Abstract
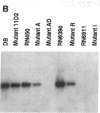
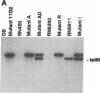
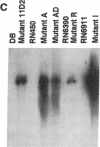
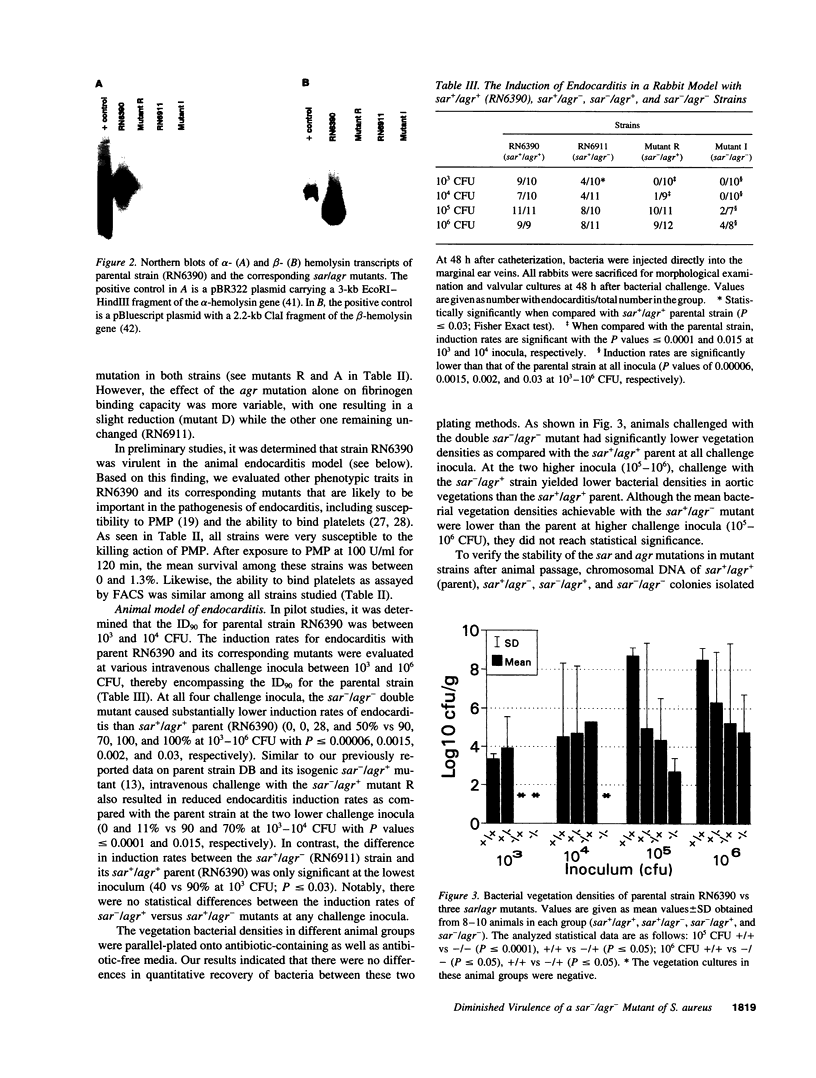
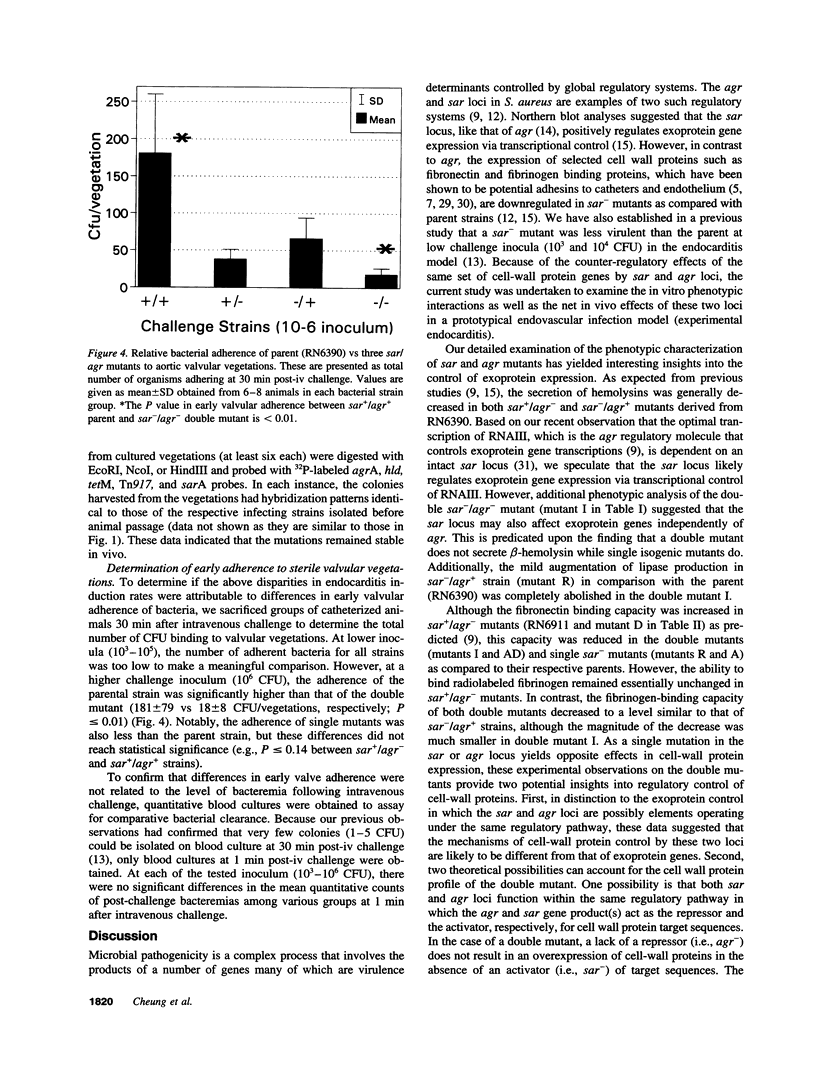
Microbial pathogenicity in Staphylococcus aureus is a complex process involving a number of virulence genes that are regulated by global regulatory systems including sar and agr. To evaluate the roles of these two loci in virulence, we constructed sar-/agr- mutants of strains RN6390 and RN450 and compared their phenotypic profiles to the corresponding single sar- and agr- mutants and parents. The secretion of all hemolysins was absent in the sar-/agr- mutants while residual beta-hemolysin activity remained in single agr- mutants. The fibronectin binding capacity was significantly diminished in both single sar- mutants and double mutants when compared with parents while the reduction in fibrinogen binding capacity in the double mutants was modest. In the rabbit endocarditis model, there was a significant decrease in both infectivity rates and intravegetation bacterial densities with the double mutant as compared to the parent (RN6390) at 10(3)-10(6) CFU inocula despite comparable levels of early bacteremia among various challenge groups. Notably, fewer bacteria in the double mutant group adhered to valvular vegetations at 30 min after challenge (10(6) CFU) than the parent group. These studies suggest that both the sar and agr loci are involved in initial valvular adherence, intravegetation persistence and multiplication of S. aureus in endocarditis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Muhly M., Mannhardt U., Hugo F., Klapettek K., Mueller-Eckhardt C., Roka L. Staphylococcal alpha toxin promotes blood coagulation via attack on human platelets. J Exp Med. 1988 Aug 1;168(2):527–542. doi: 10.1084/jem.168.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilli A., Portnoy A., Youngman P. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917 derivative that allows direct cloning of DNA flanking transposon insertions. J Bacteriol. 1990 Jul;172(7):3738–3744. doi: 10.1128/jb.172.7.3738-3744.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. L., Fischetti V. A. The role of fibrinogen in staphylococcal adherence to catheters in vitro. J Infect Dis. 1990 Jun;161(6):1177–1186. doi: 10.1093/infdis/161.6.1177. [DOI] [PubMed] [Google Scholar]
- Cheung A. L., Koomey J. M., Butler C. A., Projan S. J., Fischetti V. A. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6462–6466. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. L., Krishnan M., Jaffe E. A., Fischetti V. A. Fibrinogen acts as a bridging molecule in the adherence of Staphylococcus aureus to cultured human endothelial cells. J Clin Invest. 1991 Jun;87(6):2236–2245. doi: 10.1172/JCI115259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. L., Projan S. J. Cloning and sequencing of sarA of Staphylococcus aureus, a gene required for the expression of agr. J Bacteriol. 1994 Jul;176(13):4168–4172. doi: 10.1128/jb.176.13.4168-4172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. L., Yeaman M. R., Sullam P. M., Witt M. D., Bayer A. S. Role of the sar locus of Staphylococcus aureus in induction of endocarditis in rabbits. Infect Immun. 1994 May;62(5):1719–1725. doi: 10.1128/iai.62.5.1719-1725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. L., Ying P. Regulation of alpha- and beta-hemolysins by the sar locus of Staphylococcus aureus. J Bacteriol. 1994 Feb;176(3):580–585. doi: 10.1128/jb.176.3.580-585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clawson C. C., White J. G., Herzberg M. C. Platelet interaction with bacteria. VI. contrasting the role of fibrinogen and fibronectin. Am J Hematol. 1980;9(1):43–53. doi: 10.1002/ajh.2830090106. [DOI] [PubMed] [Google Scholar]
- Cohen M. L. Epidemiology of drug resistance: implications for a post-antimicrobial era. Science. 1992 Aug 21;257(5073):1050–1055. doi: 10.1126/science.257.5073.1050. [DOI] [PubMed] [Google Scholar]
- DiRita V. J., Mekalanos J. J. Genetic regulation of bacterial virulence. Annu Rev Genet. 1989;23:455–482. doi: 10.1146/annurev.ge.23.120189.002323. [DOI] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol. 1972 Feb;53(1):44–49. [PMC free article] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Speziale P., Hök M. Isolation and characterization of a fibronectin receptor from Staphylococcus aureus. J Biol Chem. 1987 May 15;262(14):6564–6571. [PubMed] [Google Scholar]
- Herrmann M., Suchard S. J., Boxer L. A., Waldvogel F. A., Lew P. D. Thrombospondin binds to Staphylococcus aureus and promotes staphylococcal adherence to surfaces. Infect Immun. 1991 Jan;59(1):279–288. doi: 10.1128/iai.59.1.279-288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Vaudaux P. E., Pittet D., Auckenthaler R., Lew P. D., Schumacher-Perdreau F., Peters G., Waldvogel F. A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988 Oct;158(4):693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- Herrmann M., Vaudaux P. E., Pittet D., Auckenthaler R., Lew P. D., Schumacher-Perdreau F., Peters G., Waldvogel F. A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988 Oct;158(4):693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- Herzberg M. C., Gong K., MacFarlane G. D., Erickson P. R., Soberay A. H., Krebsbach P. H., Manjula G., Schilling K., Bowen W. H. Phenotypic characterization of Streptococcus sanguis virulence factors associated with bacterial endocarditis. Infect Immun. 1990 Feb;58(2):515–522. doi: 10.1128/iai.58.2.515-522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A. Cell biology of endothelial cells. Hum Pathol. 1987 Mar;18(3):234–239. doi: 10.1016/s0046-8177(87)80005-9. [DOI] [PubMed] [Google Scholar]
- Kornblum J. S., Projan S. J., Moghazeh S. L., Novick R. P. A rapid method to quantitate non-labeled RNA species in bacterial cells. Gene. 1988;63(1):75–85. doi: 10.1016/0378-1119(88)90547-1. [DOI] [PubMed] [Google Scholar]
- Kuypers J. M., Proctor R. A. Reduced adherence to traumatized rat heart valves by a low-fibronectin-binding mutant of Staphylococcus aureus. Infect Immun. 1989 Aug;57(8):2306–2312. doi: 10.1128/iai.57.8.2306-2312.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz M. S., Allen R. D., Bounelis P., Switalski L. M., Hook M. Bacteroides gingivalis and Bacteroides intermedius recognize different sites on human fibrinogen. J Bacteriol. 1990 Feb;172(2):716–726. doi: 10.1128/jb.172.2.716-726.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesin M., Svec P., Lupski J. R., Godson G. N., Kreiswirth B., Kornblum J., Projan S. J. Cloning and nucleotide sequence of a chromosomally encoded tetracycline resistance determinant, tetA(M), from a pathogenic, methicillin-resistant strain of Staphylococcus aureus. Antimicrob Agents Chemother. 1990 Nov;34(11):2273–2276. doi: 10.1128/aac.34.11.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. The crisis in antibiotic resistance. Science. 1992 Aug 21;257(5073):1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- Nixon B. T., Ronson C. W., Ausubel F. M. Two-component regulatory systems responsive to environmental stimuli share strongly conserved domains with the nitrogen assimilation regulatory genes ntrB and ntrC. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7850–7854. doi: 10.1073/pnas.83.20.7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- O'Reilly M., de Azavedo J. C., Kennedy S., Foster T. J. Inactivation of the alpha-haemolysin gene of Staphylococcus aureus 8325-4 by site-directed mutagenesis and studies on the expression of its haemolysins. Microb Pathog. 1986 Apr;1(2):125–138. doi: 10.1016/0882-4010(86)90015-x. [DOI] [PubMed] [Google Scholar]
- Projan S. J., Kornblum J., Kreiswirth B., Moghazeh S. L., Eisner W., Novick R. P. Nucleotide sequence: the beta-hemolysin gene of Staphylococcus aureus. Nucleic Acids Res. 1989 Apr 25;17(8):3305–3305. doi: 10.1093/nar/17.8.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recsei P., Kreiswirth B., O'Reilly M., Schlievert P., Gruss A., Novick R. P. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol Gen Genet. 1986 Jan;202(1):58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- Scheld W. M., Strunk R. W., Balian G., Calderone R. A. Microbial adhesion to fibronectin in vitro correlates with production of endocarditis in rabbits. Proc Soc Exp Biol Med. 1985 Dec;180(3):474–482. doi: 10.3181/00379727-180-42205. [DOI] [PubMed] [Google Scholar]
- Scheld W. M., Valone J. A., Sande M. A. Bacterial adherence in the pathogenesis of endocarditis. Interaction of bacterial dextran, platelets, and fibrin. J Clin Invest. 1978 May;61(5):1394–1404. doi: 10.1172/JCI109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeltzer M. S., Hart M. E., Iandolo J. J. Phenotypic characterization of xpr, a global regulator of extracellular virulence factors in Staphylococcus aureus. Infect Immun. 1993 Mar;61(3):919–925. doi: 10.1128/iai.61.3.919-925.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullam P. M., Payan D. G., Dazin P. F., Valone F. H. Binding of viridans group streptococci to human platelets: a quantitative analysis. Infect Immun. 1990 Nov;58(11):3802–3806. doi: 10.1128/iai.58.11.3802-3806.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttorp N., Hessz T., Seeger W., Wilke A., Koob R., Lutz F., Drenckhahn D. Bacterial exotoxins and endothelial permeability for water and albumin in vitro. Am J Physiol. 1988 Sep;255(3 Pt 1):C368–C376. doi: 10.1152/ajpcell.1988.255.3.C368. [DOI] [PubMed] [Google Scholar]
- Vandenesch F., Kornblum J., Novick R. P. A temporal signal, independent of agr, is required for hla but not spa transcription in Staphylococcus aureus. J Bacteriol. 1991 Oct;173(20):6313–6320. doi: 10.1128/jb.173.20.6313-6320.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M. R., Norman D. C., Bayer A. S. Staphylococcus aureus susceptibility to thrombin-induced platelet microbicidal protein is independent of platelet adherence and aggregation in vitro. Infect Immun. 1992 Jun;60(6):2368–2374. doi: 10.1128/iai.60.6.2368-2374.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M. R., Puentes S. M., Norman D. C., Bayer A. S. Partial characterization and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infect Immun. 1992 Mar;60(3):1202–1209. doi: 10.1128/iai.60.3.1202-1209.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M. R., Sullam P. M., Dazin P. F., Norman D. C., Bayer A. S. Characterization of Staphylococcus aureus-platelet binding by quantitative flow cytometric analysis. J Infect Dis. 1992 Jul;166(1):65–73. doi: 10.1093/infdis/166.1.65. [DOI] [PubMed] [Google Scholar]