT1-MMP leads to a renal phenotype characterized by a moderate decrease in ureteric bud branching morphogenesis and a severe proliferation defect. The kidneys of MT1-MMP-null mice have increased deposition of collagen IV, laminins, perlecan, and nidogen and the phenotype is independent of the MT-1MMP target, MMP-2. Utilizing in vitro systems we demonstrated that MTI-MMP proteolytic activity is required for renal tubule cells to proliferate in three dimensional matrices and to migrate on collagen IV and laminins. Together these data suggest an important role for MT1-MMP in kidney development, which is mediated by its ability to regulate cell proliferation and migration by proteolytically cleaving kidney basement membrane components.
Keywords: branching morphogenesis, basement membrane, matrix metalloproteinases, kidney
Introduction
The kidney is composed of multiple filtering units known as nephrons that connect to collecting ducts which ultimately join together to form the ureter. The nephron, which consists of a glomerulus and highly differentiated tubules, is derived from the metanephric mesenchyme (MM), while the collecting system is derived from the ureteric bud (UB). Kidney development begins when the UB invades the MM which condenses and transforms into epithelial cells that ultimately form the nephrons. Simultaneously, the MM signals to the UB inducing it to undergo numerous iterations of branching morphogenesis [1].
The key function of the kidney is to maintain fluid and electrolyte homeostasis and to excrete toxins from the body, which it does by selectively filtering and reabsorbing solutes of different sizes and charges in the glomerulus as well as different nephron segments. Due to the complexity of its functions, the kidney has developed some of the most specialized basement membranes (BMs) in the body whose formation and turnover are tightly controlled both spatially and temporally. The major constituents of these BMs are the ECM proteins collagen IV, laminins and heparan sulphate proteoglycans [2]. A tight balance between extracellular matrix (ECM) synthesis and degradation is important for normal kidney development. Matrix metalloproteinases (MMP) are matrix degrading enzymes that play an important role for the normal development and turnover of these BMs.
MMPs are a family of zinc-dependent endopeptidases that are either secreted and diffuse to their sites of action or are bound to the cell surface where they mediate pericellular proteolysis [3]. Although numerous MMPs are expressed in the kidney, the most extensively studied are the gelatinases, MMP-2 and MMP-9, due to their ability to degrade collagens and laminins, which are major kidney ECM components. MMP-2 and MMP-9 are expressed in both the MM and the UB by day E11 in mice [4]. Blocking MMP-9, but not MMP-2, synthesis or activity inhibits UB branching in organotypic cultures [4], however when deoxynucleotides inhibited MMP-2 synthesis in E13 rat kidney cultures, decreased UB branching morphogenesis was observed [5]. Despite these in vitro findings, mice harboring targeted null mutations for MMP-2 [6], MMP-9 [7] or MMMP-2/MMP-9 [8] had no obvious renal abnormalities. Although MMP-9 was demonstrated to preserve vessel structure and alleviate blood pressure increases in a disease model of angiotensin-II induced hypertension [9], progression of anti-glomerular basement disease was not affected in either MMP-2 or MMP-9 null mice [10]. These minor or lack of effect on renal development or following renal injury suggest that, in addition to gelatinases, other MMP family members might modulate ECM turnover in the kidney.
MMP14, also referred to as MT1-MMP, which is the prototype membrane type (MT) MMP, has been studied in the context of renal development. This enzyme has intrinsic proteolytic capabilities and can also induce its effects by activating MMP-2 and MMP-13 [11]. Numerous ECM components, including collagens I, II and III, fibronectin, vitronectin, laminins 111 and 332, fibrin and proteoglycans are substrates for MT1-MMP [12]. In addition, MT1-MMP can cleave other cell surface proteins such as CD44 [13], transglutaminase [14], low-density lipoprotein receptor related protein [15], the integrin αv subunit [16], and syndecan-1 [17]. These highly divergent substrates for MT1-MMP make this enzyme a critical regulator of the pericellular environment and allow it to regulate multiple cellular functions. The physiological importance of MT1-MMP was demonstrated by the multiple abnormalities observed in the MT1-MMP null mice, which die shortly after birth with severe musculoskeletal abnormalities characterized by decreased chondrocyte proliferation and decreased collagenolytic activity [18, 19]. More recent investigations on the musculoskeletal system have shown that reconstitution of MT1-MMP activity in the type II collagen-expressing cells of the skeleton in MT1-MMP null mice rescues the diminished chondrocyte proliferation in these mice and ameliorates the severe skeletal dysplasia by enhancing bone formation. [20]. In addition, these null mice have submandibular gland branching morphogenesis abnormalities [21] as well as defects in lung development [21, 22], angiogenesis [23] and myeloid cell fusion [24]. These deficiencies are ascribed to a lack of MT1-MMP catalytic ability, alterations in downstream pro-MMP-2 activation and alterations in cell functions regulated by the MT1-MMP cytoplasmic tail.
MT1-MMP is widely expressed in the kidney and is found in the UB at E11 and the MM at E12 [25]. Like the gelatinases, MT1-MMP function was shown to be required for UB branching morphogenesis in kidney organ cultures, where it induced its affects, at least in part, by activating MMP-2 [5]. In contrast to the gelatinase null mice, we previously described subtle, but distinct renal abnormalities in 10-week-old out-bred MT1-MMP mice, which were characterized by a proportional decrease in both cortical and medullary mass [26]. Both the glomeruli and the tubules were slightly dysmorphic and these renal abnormalities correlated with an increase in laminin 332 deposition, suggesting that lack of laminin 332 cleavage by MT1-MMP accounted for these abnormalities [26].
Although these data defined a role for MT1-MMP in renal development and suggested its role was the cleavage of at least one ECM component in renal BMs, the mechanisms whereby the renal abnormalities occur is unclear. We therefore explored the role of MT1-MMP in renal development in more detail and demonstrate that when MT1-MMP null mice are bred onto a pure C57/B6 background, they die at P14 with small kidneys due to a severe proliferative defect and a moderate UB branching abnormality. We show that MT1-MMP does not activate MMP-2 in the kidney in vivo and the proteolytic activity of MT1-MMP is required for normal UB branching in in vitro organ culture models. We further demonstrate increased deposition of laminins, collagen IV, nidogen and perlecan in MT-MMP-null kidneys. Utilizing MT1-MMP deficient renal tubular epithelial cells we show that MT1-MMP proteolytic activity is required for normal cell migration on ECM components and proliferation in 3 dimensional gels. Thus our results suggest that pericellular cleavage of multiple BM components by MT1-MMP is important for cell proliferation and migration and plays a critical role in normal kidney development.
Materials and methods
Morphological analysis of MT1-MMP null mice
MT1-MMP mice generated by Dr. M Seiki (University of Tokyo) were bred onto a pure C57/B6 background [27]. Kidneys were isolated at different time points, fixed in 4% formaldehyde for 1 hour and embedded in paraffin. Paraffin tissue sections were stained with either hematoxylin and eosin or periodic acid-Schiff (PAS).
Glomerular count
Glomeruli were counted in the null and wild type mice as described previously [28]. Briefly, individual kidneys were isolated from 2 week old mice and minced into 2-mm cubes. Fragments were incubated in 5 ml of 6M HCl at 37°C for 90 min. Tissue was further homogenized by repeat and vigorous pipetting. 25 ml of H2O was added and after overnight incubation at 4°C, glomeruli in 1 ml of this solution were counted in a 35-mm counting dish; each sample was counted 5 times. Total glomerular number per kidney was extrapolated mathematically from the mean of these five counts.
Organ culture
Embryonic kidneys were isolated from E12.5 mice and cultured on top of transwell filters as previously described [29]. For the TIMP studies, TIMP-1 or TIMP-2 were used at a concentration of 15μg/ml. Seventy two hours later the kidneys were fixed in 4% paraformaldehyde and stained with fluorescein-conjugated E-cadherin antibodies (BD Transduction Laboratories), as described. Quantification of branching structures in 10 kidneys was performed as previously described [29].
Zymography assays
Gelatin zymograms of kidneys were performed as previously described [30]. Briefly, equal amounts of protein (30 μg/lane) were loaded on a 10% SDS PAGE containing 1 mg/ml gelatin and run under non-reducing conditions. The gels were incubated in 50 mM Tris–HCl, pH7.5, 0.1 MNaCl and 2.5% Triton-X100 for 2 h at room temperature, and then incubated in 50 mM Tris–HCl, 1 mM CaCl2 and 0.02% NaN3 for 18 h at 37°C. The gels were stained with Coomasie Blue to visualize MMP activity.
Immunoblotting
30 μg of total proteins were electrophoresed by SDS-PAGE and subsequently transferred to nitrocellulose membranes. Membranes were incubated with different primary antibodies followed by the appropriate HRP-conjugated secondary antibodies. Immunoreactive bands were identified using enhanced chemiluminescence according to the manufacturer's instructions. The following antibodies were used: Collagen IV (Biodesign International, 1:500), Laminin-ß1 chain (Mab 5A2 a gift from Dale Abrahamson, 1:100) Laminin-α3 chain (a gift from Vito Quaranta, 1:1000), Laminin-α5 chain (Chemicon International, AB8948, 1:800), Nidogen (a gift from Peter Yurchenco as described by [31], 1:500), and Perlecan (mAb a gift from Peter Yurchenco as described by [32], 1:250). FAK antibody bought (Santa Cruz Biotechnology, sc558, 1:1000).
Immunohistochemistry
5 μm kidney sections were cut from paraffin blocks or frozen sections after which immunohistochemistry was performed with the antibodies listed under the immunoblotting section.
Generation of MT1-MMP expressing cell line
Inner medullary collecting duct (IMCD) cells were isolated and cultured from 10 day old MT1-MMP null mice as described [33]. The cells were then transfected with either wild type MT1-MMP or a previously described E240A MT1-MMP mutant that has no proteolytic activity [34]. Levels of MT1-MMP expression in the reconstituted cells were verified by flow cytometry utilizing an anti-rabbit MT1-MMP antibody (AB8345 from Chemicon).
Small interference RNA assay
MT1-MMP expression in murine UB cells was downregulated utilizing 2 separate small interfering RNA duplexes corresponding to the target sequences 5’CAUCUGUGACGGGAACUUtt3’ and 5/AGUACUACCGGUUCAAUGAtt3’ (siRNAs s69919 and s69920 from Ambion). Oligonucleotides were transfected into UB cells using the Lipofectamine 2000 system (Invitrogen).
Cell migration
Cell migration was assayed as previously described [35]. Briefly, transwells with 8 μm pores were coated with different ECM components and 1×106 cells were added to the upper well in serum-free medium. The cells that migrated through the filter after 4 hours were counted.
Cell proliferation
For proliferation on specific ECM matrices, 5×103 cells were plated in 96-well plates, coated with the matrix of interest, in complete medium. After 12 hours they were incubated with DMEM (2% FBS) for 48 hours and then pulsed with 1 μCi/well [3H] thymidine for 24 hours (PerkinElmer Life Sciences). The cells were solubilized and radioactivity was measured using a scintillation counter.
For 3-Dimensional analysis, UB or IMCD cells were embedded within a 3-D collagen matrix containing Collagen I and Matrigel as previously described [35] and maintained in DMEM (10% FBS). After 12 hours, the cells were incubated in DMEM (2% FBS) for 48 hours and then pulsed with 1 μCi/well [3H] thymidine (PerkinElmer Life Sciences). Forty eight hours later, the gels were removed from the plate and dialyzed in PBS for 24 hours to remove unincorporated [3H] thymidine. The gels were subsequently solubilized in 100μl of 20% SDS. Radioactivity was measured using a scintillation counter.
Tubulogenesis Assays
Tubulogenesis of UB-derived cells was performed in 3D ECM gels as previously described [35]. The gels were composed of 0.1 mg/ml Collagen I in DMEM containing 20 mM HEPES (pH 7.2). For the Matrigel/Collagen I gels, a 1:1 mixture of the collagen solution described above was mixed with growth factor-reduced Matrigel, giving a final concentration of 0.5 mg/ml of Collagen I and 0.5 mg/ml of Matrigel. One hundred microliters of medium supplemented with 10% FBS were added to the gels after they had solidified
Statistics
Student's t-test was used for comparisons between two groups, and analysis of variance using Sigma Stat software was used for statistical differences between multiple groups. p<0.05 was considered statistically significant.
Results
Kidneys from MT1-MMP null mice are small and have a severe proliferation and mild branching morphogenesis defect
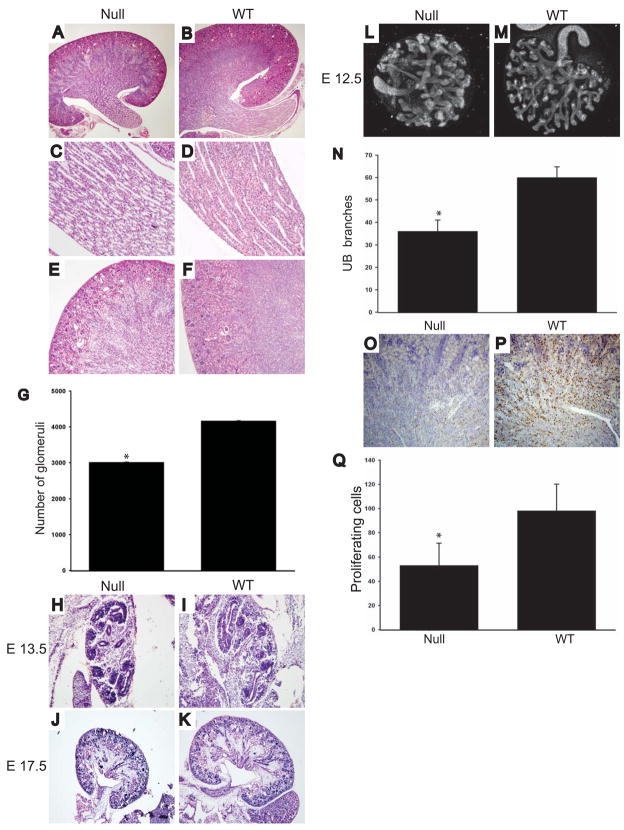
We previously showed that MT1-MMP null mice bred on a mixed background die at 10 weeks of age and their kidneys are small, dysmorphic and dysplastic [26]. To define the kidney developmental abnormalities further, we bred MT1-MMP-null mice onto a pure C57/B6 background. These mice die between 10 and 15 days of age at which time they are significantly smaller than their wild type controls and have musculoskeletal defects as previously described [18, 23]. At the time of death the kidneys were small, but the size was proportional to the decreased size of the mice (Figs.1A and B). On light microscopy the parenchyma of MT1-MMP null kidneys was less dense than that of their wild type controls, suggesting a UB branching morphogenesis defect as well as decreased nephron formation. The branching defect was clearly evident in the papilla of the kidney (Figs. 1C and D), where the tubules were loosely packed, dilated and dysmorphic. The cortex of the MT1-MMP null kidneys was small, the cortico-medullary junction poorly delineated and fewer glomeruli were evident (Figs. 1E and F). The decreased number of glomeruli was confirmed by glomerular counts (Fig. 1G). As MT1-MMP is expressed in the UB at E11.5 and MM at E12.5 [25] and this expression is seen in the kidneys at all time points till adulthood, we assessed the morphology of the kidneys from E12.5 till birth. Kidneys from MT1-MMP null mice were smaller than those of wild type mice at all time points and this was associated with a moderate UB branching and MM induction defect (examples at E13.5 and E17.5 in Figs. 1H-K). The UB branching defect in the MT1-MMP-null mice was confirmed in in vitro organ cultures of E12.5 kidneys (Figs. 1L-N), where a significant decrease in UB branches in the mutant mice was observed. Kidneys from MT1-MMP-null mice proliferated approximately half as much as kidneys from wild type mice when measured by Ki67 staining (Figs. 1O-Q) and there was no difference in apoptosis at any time point analyzed (data not shown).
Figure 1. Kidneys from MT1-MMP-null mice are dysmorphic and dysgenic.
(A-F) Microscopy of hematoxylin and eosin stained kidney slides taken from P10 mice show decreased size of the MT1-MMP null (Null) relative to wild type (WT) mice (100x) (AB). Loosely packed, dilated and dysmorphic tubules were present in the papilla (400x) (C-D) and the cortico-medullary junction is poorly delineated and fewer glomeruli were evident in the MT1-MMP null kidneys (400X) (E-F). (G) The number of glomeruli in null and WT animals were determined as stated in the methods and expressed as the average+/− the standard deviation. A significant difference in the number of glomeruli between 5 mice from each genotype was present (p<0.05). (H-K) Hematoxilin and esoin stained slides of E13.5 (100X) (H-I) and E17.5 (100X) (J-K) shows that the MT1-MMP kidneys were smaller with a moderate UB branching defect. (L-N) E12.5 MT1-MMP and wild type kidneys were isolated, grown on transwells and stained for E-cadherin as described in the methods (L-M). The number of branches in 10 kidneys of MT1-MMP null and WT mice were counted and expressed as the average +/− the standard deviation. There was a significant difference in branch number between genotypes (p<0.05). (O-P) Cell proliferation in newborn kidneys from MT1-MMP null and WT mice was defined by Ki67 staining. The number of cells in 10 high powered fields in 4 mice per genotype was counted. The average and standard deviation is presented. * denotes a significant difference in proliferating cell number between genotypes (p<0.05) (P).
These results demonstrate that the developmental phenotype in kidneys from MT1-MMP-null mice begins early and is characterized by a severe proliferative and a moderate branching defect.
The branching morphogenesis kidney phenotype of the MT1-MMP-null mice is independent of MMP-2 and MMP-9 activity
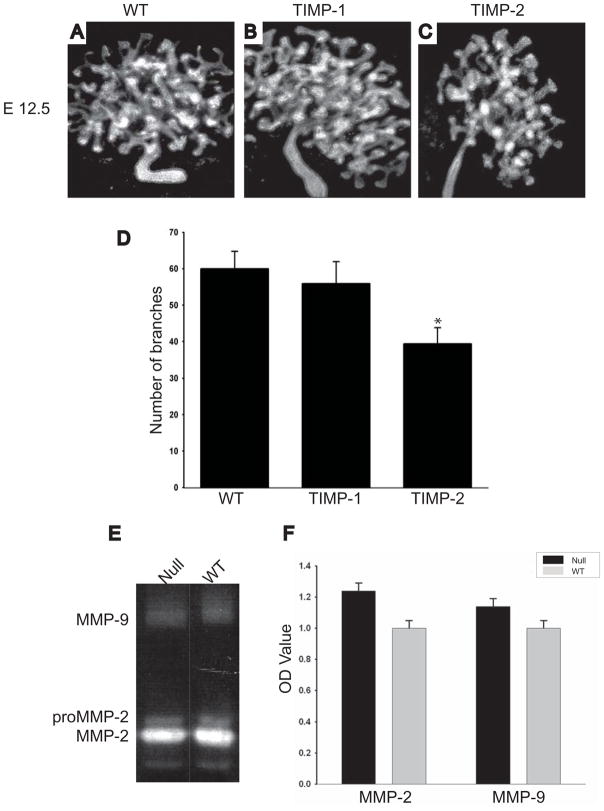
MT1-MMP exerts its proteolytic effects on ECM by both its intrinsic enzymatic functions as well as by activating MMP-2 [36]. To define which of these mechanisms caused the developmental abnormalities in the MT1-MMP-null mice we cultured E 12.5 kidneys from wild type animals with either TIMP-1, which specifically inhibits MMP-2 and MMP-9 activity, or TIMP-2 which inhibits MT1-MMP, MMP-2 and MMP-9 activity [37]. There were no differences in UB branching morphogenesis of kidneys grown in the presence or absence of TIMP-1 (Figs. 2A-B, D); however kidney branching morphogenesis was decreased by approximately 33% (p< 0.01) by TIMP-2 (Fig 2C-D). To confirm that MMP-2 and/or MMP-9 did not play a role in the MT1-MMP-induced effects on renal development we performed zymography to define the activity of these gelatinases on kidneys from MT1-MMP-null and wild type mice. There was no difference in the activity of MMP-2 and MMP-9 between these genotypes, confirming that the observed renal phenotype is independent of MMP-2 and MMP-9 activity (Figs. 2E-F).
Figure 2. The branching morphogenesis kidney phenotype of the MT1-MMP-null mice is independent of MMP-2 and MMP-9 activity.
(A-D) E12.5 kidneys of wild type mice in the presence of no inhibitor (A), TIMP-1 (B) or TIMP-2 (C) were grown on transwells as described in the methods and then stained with antibodies directed against E-cadherin. The number of branches in 10 kidneys in each group was counted and expressed as the average +/− the standard deviation (D). * denotes a significant decrease in branch number in kidneys grown in the presence of TIMP-2 relative to control (p<0.05). (E-F) An example of gelatin zymography performed on total kidney tissue lysates from 1 week-old MT1-MMP null and wild type mice show equal amounts of activated MMP-2 and MMP-9. The average and standard deviation of the O.D. of zymography performed on 5 mice in each group is demonstrated. No statistical differences were found between the two groups (F).
Together these results confirm that gelatinases are not required for UB branching morphogenesis of the kidney and that MT1-MMP does not regulate gelatinase activity in the kidney.
MT1-MMP proteolytic activity is required for renal tubular cells to proliferate and undergo tubulogenesis in vitro
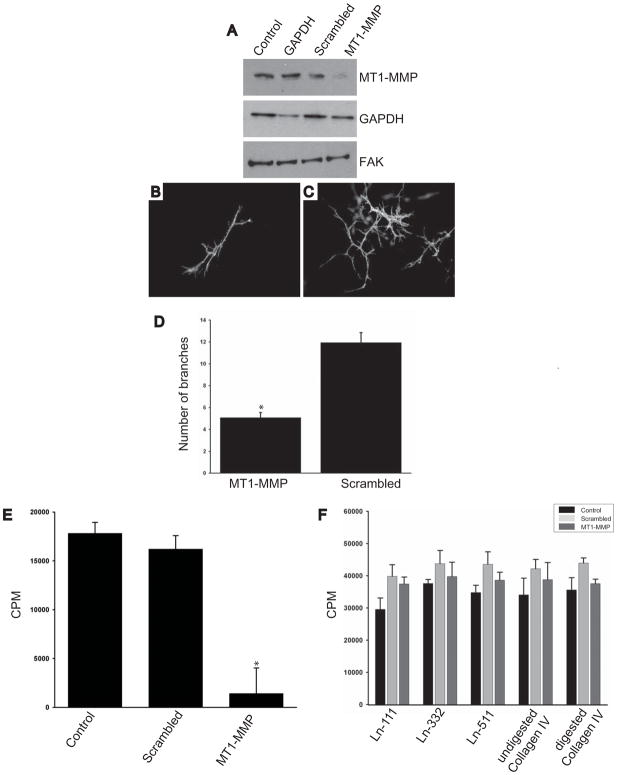
In addition to the moderate branching morphogenesis defect, a severe proliferative deficiency was evident in the kidneys of MT1-MMP-null mice. To investigate the mechanisms of both these defects we made use of a well described UB cell line derived from E12.5 mouse kidneys that is able to undergo tubulogenesis in three dimensional collagen/matrigel gels [29]. These cells endogenously express MT1-MMP (Fig. 3A), which we were able to downregulate utilizing siRNA. When MT1-MMP siRNA treated UB cells were placed in 3-dimensional gels they exhibited a moderately severe (50%) branching morphogenesis defect when compared to control cells (Figs. 3B-D). Furthermore, the tubules were smaller suggesting these cells had a proliferative defect. We therefore determined the ability of these cells to proliferate in 3-D collagen I (data not shown) or collagen I/matrigel gels by thymidine incorporation assays. As predicted, the MT1-MMP siRNA treated UB cells had a severe proliferative defect (Fig. 3E). To define whether there was a specific MT1-MMP-dependent substrate that regulated cell proliferation, we performed thymidine incorporation assays on MT1-MMP siRNA treated UB cells grown on various BM components expressed in the kidney. Interestingly, under these conditions siRNA treated UB cells proliferated as well as untreated cells irrespective of the substrate (undigested and trypsin digested collagen IV, laminins 111, 332 or 511) (Fig. 3F).
Figure 3. MT1-MMP is required for UB cells to proliferate and undergo tubulogenesis in vitro.
(A-C) (A) UB cells were transfected with either an empty vector (control) or double-strand small interfering (si) RNA oligonucleotides against GAPDH, a scrambled peptide or MT1-MMP. Western blot analysis was performed 72 hours later utilizing primary antibodies against MT1-MMP, GAPDH or focal adhesion kinase (FAK) which was used as a loading control. A representative of four separate experiments is shown. (B-C) UB cells either treated with siRNA directed against MT1-MMP or a scrambled siRNA were placed in 3D-collagen I/matrigel gels and allowed to undergo branching morphogenesis as described in the methods. The number of branches in the different UB cell populations was counted in at least 3 wells in 5 different experiments and expressed as the average number of branches/tubular structure +/ the standard deviation (D). * denotes that significantly fewer branches were observed in the UB cells treated with siRNA directed against MT1-MMP (p<0.01). (E) UB cells treated with siRNA directed against MT1-MMP and scrambled siRNA were placed in 3D-collagen I/matrigel gels and proliferation was determined utilizing 3H thymidine as described in the methods. The average and standard deviation of 3 wells in 4 experiments is shown. * denotes a significant decrease in proliferation in UB cells treated with siRNA (p<0.05) (F). When control UB cells and UB cells treated with siRNA directed against MT1-MMP or scrambled siRNA were placed in onto laminin-111, laminin-332, laminin-511, trypsin digested collagen IV (α1α1α2) and undigested collagen IV (α1α1α2) shown 3D-collagen I/matrigel gels no differences in proliferation was seen as determined by tritiated thymidine.
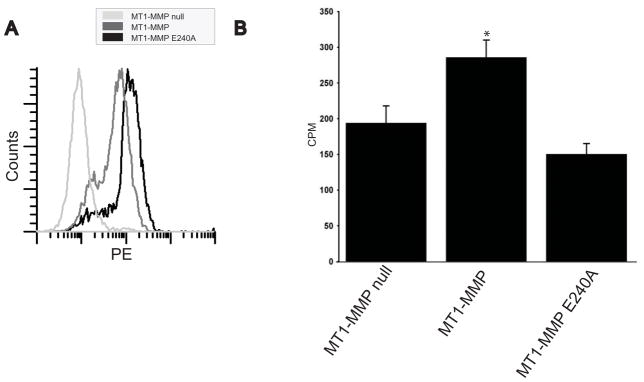
While the results presented above indicate that MT1-MMP expression is required for renal tubule cells to proliferate in a 3-dimensional matrix, they do not define the requirement of the proteolytic activity of MT1-MMP. We therefore isolated inner medullary collecting duct cells from MT1-MMP null mice at postnatal day 15 and reconstituted them with either human full length MT1-MMP or a proteolytically inactive E240A MT1-MMP mutant. IMCD cells obtained from wildtype mice express MT1-MMP (data not shown). Cells were sorted for equal expression of proteolytic enzyme by flow cytometry (Fig. 4A). When these cells were placed in 3-dimensional collagen/matrigel gels (Fig. 4B) or collagen gels (data not shown) the MT1-MMP null IMCD cells reconstituted with wild type MT1-MMP proliferated significantly more than either the MT1-MMP null IMCD cells or E240A reconstituted cells.
Figure 4. MT1-MMP proteolytic activity is required for IMCD cells to proliferate in 3-dimensional collagen I/matrigel gels.
(A) MT1-MMP-null inner medullary collecting duct cells were transfected with wild type or an E240/A mutant of MT1-MMP after which they were flow sorted for equal expression. (B) MT1-MMP-null, MT1-MMP-null reconstituted with MT1-MMP (MT1-MMP) or an E240/A mutant (MT1-MMP E240/A) inner medullary collecting duct cells were placed in 3D-collagen I/matrigel gels and proliferation was determined utilizing 3H-thymidine as described in the methods. The average and standard deviation of 3 wells in 4 experiments is shown. * denotes a significant increase in proliferation in IMCD cells expressing MT1-MMP (p<0.05).
Together these results show that MT1-MMP is required for normal branching morphogenesis of UB cells in vitro and for proliferation of renal tubular cells in 3-dimensional ECM matrices. Furthermore, we demonstrate the requirement of the proteolytic function of MT1-MMP for renal tubular cell proliferation in 3-dimensional matrices.
Kidneys of MT1-MMP null mice have excessive amounts of ECM components
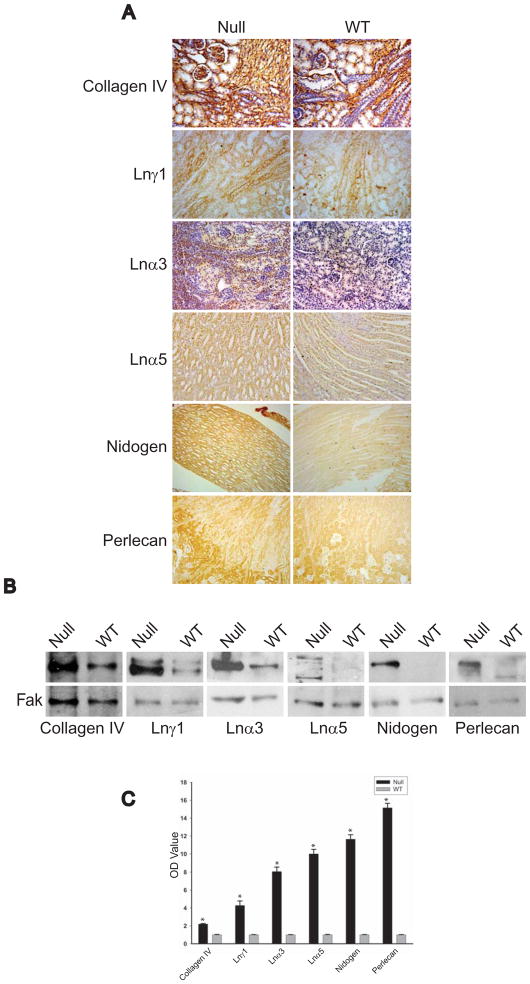
We previously demonstrated that the dysmorphic dysgenic renal phenotype of the MT1-MMP null mice correlated with increased laminin 332 in renal tubules. However in the experiments above we show that MT1-MMP is required for renal tubular cells to proliferate and undergo tubulogenesis in 3-dimensional collagenI/matrigel gels which do not contain laminin 322. This result suggests that MT1-MMP expressed by renal tubule cells exerts its proteolytic effects on multiple ECM components. To examine this possibility further, we performed immunohistochemistry on kidneys of MT1-MMP-null and wild type mice to define whether there were differences in the amount of the predominant ECM components of the kidney BM in vivo, namely collagen IV (α1α2α1), laminins 111, 332, 511/521, entactin/nidogen and sulfated proteoglycans. Relative to the wild type controls, all components of the BM were increased in either the tubules and/or the glomeruli of the MT1-MMP null animals (Fig. 4A). These increases were confirmed on immunoblots of whole kidney lysates of MT1-MMP-null mice (Figs 5B and C). Thus MT1-MMP regulates the amount of all the major components found in the renal BM.
Figure 5. Kidney BMs of MT1-MMP null mice have excessive amounts of ECM components.
(A) Kidney sections from newborn MT1-MMP and wild type mice stained with specific antibodies to collagen IV (α1α1α2), laminin-111, laminin-α3, laminin-α5, nidogen, and perlecan. (B) Total kidney lysates (30 μg) of newborn mice were immunoblotted with antibodies directed against the proteins described above. Immunoblotting with an antibody against FAK was performed as a loading control. (C) Immunoblots of the BM proteins and FAK from newborn MT1-MMP and wild type mice were scanned, normalized and expressed as the relative intensity of MT1-MMP mice compared to wild type mice. The averages and standard deviations of 4 different mice are shown. * denotes a significant increase in the amount of basement membrane proteins in the MT1-MMP-null mice.
MT1-MMP proteolytic cleavage of laminins and collagen IV modulates renal tubular epithelial cell migration
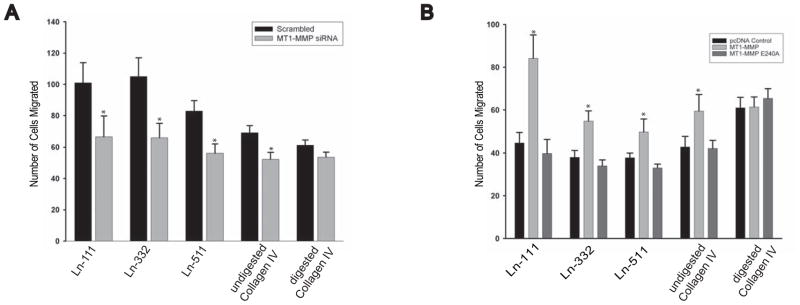
We next defined whether the lack of MT1-MMP-dependent ECM proteolysis affected cellular migration which is known to be required for renal development. Cell migration of UB cells where MT1-MMP was downregulated by siRNA was significantly decreased on Ln-111, Ln-332, Ln-511, and Collagen IV, but not on trypsin cleaved collagen IV (Fig. 6A). Conversely, MT1-MMP null IMCD cells expressing full length but not the E240A MT1-MMP mutant migrated significantly more on all these substrates but not tryptically cleaved collagen IV(Fig. 6B). Thus the proteolytic activity of MT1-MMP increases renal tubular epithelial cell migration on the major ECM components found in renal tubule BMs.
Figure 6. MT1-MMP proteolytic activity stimulates renal cell migration.
(A) UB cells were subjected to either a scrambled siRNA or siRNA directed at MT1-MMP silencing and allowed to migrate on digested and undigested collagen IV (α1α1α2), laminin-111, laminin-332 or laminin 521 (all at 10 μg/ml) for 4 hours. The cells were counted at the end of this time and the number is expressed as cells/high power field. Values are the mean and standard deviation of three experiments performed in triplicate. * denotes statistically significant differences (P<0.05) between the two cell populations. (B) IMCD cells that were either null for MT1-MMP or were reconstituted with MT1-MMPor MT1-MMP E240/A were allowed to migrate on digested and undigested collagen IV (α1α1α2), laminin-111, laminin-332 or laminin 511 (all at 10 μg/ml) for 4 hours. The cells were counted at the end of this time and the number is expressed as cells/high power field. Values are the mean and standard deviation of three experiments performed in triplicate. * denotes statistically significant differences (P<0.05) between MT1-MMP null IMCD cells reconstituted with human MT1-MMP or MT1-MMP E240A.
Discussion
MT1-MMP remodeling of the pericellular ECM environment plays a critical role in bone [18], lung [21, 22] and submandibular gland development [21]. We now demonstrate that MT1-MMP also plays a role in kidney development. Kidneys from MT1-MMP null mice exhibit a severe proliferation and a mild to moderate UB branching defect with decreased nephrogenesis. These morphological defects are associated with increased amounts of ECM components of the BM. MMP-2 and MMP-9 activation was normal in the MT1-MMP-null kidneys suggesting that MT1-MMP does not activate these gelatinases in the kidney. We also demonstrate that MT1-MMP was required for kidney branching morphogenesis in organ culture, renal tubular cell proliferation and branching morphogenesis in 3D matrix gels as well as cell migration on multiple ECM components. Together these results suggest that MT1-MMP regulates normal renal development due to its ability to cleave numerous renal BM components. Although this work is a significant advancement as it combines in vitro and in vivo assays to identify important MT1-MMP targets in BMs of the kidney, it is still difficult to define the precise pathways whereby MT1-MMP results in the observed renal phenotype as MMPs proteolytically cleave so many ECM and non-matrix molecules [38].
MT1-MMP is the only MMP shown to play a significant role in renal development both in vivo and in vitro. We demonstrate a branching defect in the MT1-MMP null mice that occurs early in development. This is consistent with the recent observation that the MT1-MMP is a downstream target of ETS transcription factors Etv4 and Etv5, which are positively regulated by Ret signaling in the UB tips [39]. The Ret-GDNF axis is one of the critical determinants for the initiation and subsequent branching of the UB in renal development [40–43].
The UB branching morphogenesis defect in MT1-MMP null mice, which is recapitulated in an ex vivo organ culture model, is similar to that seen in the submandibular gland but not in the lung where branching is normal [21, 22]. Our data confirm previous studies demonstrating a role for MT1-MMP in UB branching morphogenesis in vitro [5]. However, contrary to these in vitro reports our data demonstrate that MT1-MMP modulates renal branching morphogenesis by its inherent proteolytic activity and does not require MT1-MMP/TIMP-2-dependent MMP-2 activation. These results are similar to the observation that MMP2 activation occurs in fibroblasts isolated from MT1-MMP null animals [44] and verifies that like lung development [22], MMP2 activation is not required for normal renal development.
One of the most striking abnormalities in the MT1-MMP null kidneys is the proliferation defect, which was also seen in our in vitro organ (data not shown) and 3D but not 2D cell culture systems. These results are consistent with in vitro studies showing that pericellular collagenolysis is required for cellular proliferation due to the inability of cells to generate pericellular space for expansion rather than an intrinsic proliferation defect [45]. However it was recently demonstrated that MT1- and MT2-MMP dependent cleavage of NC1 domains of collagen IV is required for the proliferation and branching of the submandibular gland [46]. In this system, it was proposed that NC1 domains signal via ß1 integrins to induce epithelial proliferation by the induction of epithelial HB-EGF- and FGF1. In contrast, other studies have shown that MT1-MMP can cleave growth factors that are important for mediating cell proliferation. For example HB-EGF [47], decorin [48]and semaphorins [49] have been shown to be substrates for MT1-MMP. Thus it is possible that MT1-MMP increases cell proliferation by inducing both ECM proteolysis and processing growth factors like HB-EGF to a more active form. Finally it is possible but unlikely, due to the lack of TUNEL staining seen in the MT1-MMP null kidneys and the data showing that MT1-MMP needs to be active to induce its cellular effects in vitro (Figures 4 and 6), that the effects seen in the MT1-MMP null mice is caused by increased cell apoptosis. MT1-MMP has been demonstrated to induce apoptosis by cleaving the ectodomain of death receptor-6 by signal transduction through its cytoplasmic domain [50].
The kidney is the only organ in MT1-MMP null mice to show BM abnormalities. We previously demonstrated an increased deposition of the laminin γ2 chain [26] and now we show that all the major components of the BM are increased in vivo. This study is the first demonstration of increased nidogen and perlecan deposition in any organ in the absence of MT1-MMP and is consistent with the ability of MT1-MMP to digest these ECM components in vitro [51]. Our observation of increased BM components in the kidney in the MT1-MMP mice contrasts with data on the submandibular gland which shows that BM components were increased only when MT2- but not MT1-MMP was downregulated by siRNA [46]. In the submandibular culture model with MT2-MMP siRNA, the α2 chain of collagen IV and the laminin α5 chain were increased due to both decreased degradation and an increase in production. The increased collagen IV in the MT1-MMP null kidney was less than that of other BM components, suggesting that MT2-MMP might also be the major MT-MMP for collagen IV degradation in the kidney in vivo. The role of MT2-MMP in renal development has not been defined, however based on the data from the submandibular gland where it has been shown that MT2- and MT1-MMP negatively regulate each other's expression levels it is likely that this kind of regulation also exists in the kidneys
We show that migration of renal tubular cells that express MT1-MMP is increased on laminins 111, 322, 511 and collagen IV (α1α1α2). These data confirm multiple other studies demonstrating that MT1-MMP plays a role in mediating cell migration on two-dimensional collagen substrates [52, 53]. In addition this study verifies our previous work showing that MT1-MMP modulates cell migration on laminin 332 [26] and extends these observations to laminins 111 and 511. The mechanism whereby MT1-MMP modulates cell migration is unclear, however our data utilizing the cleaved and uncleaved collagen IV suggests it is by exposing cryptic sites within cleaved ECM.
In conclusion we have shown that MT1-MMP plays an important role in renal development, where it regulates both cell proliferation and branching morphogenesis. These affects are at least in part mediated by the direct ability of MT1-MMP to proteolytically cleave multiple ECM components of the BM. Based on the critical role of MT2-MMP in submandibular gland development in vitro, it is likely that both MT1- and MT2-MMP regulate renal development. The specific roles of the different MT-MMPs will only be defined when floxed mice for these proteases are generated so that compound and cell specific mutants can be analyzed in detail.
Acknowledgments
We would like to thank Jeff Miner for the Ln-α5 antibody and Peter Yurchenco for perlecan and nidogen antibodies. This work was supported by DK065123 (AP and RZ); DK075594 (RZ), DK65123 (RZ), DK083187 (RZ), AHA established investigator award (RZ); a Merit award from the Department of Veterans Affairs (AP and RZ) and the George O’Brien Center Grant (AP and RZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Costantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miner J. Renal basement membrane components. Kidney Int. 1999;56:2016–2024. doi: 10.1046/j.1523-1755.1999.00785.x. [DOI] [PubMed] [Google Scholar]
- 3.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 4.Lelongt BT, Murphy G, Ronco G, PM Matrix metalloproteinases MMP2 and MMP9 are produced in early stages of kidney morphogenesis but only MMP9 is required for renal organogenesis in vitro. J Cell Biol. 1997;136:1363–1373. doi: 10.1083/jcb.136.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanwar OKYS, Yang Q, Wada J, Kashihara N, Tian Y, Wallner EI. Role of membrane-type matrix metalloproteinase 1 (MT-1-MMP), MMP-2, and its inhibitor in nephrogenesis. Am J Physiol. 1999;277:934–947. doi: 10.1152/ajprenal.1999.277.6.F934. [DOI] [PubMed] [Google Scholar]
- 6.Itoh TIT, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem. 1997;272:22389–22392. doi: 10.1074/jbc.272.36.22389. [DOI] [PubMed] [Google Scholar]
- 7.Andrews K, Betsuyaku T, Rogers S, Shipley JM, Senior RM, Miner JH. Gelatinase B (MMP-9) is not essential in the normal kidney and does not influence progression of renal disease in a mouse model of Alport syndrome. Am J Pathol. 2000;157:303–311. doi: 10.1016/S0002-9440(10)64541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeisberg M, Khurana M, Rao VH, Cosgrove D, Rougier JP, Werner MC, Shield CF, Werb Z, Kalluri R. Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flamant M, Placier S, Dubroca C, Esposito B, Lopes I, Chatziantoniou C, Tedgui A, Dussaule JC, Lehoux S. Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension. 2007;50:212–218. doi: 10.1161/HYPERTENSIONAHA.107.089631. [DOI] [PubMed] [Google Scholar]
- 10.Lambert WBV, Munaut C, Galopin C, Jost M, Itoh T, Werb Z, Baker A, Libert C, Krell HW, Foidart JM, Noël A, Rakic JM. MMP-2 and MMP-9 synergize in promoting choroidal neovascularization. FASEB J. 2003;17:2290–2292. doi: 10.1096/fj.03-0113fje. [DOI] [PubMed] [Google Scholar]
- 11.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 12.Seiki M. Membrane-type 1 matrix metalloproteinase: a key enzyme for tumor invasion. Cancer Lett. 2003;194:1–11. doi: 10.1016/s0304-3835(02)00699-7. [DOI] [PubMed] [Google Scholar]
- 13.Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, Seiki M. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153:893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belkin A, Akimov SS, Zaritskaya LS, Ratnikov BI, Deryugina EI, Strongin AY. Matrix-dependent proteolysis of surface transglutaminase by membrane-type metalloproteinase regulates cancer cell adhesion and locomotion. J Biol Chem. 2001;276:18415–18422. doi: 10.1074/jbc.M010135200. [DOI] [PubMed] [Google Scholar]
- 15.Rozanov D, Hahn-Dantona E, Strickland DK, Strongin AY. The low density lipoprotein receptor-related protein LRP is regulated by membrane type-1 matrix metalloproteinase (MT1-MMP) proteolysis in malignant cells. J Biol Chem. 2004;279:4260–4268. doi: 10.1074/jbc.M311569200. [DOI] [PubMed] [Google Scholar]
- 16.Deryugina E, Ratnikov BI, Postnova TI, Rozanov DV, Strongin AY. Processing of integrin alpha(v) subunit by membrane type 1 matrix metalloproteinase stimulates migration of breast carcinoma cells on vitronectin and enhances tyrosine phosphorylation of focal adhesion kinase. J Bio Chem. 2002;277:9749–9756. doi: 10.1074/jbc.M110269200. [DOI] [PubMed] [Google Scholar]
- 17.Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, Sato H. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J Bio Chem. 2003;278:40764–40770. doi: 10.1074/jbc.M306736200. [DOI] [PubMed] [Google Scholar]
- 18.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szabova L, Yamada SS, Wimer H, Chrysovergis K, Ingvarsen S, Behrendt N, Engelholm LH, Holmbeck K. MT1-MMP and type II collagen specify skeletal stem cells and their bone and cartilage progeny. J Bone Miner Res. 2009;24:1905–1916. doi: 10.1359/JBMR.090510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oblander S, Zhou Z, Gálvez BG, Starcher B, Shannon JM, Durbeej M, Arroyo AG, Tryggvason K, Apte SS. Distinctive functions of membrane type 1 matrix-metalloprotease (MT1-MMP or MMP-14) in lung and submandibular gland development are independent of its role in pro-MMP-2 activation. Dev Biol. 2005;277:255–269. doi: 10.1016/j.ydbio.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 22.Atkinson J, Holmbeck K, Yamada S, Birkedal-Hansen H, Parks WC, Senior RM. Membrane-type 1 matrix metalloproteinase is required for normal alveolar development. Developmental Dynamics. 2005;232:1079–1090. doi: 10.1002/dvdy.20267. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalo GMP, Hernández-Riquer MV, Pollán A, Grande-García A, Bartolomé RA, Vasanji A, Ambrogio C, Chiarle R, Teixidó J, Risteli J, Apte SS, del Pozo MA, Arroyo AG. MT1-MMP is required for myeloid cell fusion via regulation of Rac1 signaling. Dev Cell. 2010;18:77–89. doi: 10.1016/j.devcel.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Legallicier TGB, Murphy G, Lelongt B, Ronco P. Expression of the Type IV Collagenase System during Mouse Kidney Development and Tubule Segmentation. J Am Soc Nephrol. 2001;12:2358–2369. doi: 10.1681/ASN.V12112358. [DOI] [PubMed] [Google Scholar]
- 26.Koshikawa N, Schenk S, Moeckel G, Sharabi A, Miyazaki K, Gardner H, Zent R, Quaranta V. Proteolytic processing of laminin-5 by MT1-MMP in tissues and its effects on epithelial cell morphology. FASEB J. 2004;18:364–366. doi: 10.1096/fj.03-0584fje. [DOI] [PubMed] [Google Scholar]
- 27.Irie K, Komori K, Seiki M, Tsuruga E, Sakakura Y, Kaku T, Yajima T. Impaired alveolization in mice deficient in membrane-type matrix metalloproteinase 1 (MT1-MMP) Med Mol Morphol. 2005;38:43–46. doi: 10.1007/s00795-004-0277-9. [DOI] [PubMed] [Google Scholar]
- 28.Boyle S, Shioda T, Perantoni AO, de Caestecker M. Cited1 and Cited2 are differentially expressed in the developing kidney but are not required for nephrogenesis. Dev Dyn. 2007;236:2321–2330. doi: 10.1002/dvdy.21242. [DOI] [PubMed] [Google Scholar]
- 29.Zent R, Bush KT, Pohl ML, Quaranta V, Koshikawa N, Wang Z, Kreidberg JA, Sakurai H, Stuart RO, Nigam SK. Involvement of laminin binding integrins and laminin-5 in branching morphogenesis of the ureteric bud during kidney development. Dev Biol. 2001;238:289–302. doi: 10.1006/dbio.2001.0391. [DOI] [PubMed] [Google Scholar]
- 30.Pozzi A, Moberg PE, Miles LA, Wagner S, Soloway P, Gardner HA. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci U S A. 2000;97:2202–2207. doi: 10.1073/pnas.040378497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Bordoy R, Stanchi F, Moser M, Braun A, Kudlacek O, Wewer UM, Yurchenco PD, Fassler R. PINCH1 regulates cell-matrix and cell-cell adhesions, cell polarity and cell survival during the peri-implantation stage. J Cell Sci. 2005;118:2913–2921. doi: 10.1242/jcs.02422. [DOI] [PubMed] [Google Scholar]
- 32.Yurchenco PD, Cheng YS, Ruben GC. Self-assembly of a high molecular weight basement membrane heparan sulfate proteoglycan into dimers and oligomers. J Biol Chem. 1987;262:17668–17676. [PubMed] [Google Scholar]
- 33.Husted RF, Hayashi M, Stokes JB. Characteristics of papillary collecting duct cells in primary culture. Am J Physiol. 1988;255:F1160–1169. doi: 10.1152/ajprenal.1988.255.6.F1160. [DOI] [PubMed] [Google Scholar]
- 34.Atkinson SJ, Crabbe T, Cowell S, Ward RV, Butler MJ, Sato H, Seiki M, Reynolds JJ, Murphy G. Intermolecular autolytic cleavage can contribute to the activation of progelatinase A by cell membranes. J Biol Chem. 1995;270:30479–30485. doi: 10.1074/jbc.270.51.30479. [DOI] [PubMed] [Google Scholar]
- 35.Chen D, Roberts R, Pohl M, Nigam S, Kreidberg J, Wang Z, Heino J, Ivaska J, Coffa S, Harris RC, Pozzi A, Zent R. Differential expression of collagen- and laminin-binding integrins mediates ureteric bud and inner medullary collecting duct cell tubulogenesis. Am J Physiol Renal Physiol. 2004;287:602–611. doi: 10.1152/ajprenal.00015.2004. [DOI] [PubMed] [Google Scholar]
- 36.Sato TTH, Miyamori H. Roles of membrane-type matrix metalloproteinase-1 in tumor invasion and metastasis. Cancer Science. 2005;96:212–217. doi: 10.1111/j.1349-7006.2005.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagase HaMG. Tailoring TIMPs for selective metalloproteinase inhibition. Springer Science; New York: 2008. [Google Scholar]
- 38.Rodriguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim Biophys Acta. 2010;1803:39–54. doi: 10.1016/j.bbamcr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Lu CCBC, Chi X, Kuure S, Kuo R, Bates CM, Arber S, Hassell J, MacNeil L, Hoshi M, Jain S, Asai N, Takahashi M, Schmidt-Ott KM, Barasch J, D'Agati V, Costantini F. Etv4 and Etv5 are required downstream of GDNF and Ret for kidney branching morphogenesis. Nat Genet. 2009;41:1295–1302. doi: 10.1038/ng.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pichel SLJG, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 41.Schuchardt DAVA, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 42.Moore KRMW, Fariñas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 43.Sánchez S-SIMP, Frisén J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 44.Hotary YIKB, Sabeh F, Li XY, Holmbeck K, Birkedal-Hansen H, Allen ED, Hiraoka N, Weiss SJ. Matrix metalloproteinases (MMPs) regulate fibrin-invasive activity via MT1-MMP-dependent and -independent processes. J Exp Med. 2002;195:295–308. doi: 10.1084/jem.20010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hotary K, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 46.Rebustini MCIT, Lassiter KS, Surmak A, Szabova L, Holmbeck K, Pedchenko V, Hudson BG, Hoffman MP. MT2-MMP-dependent release of collagen IV NC1 domains regulates submandibular gland branching morphogenesis. Dev Cell. 2009;17:482–493. doi: 10.1016/j.devcel.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koshikawa N, Mizushima H, Minegishi T, Iwamoto R, Mekada E, Seiki M. Membrane type 1-matrix metalloproteinase cleaves off the NH2-terminal portion of heparin-binding epidermal growth factor and converts it into a heparin-independent growth factor. Cancer Res. 2010;70:6093–6103. doi: 10.1158/0008-5472.CAN-10-0346. [DOI] [PubMed] [Google Scholar]
- 48.Mimura T, Han KY, Onguchi T, Chang JH, Kim TI, Kojima T, Zhou Z, Azar DT. MT1-MMP-mediated cleavage of decorin in corneal angiogenesis. J Vasc Res. 2009;46:541–550. doi: 10.1159/000226222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basile JR, Holmbeck K, Bugge TH, Gutkind JS. MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J Biol Chem. 2007;282:6899–6905. doi: 10.1074/jbc.M609570200. [DOI] [PubMed] [Google Scholar]
- 50.Tam EM, Morrison CJ, Wu YI, Stack MS, Overall CM. Membrane protease proteomics: Isotope-coded affinity tag MS identification of undescribed MT1-matrix metalloproteinase substrates. Proc Natl Acad Sci U S A. 2004;101:6917–6922. doi: 10.1073/pnas.0305862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.d'Ortho M, Will H, Atkinson S, Butler G, Messent A, Gavrilovic J, Smith B, Timpl R, Zardi L, Murphy G. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. 1997;250(3):751–757. doi: 10.1111/j.1432-1033.1997.00751.x. [DOI] [PubMed] [Google Scholar]
- 52.Chen P, Parks WC. Role of matrix metalloproteinases in epithelial migration. J Cell Biochem. 2009;108:1233–1243. doi: 10.1002/jcb.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Genis L, Galvez BG, Gonzalo P, Arroyo AG. MT1-MMP: universal or particular player in angiogenesis? Cancer Metastasis Rev. 2006;25:77–86. doi: 10.1007/s10555-006-7891-z. [DOI] [PubMed] [Google Scholar]