Abstract
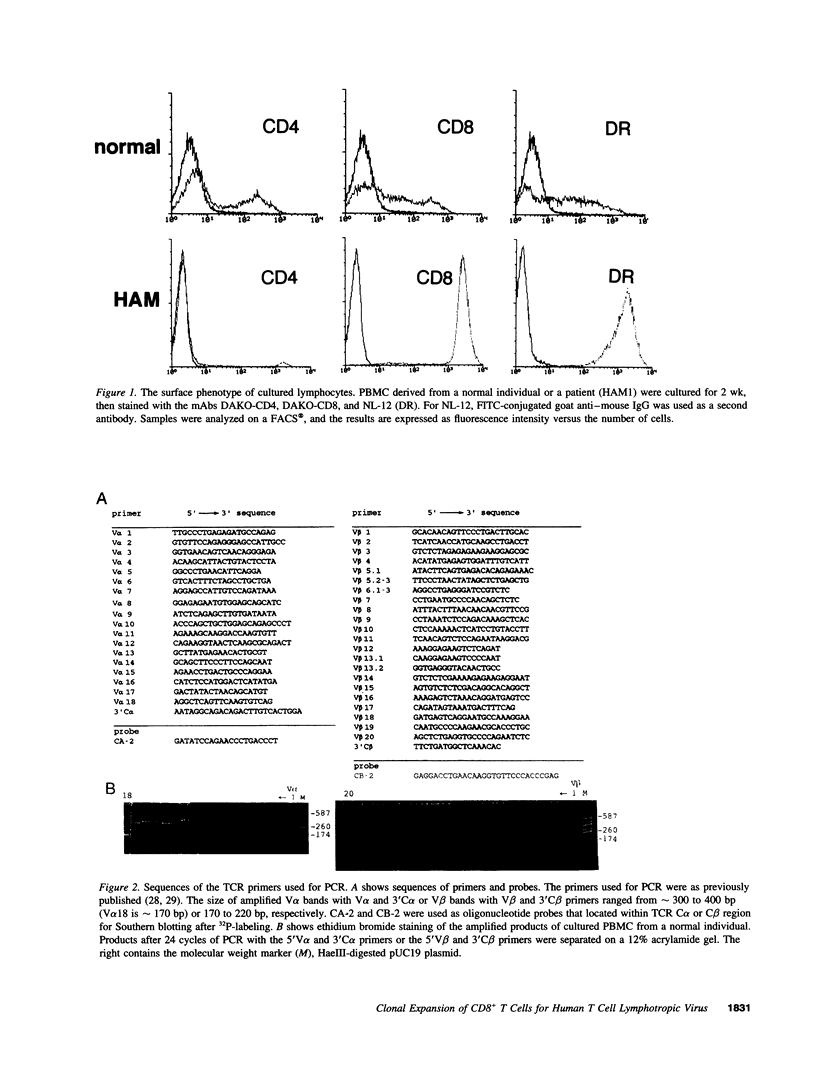
Short-term culture of peripheral blood mononuclear cells (PBMC) derived from patients with human T cell lymphotropic virus type I-associated myelopathy (HAM)/tropical spastic paraparesis resulted in dominance by DR+ activated CD8+ T cells. Variations in the T cell receptor (TCR) V alpha and V beta chains in these cells were analyzed, and in all 10 patients examined, 2-3 V gene families were dominant in both TCR V alpha and V beta. In five patients we examined, cultured lymphocytes contained cytotoxic lymphocytes for p40tax (patients HAM2, 3, 7, and 8) or env protein (patient HAM4) of human T lymphotropic virus type I. In patients HAM2 and HAM8, cultured lymphocytes contained a large proportion of V beta 8+ CD8+ and/or V beta 12+ CD8+ cells. The sequence of V beta 8+ and V beta 12+ cDNA revealed that they were oligoclonal with identical or similar sequences in each patient. Elimination experiments with monoclonal antibodies for TCR V beta 8 and V beta 12 showed that they were CD8+ cytotoxic T lymphocytes (CTL) for p40tax. In addition, flow cytometry and sequencing analysis of uncultured PBMC revealed that in HAM2, V beta 8+ CTL and their precursors account for 7% and V beta 12+ CTL and their precursors account for 18% of total CD8+ cells. This indicates the presence of two markedly expanded clones in vivo. No common dominant TCR V alpha or V beta were observed among 10 HAM patients analyzed.
Full text
PDF

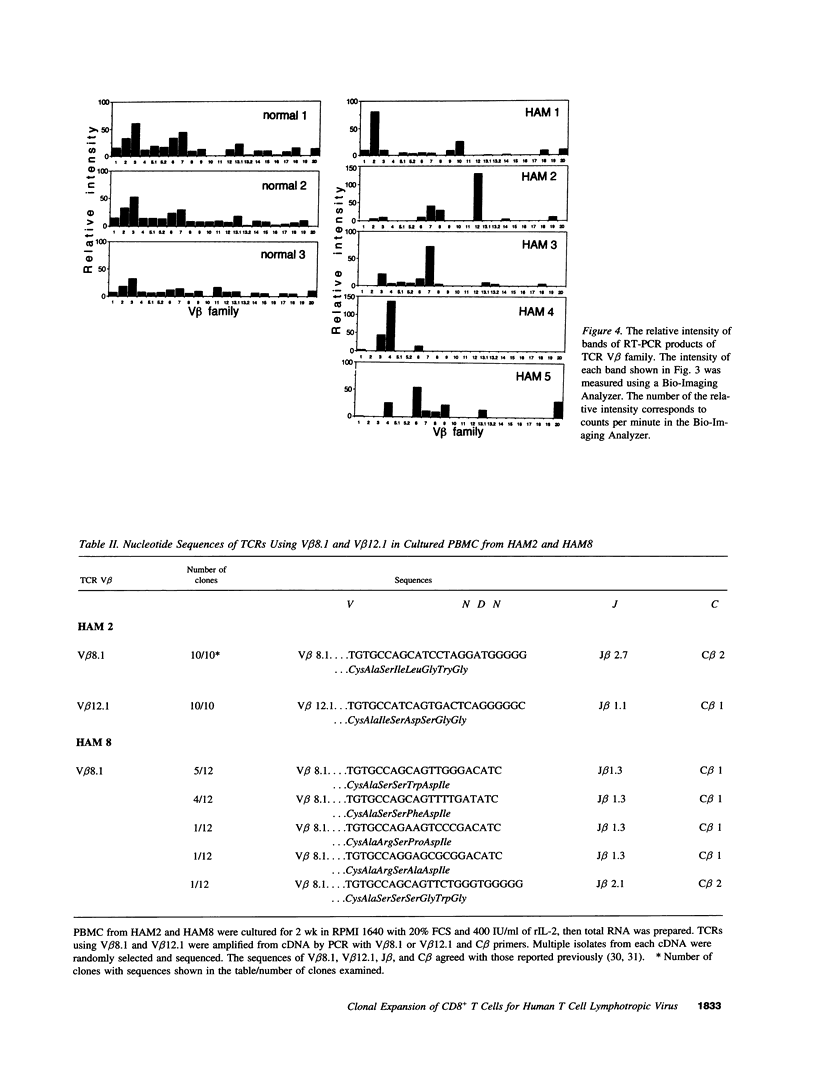
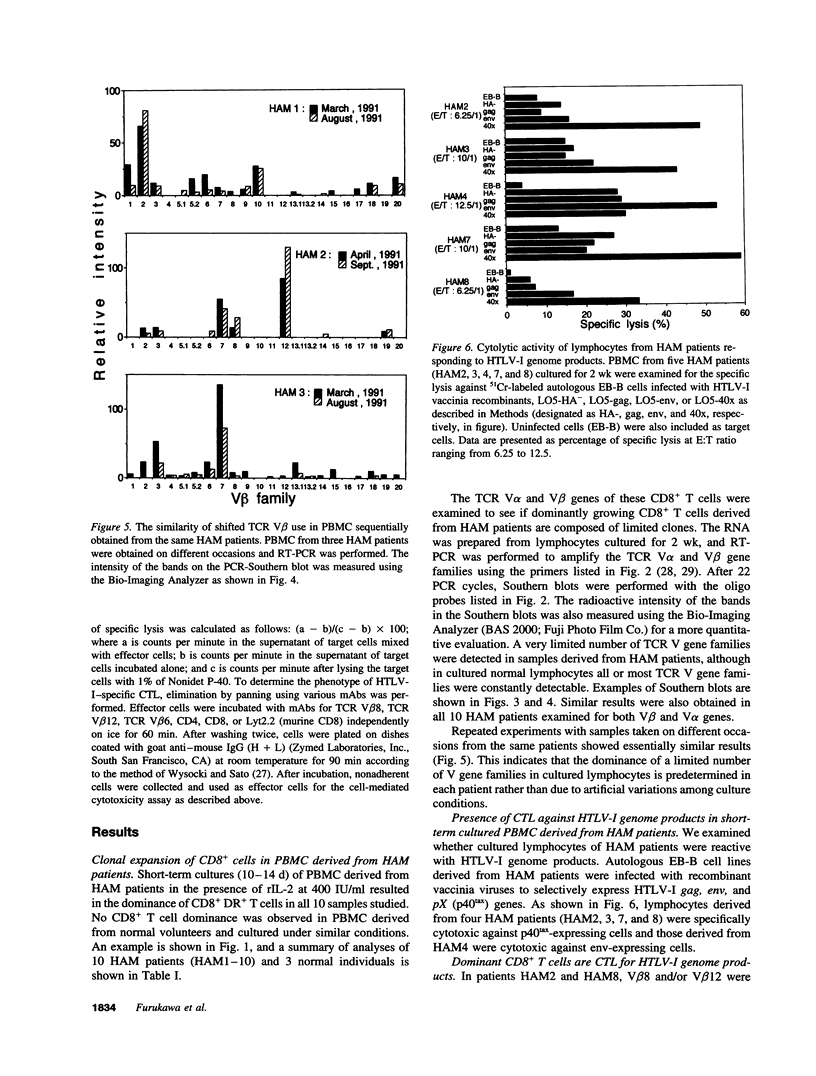
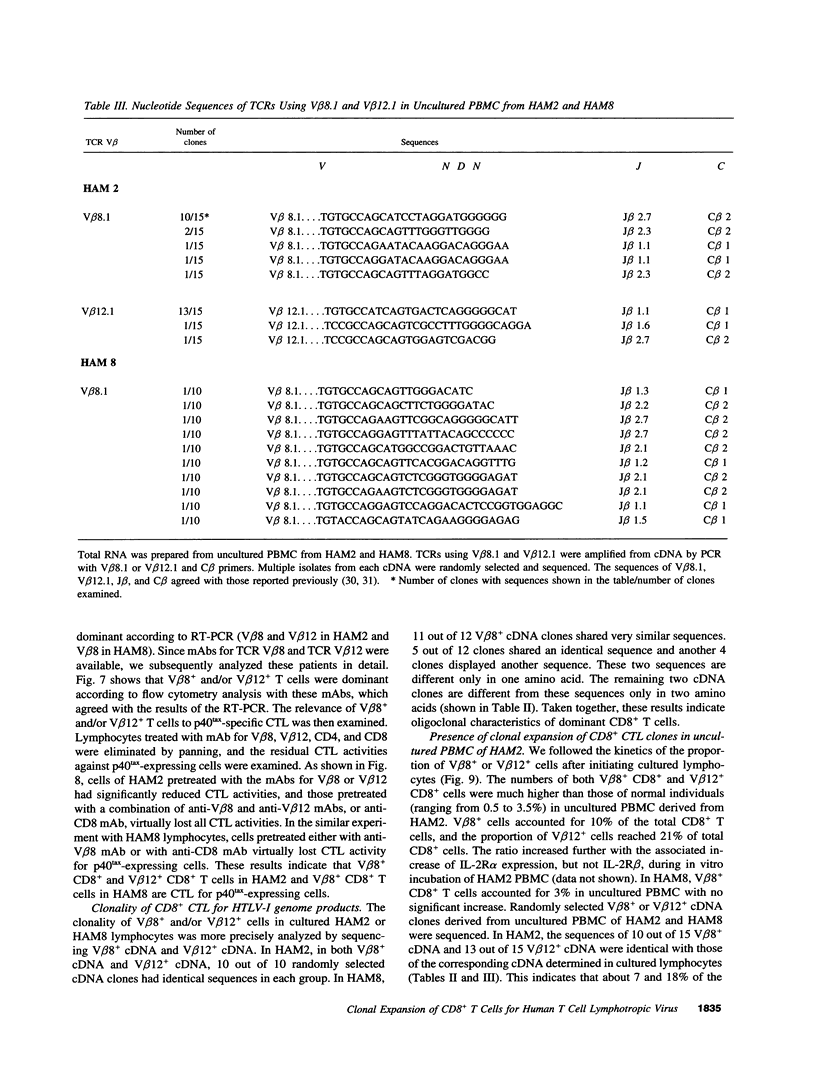

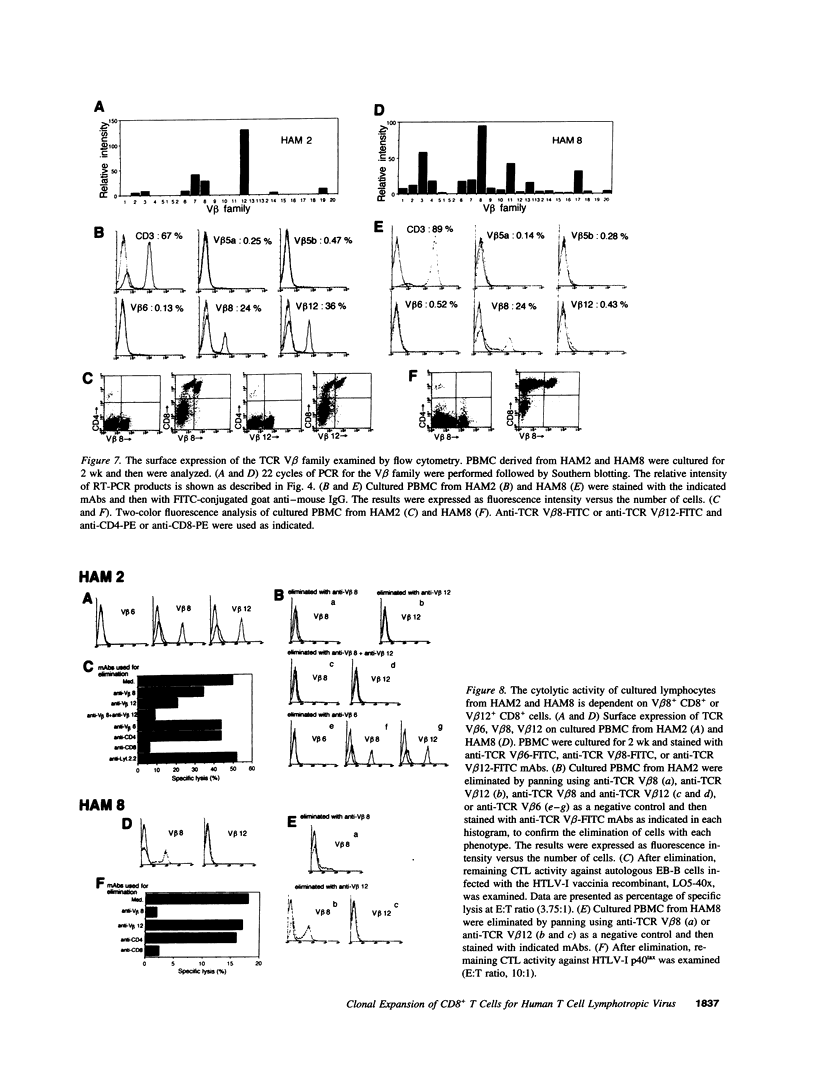

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alp N. J., Sissons J. G., Borysiewicz L. K. Automation of limiting dilution cytotoxicity assays. J Immunol Methods. 1990 May 25;129(2):269–276. doi: 10.1016/0022-1759(90)90447-4. [DOI] [PubMed] [Google Scholar]
- Borysiewicz L. K., Graham S., Hickling J. K., Mason P. D., Sissons J. G. Human cytomegalovirus-specific cytotoxic T cells: their precursor frequency and stage specificity. Eur J Immunol. 1988 Feb;18(2):269–275. doi: 10.1002/eji.1830180214. [DOI] [PubMed] [Google Scholar]
- Borysiewicz L. K., Hickling J. K., Graham S., Sinclair J., Cranage M. P., Smith G. L., Sissons J. G. Human cytomegalovirus-specific cytotoxic T cells. Relative frequency of stage-specific CTL recognizing the 72-kD immediate early protein and glycoprotein B expressed by recombinant vaccinia viruses. J Exp Med. 1988 Sep 1;168(3):919–931. doi: 10.1084/jem.168.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichard V., Van Pel A., Wölfel T., Wölfel C., De Plaen E., Lethé B., Coulie P., Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993 Aug 1;178(2):489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael A., Jin X., Sissons P., Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J Exp Med. 1993 Feb 1;177(2):249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Concannon P., Pickering L. A., Kung P., Hood L. Diversity and structure of human T-cell receptor beta-chain variable region genes. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6598–6602. doi: 10.1073/pnas.83.17.6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovaara I., Koenig S., Brewah A. Y., Woods R. M., Lehky T., Jacobson S. High human T cell lymphotropic virus type 1 (HTLV-1)-specific precursor cytotoxic T lymphocyte frequencies in patients with HTLV-1-associated neurological disease. J Exp Med. 1993 Jun 1;177(6):1567–1573. doi: 10.1084/jem.177.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M., Sassone-Corsi P., Verma I. M. c-fos promoter trans-activation by the tax1 protein of human T-cell leukemia virus type I. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8526–8530. doi: 10.1073/pnas.85.22.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Akagi T., Nagata Y., Yamada Y., Shimotohno K., Cheung N. K., Shiku H., Furukawa K. GD2 ganglioside on human T-lymphotropic virus type I-infected T cells: possible activation of beta-1,4-N-acetylgalactosaminyltransferase gene by p40tax. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1972–1976. doi: 10.1073/pnas.90.5.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Furukawa K., Shiku H. Alternatively spliced mRNA of the pX region of human T lymphotropic virus type I proviral genome. FEBS Lett. 1991 Dec 16;295(1-3):141–145. doi: 10.1016/0014-5793(91)81404-v. [DOI] [PubMed] [Google Scholar]
- Gessain A., Barin F., Vernant J. C., Gout O., Maurs L., Calender A., de Thé G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985 Aug 24;2(8452):407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijichi S., Eiraku N., Osame M., Izumo S., Kubota R., Maruyama I., Matsumoto M., Niimura T., Sonoda S. Activated T lymphocytes in cerebrospinal fluid of patients with HTLV-I-associated myelopathy (HAM/TSP). J Neuroimmunol. 1989 Dec;25(2-3):251–254. doi: 10.1016/0165-5728(89)90143-4. [DOI] [PubMed] [Google Scholar]
- Jacobson S., Gupta A., Mattson D., Mingioli E., McFarlin D. E. Immunological studies in tropical spastic paraparesis. Ann Neurol. 1990 Feb;27(2):149–156. doi: 10.1002/ana.410270209. [DOI] [PubMed] [Google Scholar]
- Jacobson S., Reuben J. S., Streilein R. D., Palker T. J. Induction of CD4+, human T lymphotropic virus type-1-specific cytotoxic T lymphocytes from patients with HAM/TSP. Recognition of an immunogenic region of the gp46 envelope glycoprotein of human T lymphotropic virus type-1. J Immunol. 1991 Feb 15;146(4):1155–1162. [PubMed] [Google Scholar]
- Jacobson S., Shida H., McFarlin D. E., Fauci A. S., Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990 Nov 15;348(6298):245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- Kannagi M., Harada S., Maruyama I., Inoko H., Igarashi H., Kuwashima G., Sato S., Morita M., Kidokoro M., Sugimoto M. Predominant recognition of human T cell leukemia virus type I (HTLV-I) pX gene products by human CD8+ cytotoxic T cells directed against HTLV-I-infected cells. Int Immunol. 1991 Aug;3(8):761–767. doi: 10.1093/intimm/3.8.761. [DOI] [PubMed] [Google Scholar]
- Lee B., Tanaka Y., Tozawa H. Monoclonal antibody defining tax protein of human T-cell leukemia virus type-I. Tohoku J Exp Med. 1989 Jan;157(1):1–11. doi: 10.1620/tjem.157.1. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Casanova J. L., Maryanski J. L., Cerottini J. C. Oligoclonal expansion of major histocompatibility complex class I-restricted cytolytic T lymphocytes during a primary immune response in vivo: direct monitoring by flow cytometry and polymerase chain reaction. J Exp Med. 1993 May 1;177(5):1487–1492. doi: 10.1084/jem.177.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake S., Seiki M., Malefijt R. D., Heike T., Fujisawa J., Takebe Y., Nishida J., Shlomai J., Yokota T., Yoshida M. Activation of T cell-derived lymphokine genes in T cells and fibroblasts: effects of human T cell leukemia virus type I p40x protein and bovine papilloma virus encoded E2 protein. Nucleic Acids Res. 1988 Jul 25;16(14A):6547–6566. doi: 10.1093/nar/16.14.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Kinoshita K., Ban N., Yamada Y., Shiku H. Activated T-lymphocytes with polyclonal gammopathy in patients with human T-lymphotropic virus type I--associated myelopathy. Ann Neurol. 1988 Aug;24(2):280–282. doi: 10.1002/ana.410240220. [DOI] [PubMed] [Google Scholar]
- Nakayama E., Shiku H., Takahashi T., Oettgen H. F., Old L. J. Definition of a unique cell surface antigen of mouse leukemia RL male 1 by cell-mediated cytotoxicity. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3486–3490. doi: 10.1073/pnas.76.7.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S. H., Kidokoro M., Shida H., Hatanaka M. Processing of gag precursor polyprotein of human T-cell leukemia virus type I by virus-encoded protease. J Virol. 1988 Oct;62(10):3718–3728. doi: 10.1128/jvi.62.10.3718-3728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T., Oksenberg J. R., Rao N. A., Steinman L. Predominant expression of T cell receptor V alpha 7 in tumor-infiltrating lymphocytes of uveal melanoma. Science. 1990 Aug 10;249(4969):672–674. doi: 10.1126/science.2382141. [DOI] [PubMed] [Google Scholar]
- Noguchi Y., Tateno M., Kondo N., Yoshiki T., Shida H., Nakayama E., Shiku H. Rat cytotoxic T lymphocytes against human T-lymphotropic virus type 1-infected cells recognize gag gene and env gene encoded antigens. J Immunol. 1989 Dec 1;143(11):3737–3742. [PubMed] [Google Scholar]
- Osame M., Matsumoto M., Usuku K., Izumo S., Ijichi N., Amitani H., Tara M., Igata A. Chronic progressive myelopathy associated with elevated antibodies to human T-lymphotropic virus type I and adult T-cell leukemialike cells. Ann Neurol. 1987 Feb;21(2):117–122. doi: 10.1002/ana.410210203. [DOI] [PubMed] [Google Scholar]
- Osame M., Usuku K., Izumo S., Ijichi N., Amitani H., Igata A., Matsumoto M., Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986 May 3;1(8488):1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- Parker C. E., Daenke S., Nightingale S., Bangham C. R. Activated, HTLV-1-specific cytotoxic T-lymphocytes are found in healthy seropositives as well as in patients with tropical spastic paraparesis. Virology. 1992 Jun;188(2):628–636. doi: 10.1016/0042-6822(92)90517-s. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román G. C., Osame M. Identity of HTLV-I-associated tropical spastic paraparesis and HTLV-I-associated myelopathy. Lancet. 1988 Mar 19;1(8586):651–651. doi: 10.1016/s0140-6736(88)91452-3. [DOI] [PubMed] [Google Scholar]
- Shida H., Tochikura T., Sato T., Konno T., Hirayoshi K., Seki M., Ito Y., Hatanaka M., Hinuma Y., Sugimoto M. Effect of the recombinant vaccinia viruses that express HTLV-I envelope gene on HTLV-I infection. EMBO J. 1987 Nov;6(11):3379–3384. doi: 10.1002/j.1460-2075.1987.tb02660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinzato O., Kamihira S., Ikeda S., Kondo H., Kanda T., Nagata Y., Nakayama E., Shiku H. Relationship between the anti-HTLV-1 antibody level, the number of abnormal lymphocytes and the viral-genome dose in HTLV-1-infected individuals. Int J Cancer. 1993 May 8;54(2):208–212. doi: 10.1002/ijc.2910540208. [DOI] [PubMed] [Google Scholar]
- Siomi H., Shida H., Nam S. H., Nosaka T., Maki M., Hatanaka M. Sequence requirements for nucleolar localization of human T cell leukemia virus type I pX protein, which regulates viral RNA processing. Cell. 1988 Oct 21;55(2):197–209. doi: 10.1016/0092-8674(88)90043-8. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Yasumoto M., Nyunoya H., Ogura T., Kikuchi M., Shimotohno K., Shiraki H., Kuroda N., Shida H., Tozawa H. Generation and characterization of monoclonal antibodies against multiple epitopes on the C-terminal half of envelope gp46 of human T-cell leukemia virus type-I (HTLV-I). Int J Cancer. 1990 Oct 15;46(4):675–681. doi: 10.1002/ijc.2910460421. [DOI] [PubMed] [Google Scholar]
- Toyonaga B., Yoshikai Y., Vadasz V., Chin B., Mak T. W. Organization and sequences of the diversity, joining, and constant region genes of the human T-cell receptor beta chain. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8624–8628. doi: 10.1073/pnas.82.24.8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Osame M., Kawai H., Toita M., Kuwasaki N., Nishida Y., Hiraki Y., Takahashi K., Nomura K., Sonoda S. Increased replication of HTLV-I in HTLV-I-associated myelopathy. Ann Neurol. 1989 Sep;26(3):331–335. doi: 10.1002/ana.410260304. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Seiki M. Recent advances in the molecular biology of HTLV-1: trans-activation of viral and cellular genes. Annu Rev Immunol. 1987;5:541–559. doi: 10.1146/annurev.iy.05.040187.002545. [DOI] [PubMed] [Google Scholar]
- van der Bruggen P., Traversari C., Chomez P., Lurquin C., De Plaen E., Van den Eynde B., Knuth A., Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991 Dec 13;254(5038):1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]