Abstract
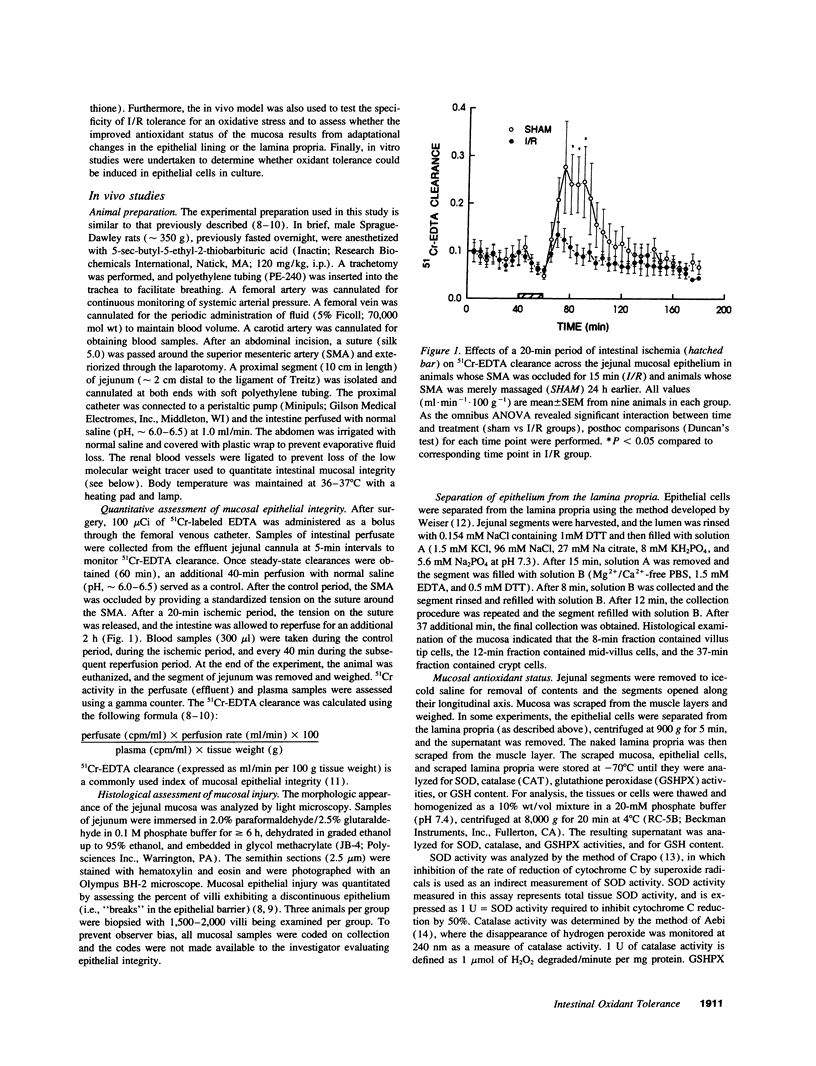
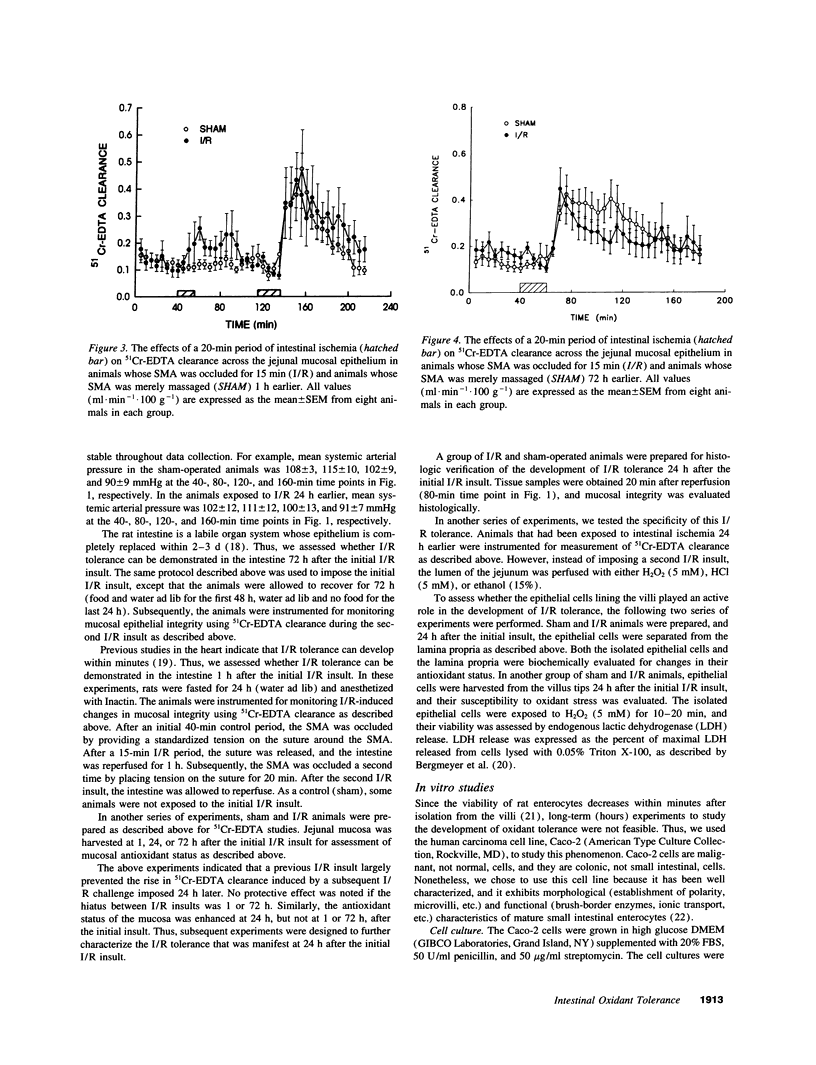
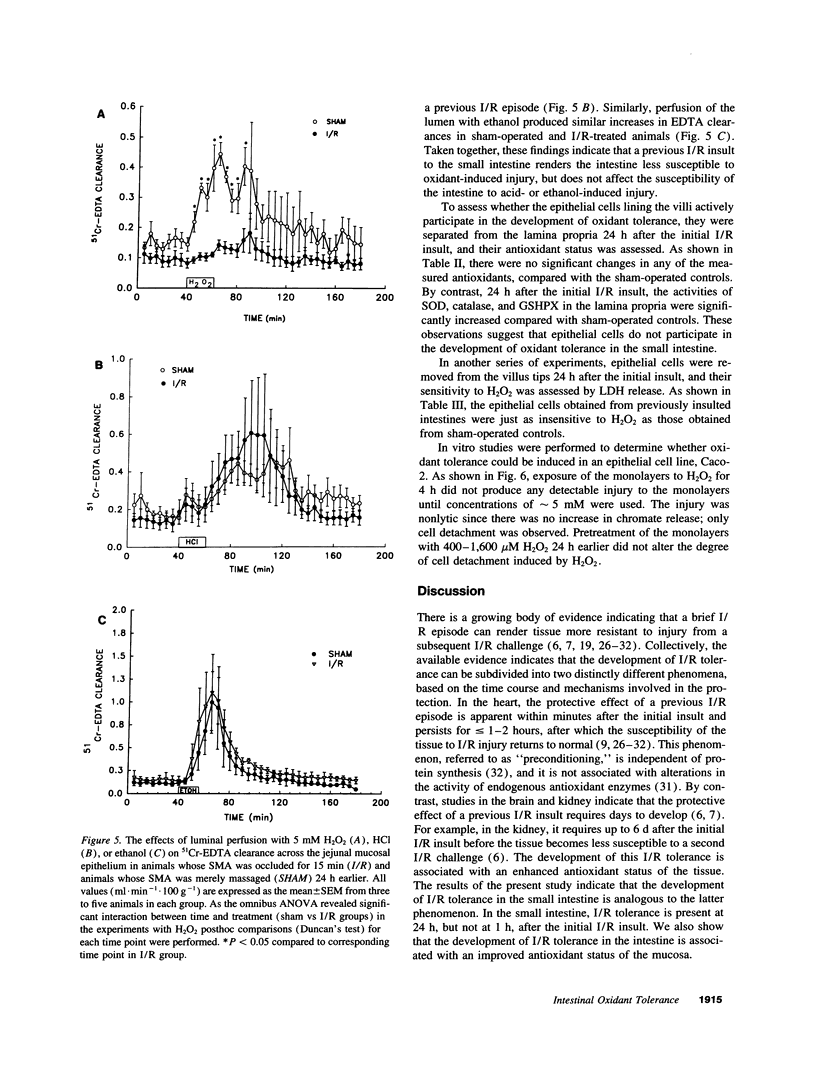
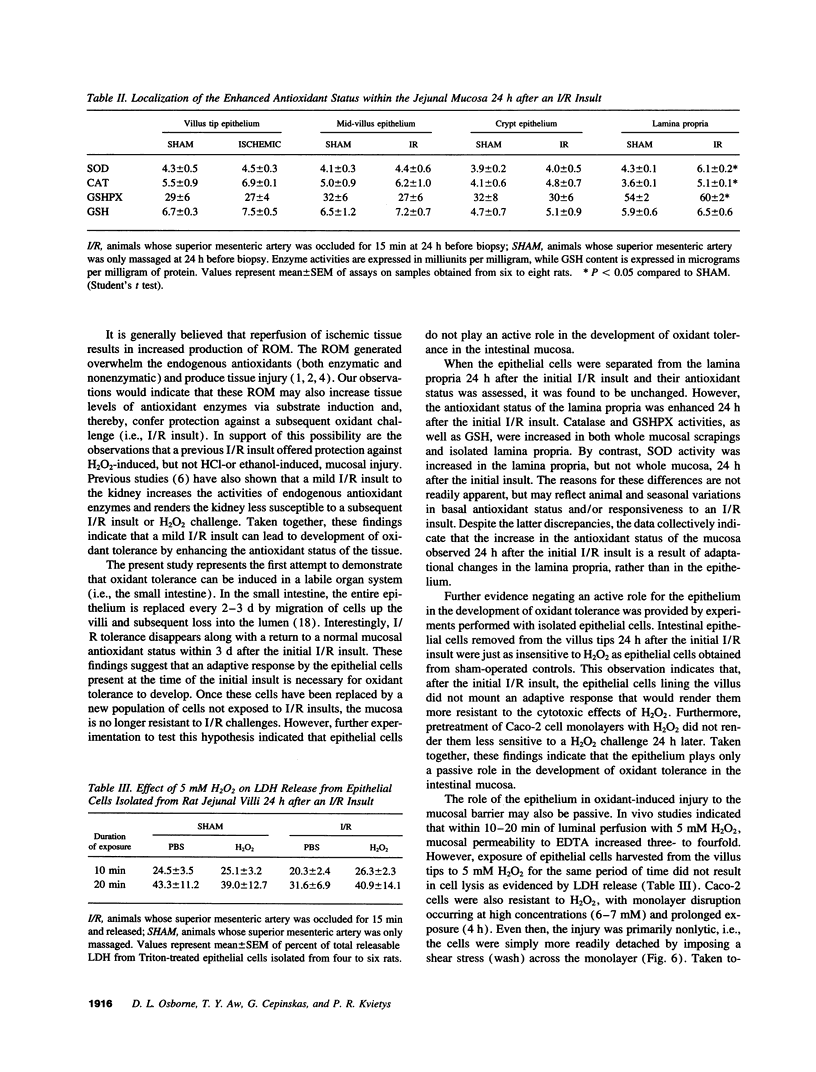
In stable organ systems, such as the heart and kidneys, an oxidant stress induces an increase in endogenous antioxidant systems resulting in an increased resistance of the tissue to a subsequent oxidant challenge. The development of this oxidant tolerance requires 1.5-6 d. The aim of the present study was to determine whether oxidant tolerance can be induced in the small intestinal mucosa, a labile system whose epithelium turns over every 2-3 d. Ischemia/reperfusion-induced epithelial barrier dysfunction of the small intestinal mucosa was monitored in Sprague-Dawley rats whose intestines had been exposed to an ischemic insult 1, 24, or 72 h previously. At 24 h, but not 1 or 72 h after the initial ischemic insult, the mucosa was more resistant to ischemia/reperfusion-induced barrier dysfunction. The antioxidant status of the mucosa was enhanced at 24 h, but not at 1 or 72 h after the initial ischemic insult. This adaptation appears to be specific for oxidants, since an initial ischemic insult imposed 24 h earlier also protected against H2O2-induced, but not acid- or ethanol-induced, barrier dysfunction. Further studies indicated that the increase in antioxidant status of the mucosa observed 24 h after the initial ischemic insult was a result of adaptational changes in the lamina propria, rather than the epithelium. In vitro studies with isolated epithelial cells also indicated that epithelial cells do not develop oxidant tolerance. We conclude that the development of oxidant tolerance in the small intestinal mucosa does not involve an active participation of the epithelial lining.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Bensard D. D., Brown J. M., Anderson B. O., Banerjee A., Shanley P. F., Grosso M. A., Whitman G. J., Harken A. H. Induction of endogenous tissue antioxidant enzyme activity attenuates myocardial reperfusion injury. J Surg Res. 1990 Aug;49(2):126–131. doi: 10.1016/0022-4804(90)90250-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cepinskas G., Specian R. D., Kvietys P. R. Adaptive cytoprotection in the small intestine: role of mucus. Am J Physiol. 1993 May;264(5 Pt 1):G921–G927. doi: 10.1152/ajpgi.1993.264.5.G921. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., McCord J. M., Fridovich I. Preparation and assay of superoxide dismutases. Methods Enzymol. 1978;53:382–393. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]
- Crissinger K. D., Kvietys P. R., Granger D. N. Pathophysiology of gastrointestinal mucosal permeability. J Intern Med Suppl. 1990;732:145–154. doi: 10.1111/j.1365-2796.1990.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Harker L. A., Striker G. E., Wright D. G. Neutrophil-mediated endothelial injury in vitro mechanisms of cell detachment. J Clin Invest. 1981 Dec;68(6):1394–1403. doi: 10.1172/JCI110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K., Matsumoto M., Kuwabara K., Tagaya M., Ohtsuki T., Hata R., Ueda H., Handa N., Kimura K., Kamada T. 'Ischemic tolerance' phenomenon detected in various brain regions. Brain Res. 1991 Oct 11;561(2):203–211. doi: 10.1016/0006-8993(91)91596-s. [DOI] [PubMed] [Google Scholar]
- Kumagai J., Jain R., Johnson L. R. Characteristics of spermidine uptake by isolated rat enterocytes. Am J Physiol. 1989 May;256(5 Pt 1):G905–G910. doi: 10.1152/ajpgi.1989.256.5.G905. [DOI] [PubMed] [Google Scholar]
- Kvietys P. R., Specian R. D., Grisham M. B., Tso P. Jejunal mucosal injury and restitution: role of hydrolytic products of food digestion. Am J Physiol. 1991 Sep;261(3 Pt 1):G384–G391. doi: 10.1152/ajpgi.1991.261.3.G384. [DOI] [PubMed] [Google Scholar]
- Li G. C., Vasquez J. A., Gallagher K. P., Lucchesi B. R. Myocardial protection with preconditioning. Circulation. 1990 Aug;82(2):609–619. doi: 10.1161/01.cir.82.2.609. [DOI] [PubMed] [Google Scholar]
- Lu D., Maulik N., Moraru I. I., Kreutzer D. L., Das D. K. Molecular adaptation of vascular endothelial cells to oxidative stress. Am J Physiol. 1993 Mar;264(3 Pt 1):C715–C722. doi: 10.1152/ajpcell.1993.264.3.C715. [DOI] [PubMed] [Google Scholar]
- MESSIER B., LEBLOND C. P. Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. Am J Anat. 1960 May;106:247–285. doi: 10.1002/aja.1001060305. [DOI] [PubMed] [Google Scholar]
- Moldow C. F., Jacob H. S. Endothelial culture, neutrophil or enzymic generation of free radicals: in vitro methods for the study of endothelial injury. Methods Enzymol. 1984;105:378–385. doi: 10.1016/s0076-6879(84)05051-5. [DOI] [PubMed] [Google Scholar]
- Mullane K. Myocardial preconditioning. Part of the adenosine revival. Circulation. 1992 Feb;85(2):845–847. doi: 10.1161/01.cir.85.2.845. [DOI] [PubMed] [Google Scholar]
- Murry C. E., Jennings R. B., Reimer K. A. New insights into potential mechanisms of ischemic preconditioning. Circulation. 1991 Jul;84(1):442–445. doi: 10.1161/01.cir.84.1.442. [DOI] [PubMed] [Google Scholar]
- Nylander O., Kvietys P., Granger D. N. Effects of hydrochloric acid on duodenal and jejunal mucosal permeability in the rat. Am J Physiol. 1989 Oct;257(4 Pt 1):G653–G660. doi: 10.1152/ajpgi.1989.257.4.G653. [DOI] [PubMed] [Google Scholar]
- OWENS C. W., BELCHER R. V. A COLORIMETRIC MICRO-METHOD FOR THE DETERMINATION OF GLUTATHIONE. Biochem J. 1965 Mar;94:705–711. doi: 10.1042/bj0940705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly P. M., Schiller H. J., Bulkley G. B. Pharmacologic approach to tissue injury mediated by free radicals and other reactive oxygen metabolites. Am J Surg. 1991 Apr;161(4):488–503. doi: 10.1016/0002-9610(91)91120-8. [DOI] [PubMed] [Google Scholar]
- Reimer K. A., Murry C. E., Jennings R. B. Cardiac adaptation to ischemia. Ischemic preconditioning increases myocardial tolerance to subsequent ischemic episodes. Circulation. 1990 Dec;82(6):2266–2268. doi: 10.1161/01.cir.82.6.2266. [DOI] [PubMed] [Google Scholar]
- Sies H. Oxidative stress: from basic research to clinical application. Am J Med. 1991 Sep 30;91(3C):31S–38S. doi: 10.1016/0002-9343(91)90281-2. [DOI] [PubMed] [Google Scholar]
- Tappel A. L. Glutathione peroxidase and hydroperoxides. Methods Enzymol. 1978;52:506–513. doi: 10.1016/s0076-6879(78)52055-7. [DOI] [PubMed] [Google Scholar]
- Thornton J., Striplin S., Liu G. S., Swafford A., Stanley A. W., Van Winkle D. M., Downey J. M. Inhibition of protein synthesis does not block myocardial protection afforded by preconditioning. Am J Physiol. 1990 Dec;259(6 Pt 2):H1822–H1825. doi: 10.1152/ajpheart.1990.259.6.H1822. [DOI] [PubMed] [Google Scholar]
- Turrens J. F., Thornton J., Barnard M. L., Snyder S., Liu G., Downey J. M. Protection from reperfusion injury by preconditioning hearts does not involve increased antioxidant defenses. Am J Physiol. 1992 Feb;262(2 Pt 2):H585–H589. doi: 10.1152/ajpheart.1992.262.2.H585. [DOI] [PubMed] [Google Scholar]
- Walker D. M., Yellon D. M. Ischaemic preconditioning: from mechanisms to exploitation. Cardiovasc Res. 1992 Aug;26(8):734–739. doi: 10.1093/cvr/26.8.734. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Yoshida N., Takemura T., Granger D. N., Anderson D. C., Wolf R. E., McIntire L. V., Kvietys P. R. Molecular determinants of aspirin-induced neutrophil adherence to endothelial cells. Gastroenterology. 1993 Sep;105(3):715–724. doi: 10.1016/0016-5085(93)90888-j. [DOI] [PubMed] [Google Scholar]
- Yoshioka T., Bills T., Moore-Jarrett T., Greene H. L., Burr I. M., Ichikawa I. Role of intrinsic antioxidant enzymes in renal oxidant injury. Kidney Int. 1990 Aug;38(2):282–288. doi: 10.1038/ki.1990.197. [DOI] [PubMed] [Google Scholar]




