Abstract
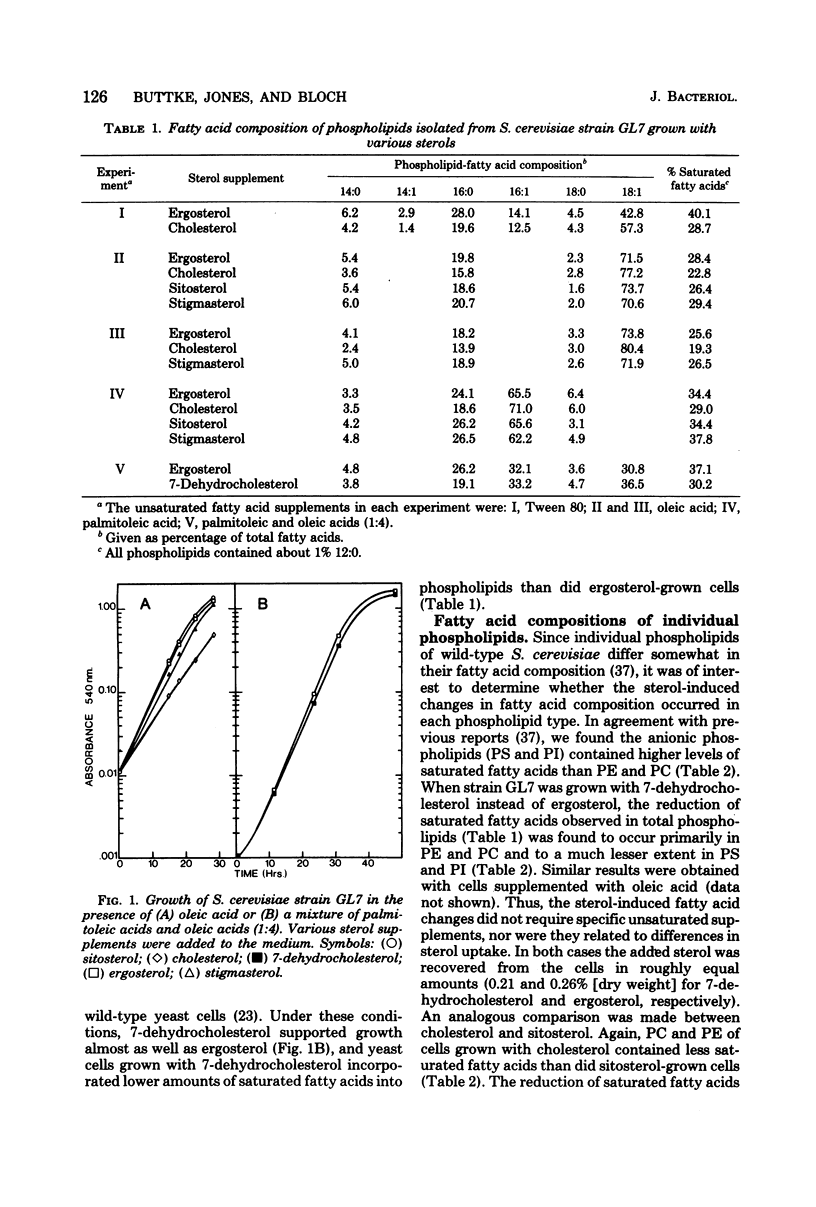
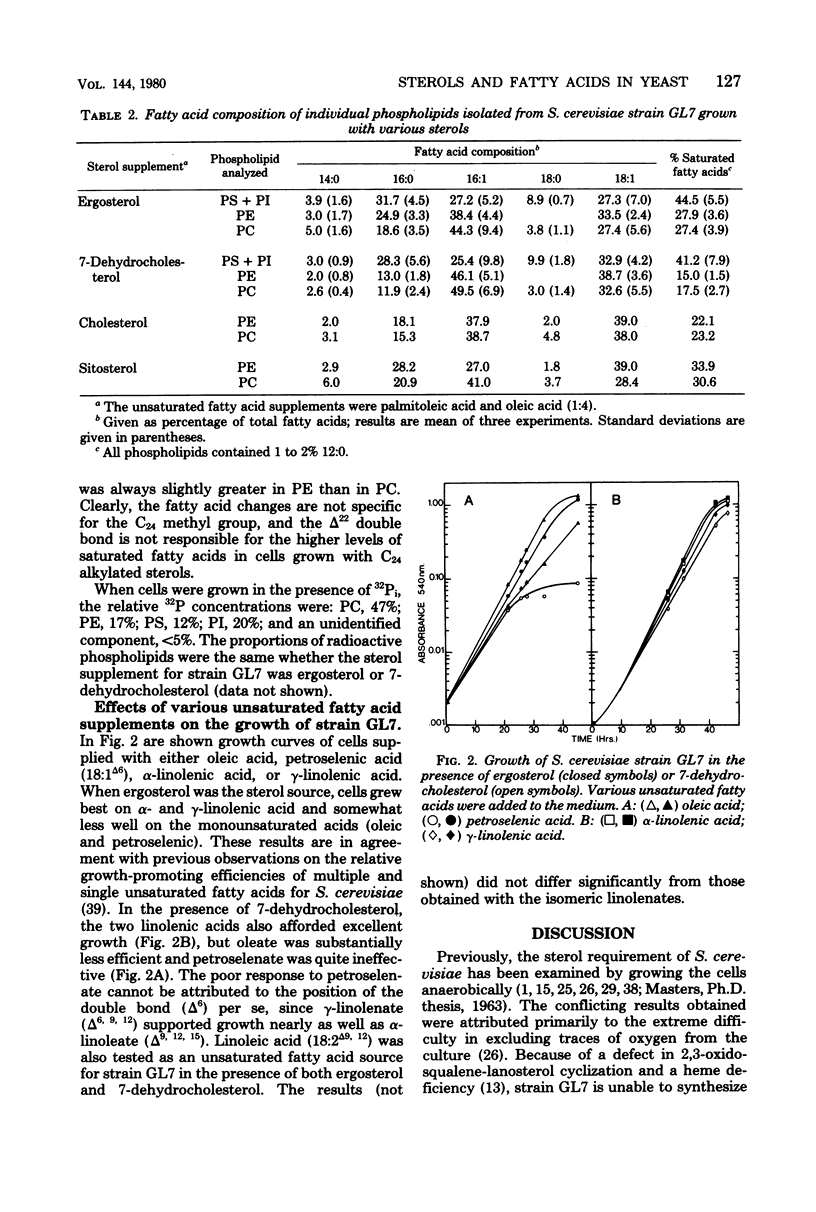
Saccharomyces cerevisiae GL7 cells require exogenous sterol and unsaturated fatty acid for growth. When grown in the presence of cholesterol or 7-dehydrocholesterol, the cells incorporated less saturated fatty acid into phospholipids than cells grown with ergosterol, stigmasterol, or beta-sitosterol as the sterol source. This lower saturated fatty acid content was most pronounced in phosphatidylethanolamine, slightly less so in phosphatidylcholine, and least evident in phosphatidylserine and phosphatidylinositol. Growing the cells with the various sterols did not affect the ratios of individual phospholipids. The ability of strain GL7 to use 7-dehydrocholesterol as the only sterol supplement for growth was dependent upon the nature of the unsaturated fatty acids added to the growth medium. In the presence of linoleic, linolenic, or a mixture of palmitoleic and oleic acids, excellent growth was observed with either ergosterol, cholesterol, or 7-dehydrocholesterol. However, when the medium was supplemented with either oleic or petroselenic acid, the cells grew more slowly (oleic) or much more poorly (petroselenic) with 7-dehydrocholesterol than with ergosterol. A specific relationship between sterol structure and membrane fatty acid composition in yeast cells is implied.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREASEN A. A., STIER T. J. B. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J Cell Physiol. 1953 Feb;41(1):23–36. doi: 10.1002/jcp.1030410103. [DOI] [PubMed] [Google Scholar]
- Baldassare J. J., Saito Y., Silbert D. F. Effect of sterol depletion on LM cell sterol mutants. Changes in the lipid composition of the plasma membrane and their effects on 3-O-methlglucose transport. J Biol Chem. 1979 Feb 25;254(4):1108–1113. [PubMed] [Google Scholar]
- Baldassare J. J., Silbert D. F. Membrane phospholipid metabolism in response to sterol depletion. Compensatory compositional changes which maintain 3-O-methylglucose transport. J Biol Chem. 1979 Oct 25;254(20):10078–10083. [PubMed] [Google Scholar]
- Bard M., Lees N. D., Burrows L. S., Kleinhans F. W. Differences in crystal violet uptake and cation-induced death among yeast sterol mutants. J Bacteriol. 1978 Sep;135(3):1146–1148. doi: 10.1128/jb.135.3.1146-1148.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttke T. M., Bloch K. Comparative responses of the yeast mutant strain GL7 to lanosterol, cycloartenol, and cyclolaudenol. Biochem Biophys Res Commun. 1980 Jan 15;92(1):229–236. doi: 10.1016/0006-291x(80)91543-0. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Demel R. A., Bruckdorfer K. R., van Deenen L. L. Structural requirements of sterols for the interaction with lecithin at the air water interface. Biochim Biophys Acta. 1972 Jan 17;255(1):311–320. doi: 10.1016/0005-2736(72)90030-2. [DOI] [PubMed] [Google Scholar]
- Demel R. A., Bruckdorfer K. R., van Deenen L. L. The effect of sterol structure on the permeability of lipomes to glucose, glycerol and Rb + . Biochim Biophys Acta. 1972 Jan 17;255(1):321–330. doi: 10.1016/0005-2736(72)90031-4. [DOI] [PubMed] [Google Scholar]
- Demel R. A., De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976 Oct 26;457(2):109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Ferguson K. A., Davis F. M., Conner R. L., Landrey J. R., Mallory F. B. Effect of sterol replacement in vivo on the fatty acid composition of Tetrahymena. J Biol Chem. 1975 Sep 10;250(17):6998–7005. [PubMed] [Google Scholar]
- Freter C. E., Ladenson R. C., Silbert D. F. Membrane phospholipid alterations in response to sterol depletion of LM cells. Metabolic studies. J Biol Chem. 1979 Aug 10;254(15):6909–6916. [PubMed] [Google Scholar]
- Gollub E. G., Liu K. P., Dayan J., Adlersberg M., Sprinson D. B. Yeast mutants deficient in heme biosynthesis and a heme mutant additionally blocked in cyclization of 2,3-oxidosqualene. J Biol Chem. 1977 May 10;252(9):2846–2854. [PubMed] [Google Scholar]
- Haslam J. M., Mahdawi S. A. The use of lipid mutants of Saccharomyces cerevisiae to investigate the role of unsaturated fatty acids and sterols in membrane functions. Biochem Soc Trans. 1980 Feb;8(1):34–37. doi: 10.1042/bst0080034. [DOI] [PubMed] [Google Scholar]
- Hossack J. A., Rose A. H. Fragility of plasma membranes in Saccharomyces cerevisiae enriched with different sterols. J Bacteriol. 1976 Jul;127(1):67–75. doi: 10.1128/jb.127.1.67-75.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst F., Lacroute F. Isolation of pleiotropic yeast mutants requiring ergosterol for growth. Biochem Biophys Res Commun. 1973 Jun 8;52(3):741–747. doi: 10.1016/0006-291x(73)90999-6. [DOI] [PubMed] [Google Scholar]
- Lala A. K., Buttke T. M., Bloch K. On the role of the sterol hydroxyl group in membranes. J Biol Chem. 1979 Nov 10;254(21):10582–10585. [PubMed] [Google Scholar]
- Lees N. D., Bard M., Kemple M. D., Haak R. A., Kleinhans F. W. ESR determination of membrane order parameter in yeast sterol mutants. Biochim Biophys Acta. 1979 Jun 2;553(3):469–475. doi: 10.1016/0005-2736(79)90302-x. [DOI] [PubMed] [Google Scholar]
- Madden T. D., Chapman D., Quinn P. J. Cholesterol modulates activity of calcium-dependent ATPase of the sarcoplasmic reticulum. Nature. 1979 Jun 7;279(5713):538–541. doi: 10.1038/279538a0. [DOI] [PubMed] [Google Scholar]
- Mallory F. B., Conner R. L. Dehydrogenation and dealkylation of various sterols by Tetrahymena pyriformis. Lipids. 1971 Mar;6(3):149–153. doi: 10.1007/BF02533028. [DOI] [PubMed] [Google Scholar]
- Marzuki S., Linnane A. W. Modification of yeast mitochondria by diet in specific mutants. Methods Enzymol. 1979;56:568–577. doi: 10.1016/0076-6879(79)56055-8. [DOI] [PubMed] [Google Scholar]
- Melchior D. L., Czech M. P. Sensitivity of the adipocyte D-glucose transport system to membrane fluidity in reconstituted vesicles. J Biol Chem. 1979 Sep 25;254(18):8744–8747. [PubMed] [Google Scholar]
- Nes W. R., Sekula B. C., Nes W. D., Adler J. H. The functional importance of structural features of ergosterol in yeast. J Biol Chem. 1978 Sep 10;253(17):6218–6225. [PubMed] [Google Scholar]
- Nozawa Y., Fukushima H., Iida H. Studies on tetrahymena membranes. Modification of surface membrane lipids by replacement of tetrahymanol by exogenous ergosterol in Tetrahymena pyriformis. Biochim Biophys Acta. 1975 Oct 6;406(2):248–263. doi: 10.1016/0005-2736(75)90008-5. [DOI] [PubMed] [Google Scholar]
- Parks L. W. Metabolism of sterols in yeast. CRC Crit Rev Microbiol. 1978;6(4):301–341. doi: 10.3109/10408417809090625. [DOI] [PubMed] [Google Scholar]
- Proudlock J. W., Wheeldon L. W., Jollow D. J., Linnane A. W. Role of sterols in Saccharomyces cerevisiae. Biochim Biophys Acta. 1968 Mar 4;152(2):434–437. doi: 10.1016/0005-2760(68)90060-x. [DOI] [PubMed] [Google Scholar]
- Rattray J. B., Schibeci A., Kidby D. K. Lipids of yeasts. Bacteriol Rev. 1975 Sep;39(3):197–231. doi: 10.1128/br.39.3.197-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintoul D. A., Chou S. M., Silbert D. F. Physical characterization of sterol-depleted LM-cell plasma membranes. J Biol Chem. 1979 Oct 25;254(20):10070–10077. [PubMed] [Google Scholar]
- Saito Y., Silbert D. F. Selective effects of membrane sterol depletion on surface function thymidine and 3-O-methyl-D-glucose transport in a sterol auxotroph. J Biol Chem. 1979 Feb 25;254(4):1102–1107. [PubMed] [Google Scholar]
- Sinensky M. Defective regulation of cholesterol biosynthesis and plasma membrane fluidity in a Chinese hamster ovary cell mutant. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1247–1249. doi: 10.1073/pnas.75.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M., Minneman K. P., Molinoff P. B. Increased membrane acyl chain ordering activates adenylate cyclase. J Biol Chem. 1979 Sep 25;254(18):9135–9141. [PubMed] [Google Scholar]
- Sinensky M., Pinkerton F., Sutherland E., Simon F. R. Rate limitation of (Na+ + K+)-stimulated adenosinetriphosphatase by membrane acyl chain ordering. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4893–4897. doi: 10.1073/pnas.76.10.4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. S., Hossack J. A., Rose A. H. Plasma-membrane lipid composition and ethanol tolerance in Saccharomyces cerevisiae. Arch Microbiol. 1978 Jun 26;117(3):239–245. doi: 10.1007/BF00738541. [DOI] [PubMed] [Google Scholar]
- Walenga R. W., Lands W. E. Effectiveness of various unsaturated fatty acids in supporting growth and respiration in Saccharomyces cerevisiae. J Biol Chem. 1975 Dec 10;250(23):9121–9129. [PubMed] [Google Scholar]
- Yavin E., Zutra A. Separation and analysis of 32P-labeled phospholipids by a simple and rapid thin-layer chromatographic procedure and its application to cultured neuroblastoma cells. Anal Biochem. 1977 Jun;80(2):430–437. doi: 10.1016/0003-2697(77)90665-0. [DOI] [PubMed] [Google Scholar]


