Abstract
The field of tissue engineering has made considerable strides since it was first described in the late 1980s. The advent and subsequent boom in stem cell biology, emergence of novel technologies for biomaterial development and further understanding of developmental biology have contributed to this accelerated progress. However, continued efforts to translate tissue-engineering strategies into clinical therapies have been hampered by the problems associated with scaling up laboratory methods to produce large, complex tissues. The significant challenges faced by tissue engineers include the production of an intact vasculature within a tissue-engineered construct and recapitulation of the size and complexity of a whole organ. Here we review the basic components necessary for bioengineering organs—biomaterials, cells and bioactive molecules—and discuss various approaches for augmenting these principles to achieve organ level tissue engineering. Ultimately, the successful translation of tissue-engineered constructs into everyday clinical practice will depend upon the ability of the tissue engineer to “scale up” every aspect of the research and development process.
Key words: tissue engineering, biomaterials, stem cells, adipose-derived stromal cells, growth factors, regenerative medicine, scale up
The unmet demand for organs and replacement tissue is well known. Millions of surgical procedures are performed each year to replace or reconstruct damaged tissue resulting from chronic disease, injury, congenital malformations and cancer. However, the gap between those patients waiting for an organ and those actually receiving a transplant continues to widen each year.1 Reconstructive and reparative procedures are limited in efficacy because of the underlying disease or injury and synthetic devices are plagued by infection, rejection and breakdown.
Tissue engineering offers a potential solution to the shortage of organ donors and the problems inherent in trying to repair damaged tissues.2 While substantial progress has been made in the field of tissue engineering, its impact in the clinic has been modest.3 Only recently have a significant number of bioengineered products entered clinical trials or become commercially available.4 Multiple small-scale studies have proven that tissue engineering can, in fact, be taken to the bedside,5–9 but primarily for very specialized indications. Further progress is needed before the utilization of bioengineered products becomes a widespread clinical reality.
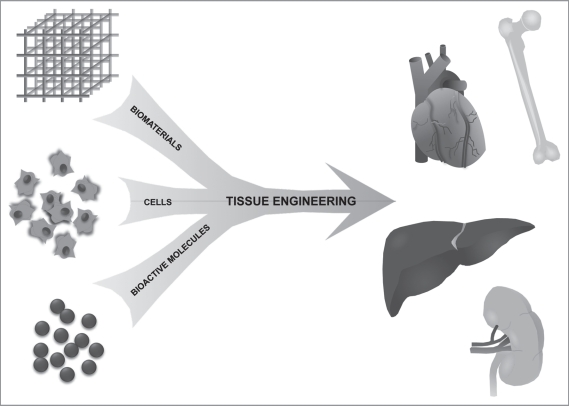
One of the principle challenges to clinical translation of tissue engineering is the difficulty recreating the complexity and scale of human-sized, clinically effective tissues and organs. In this article, we will review the three building blocks of tissue engineering strategies: biomaterials, cells and bioactive molecules (Fig. 1) and discuss strategies for using each of these components to create complex, clinically translatable tissues and organs.
Figure 1.
Organ-level tissue engineering paradigm. The basic strategy used by scientists to bioengineer tissues has changed little from the very beginnings of the field of tissue engineering. A biomaterial is utilized as a structural and mechanical scaffold into which a specific cell population is incorporated. Growth factors and other bioactive molecules can be added to the construct. After a period of maturation either in vivo or in a bioreactor, the anticipated end product is a tissue-engineered organ that serves as a functional replacement for damaged or missing tissue.
Biomaterials-Bioactive Scaffolds for Engineering Complex Tissues
The role of biomaterials in the field of tissue engineering has evolved from the early ideas of an inert scaffolding device10 to one in which biomatrices are actively contributing to the regeneration of tissue.11,12 Ideally, a biomaterial must provide a structured environment with tissue-specific mechanical properties and a biocompatible, porous niche for cells and small molecules that are capable of integrating with surrounding tissues and stimulating regeneration of normal tissue.13 As knowledge of the complex functions served by the extracellular matrix (ECM) expands, further insight is gained into the challenge for the tissue engineer in making a truly biomimetic scaffold. Given the ECM’s role in cell proliferation, differentiation, migration and polarity,14,15 the choice of a biomaterial in all tissue engineering applications is as important in guiding cell behavior as soluble factors in the wound environment or bioactive molecules added to the engineered construct.
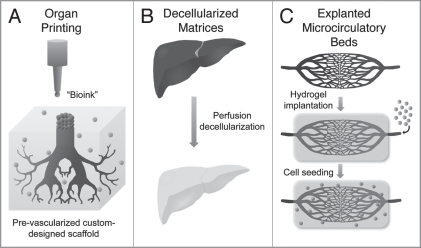
The vast collection of synthetic and natural biomaterials used currently for tissue engineering studies continues to expand and evolve as new technologies emerge and old materials are used in new ways. Commonly used natural products such as alginate and collagen have been patterned using inkjet printing technology to construct custom-designed scaffolds and to direct cellular positioning and growth via patterned collagen placement.16–18 Inkjet printing has also been used to print individual live cells in a defined two-dimensional pattern19 and to direct stem cell fate via growth factor patterning on a hydrogel substrate.20 Another jet-based technology, bio-electrospraying, has been used to viably process primary smooth muscle cells and adult stem cells and has the potential to deliver cells in situ within simultaneously fabricated fiber-based scaffolds.21–23 These promising technologies allow precise spatial control of cells, polymers and growth factors, which theoretically can reproduce organ-level complexity.17 Organ printing techniques have been suggested to potentially eliminate the solid biomaterial component from the traditional tissue engineering paradigm.24 In a process referred to as “directed tissue self-assembly,” individual tissue spheroids can be bioprinted in pre-designed patterns such that upon fusion of the spheroids specific structural elements can form including tubular vascular-like structures (Fig. 2A).25
Figure 2.
Biomaterial strategies for organ-level tissue engineering. Several emerging technologies in biomaterial scaffolds offer the promise of scaling up the size and complexity of tissue engineered constructs. “Organ printing” (A) is the use of inkjet printing techniques, which allows precise control over placement of cells and polymers deposited within a “bioink” and has been used to form tubular vascular structures. Recent advances in decellularization protocols using detergent perfusion have demonstrated promising results in the complete removal of cells from large visceral organs (B). These decellularized matrices maintain their structure and mechanical properties without containing immunogenic donor cells. The use of autologous explanted microcirculatory beds (EMBs) allows tissue engineers to circumvent the challenges of creating vasculature de novo (C). EMBs can be harvested, implanted within a hydrogel scaffold, manipulated ex vivo including cell perfusion and reimplanted at the site of tissue damage.
Another biomaterial having the potential to accelerate progress in organ level tissue engineering is decellularized tissue matrices (Fig. 2B). By removing cellular components from donor tissue, all traces of human antigens are removed thus making the remaining matrix immunocompatible and eliminating the need for immunosuppression after transplantation.4,17 These decellularized scaffolds enable exploitation of the intact three-dimensional structure of the ECM as a template for whole organ regeneration. Acellular dermal and bladder matrices have been widely used as natural materials for bioengineering of these relatively thin tissues,26–30 but current decellularization protocols have been difficult to translate to larger, more complex tissues and organs. Recently, functional architecture was maintained in decellularized cadaveric rat hearts that were seeded with neonatal cardiac cells and shown to have contractile activity after eight days in a perfusion bioreactor, indicating that whole organ decellularization is possible.31 This impressive feat has been emulated with the successful whole organ decellularization of multiple other larger organ types including liver, kidney, pancreas and intestine, which ranged in size up to 30 cm.32
One major challenge as size scale increases is vascularizing cells in the center of a given construct. The development of a functional microvascular network is a prerequisite for the integration and formation of surrounding stromal tissue. The issue of vascularization in tissue engineering continues be one of the greatest unsolved yet intensely investigated areas in the field. The de novo creation of a functional vasculature is a daunting task that continues to be an obstacle for tissue engineers. We believe the challenge of scaffold vascularization can be addressed with the use of native tissue to provide an intact vascular network (Fig. 2C). While some progress has been made in this arena,24,33 we have developed a method to circumvent this issue of de novo vasculature creation using autologous microcirculatory beds.34 These explanted microcirculatory beds (EMBs) consisting of an afferent artery, capillary beds and a single efferent vein can be harvested, maintained and seeded with mesenchymal stem cells in a bioreactor and then reimplanted. As expendable microcirculatory beds are routinely used by surgeons as microvascular free flaps, this technique offers the potential of harvesting autologous tissue, manipulating the tissue ex vivo with tissue-specific cells and growth factors and reimplanting the tissue at a heterotopic location using standard microsurgical techniques. Our laboratory is pursuing this strategy for organ-level tissue engineering in combination with traditional biomaterials in an effort to further expand and manipulate these microcirculatory beds. Strategies have also been developed to expand the encapsulated native vasculature and further increase the dimensions of the resulting bioengineered construct.
Cells-Facilitating the Repair of Heterogeneous Organs
The concept of using cells to replace damaged or missing tissue based on their functional characteristics35,36 preceded the field of tissue engineering as it was described by Langer and Vacanti in 1993.2 The use of cells in bioengineering tissue has since undergone remarkable expansion and evolution with the development of and continuing advances in stem cell biology. The breadth of cell populations available for use in tissue engineering constructs has increased tremendously from early reports using adult differentiated cell types.37,38 Most recently embryonic stem cells,39,40 adult mesenchymal stem cells,41,42 and induced pluripotent stem cells (iPS)43,44 have been used in tissue engineering efforts.
Our laboratories have recently focused on adipose-derived stromal cells (ASCs) for tissue engineering.41,45–50 Research from the early 1960s described a stromal-vascular fraction (SVF) isolated from fat that contained fibroblast-like cells.51 Subsequent examination of this SVF cell type led scientists to believe that they represented an adipose progenitor cell whose fate was limited to adipose tissue.52 In 2001, the understanding of the potential of these SVF cells greatly changed as Zuk et al. demonstrated the ability of these cells to undergo adipogenic differentiation but also chondrogenic, myogenic and osteogenic differentiation.53 The potential multipotency of ASCs has expanded tremendously since these early studies as scientists continue to fine tune and improve the differentiation of each pathway.54–60 Cell surface receptors in ASCs have been shown to be similar to those of bone marrow-derived mesenchymal stem cells (BM-MSCs).60,61 However, ASCs hold several advantages over BM-MSCs in that they are readily available in large quantities and can be harvested with minimally invasive procedures, thus making them more amenable to clinical use. Like BM-MSCs, ASCs can also be safely and effectively transplanted into an autologous host.
Recent clinical studies have focused on the use of human adipose-derived stromal cells to replace bone loss.62 Specifically, hASCs seeded on a PLGA scaffold were recently used to treat a young girl after severe mandibular trauma. In small pilot studies, defects of the cranium,63 maxilla64 and mandible65 have shown accelerated healing with the use of hASCs.66 In these case reports, however, the method of hASC usage has varied dramatically, from the combination with bone chips to the use of recombinant proteins. Such reconstructions eliminate the need for alloplastic materials, and thus reduce the risk of infection, breakdown or rejection. In our laboratory, we have observed convincingly that ASCs, whether derived from mouse or human origin, contribute to osseous healing of mouse cranial defects.41,67
Despite these intriguing case reports and accumulating translational research, there is a paucity of data defining the mechanisms through which ASCs and other stem and progenitor cells contribute to regeneration of damaged tissue. Do cells directly form the tissue of interest to heal a defect? Do engrafted cells exhibit mainly paracrine functions to produce potent pro-regenerative cytokines? In the case of our calvarial defect model, careful examination of calvarial defects engrafted with ASCs yields some valuable insights into the potential derivation of healing. In this case, bone is often observed to mineralize from the edges of a cranial defect inwards, which suggests that the host calvarium is the prime contributor to the bone regenerate.
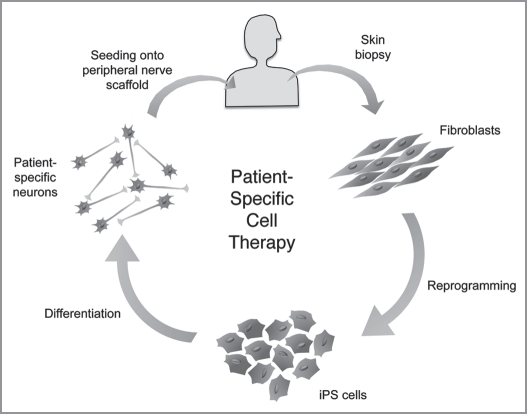
One exciting advance in tissue engineering is the recent discovery of the ability to transform adult somatic cells, back to an embryonic-like pluripotent cell that can differentiate into ectoderm, endoderm as well as mesodermal tissues creating “induced” pluripotent stem (iPS) cells.68,69 Considering the ease and reproducibility of generating iPS cells as well as the lack of ethical concerns, experts have raised the hope that iPS cells might fulfill much of the promise of human ES cells in regenerative medicine.70 These cells represent a potential mechanism by which to use readily available cells from a patient in need of an organ, transform them into iPS cells, expand them and design a tissue-engineered construct containing entirely autologous cells (Fig. 3).
Figure 3.
Patient-specific tissue engineering. The recent discovery of iPS technology has offered the potential of patient-specific cell therapy. A small skin biopsy could be obtained from a patient in need of an organ or tissue replacement, from which dermal fibroblasts would be isolated and expanded in vitro. These fibroblasts can then be reprogrammed into iPS cells whose pluripotency could be exploited to differentiate the iPS cells into any patient-specific cell type (i.e., neurons, hepatocytes, etc.). The patient-specific cells can then be incorporated into a biomaterial scaffold and implanted back to the patient at the damaged tissue site.
One of the primary obstacles in transitioning a cell-based tissue engineered construct to commercial production is the scale up of cultured cell populations.71 In an early report by Vacanti et al. taking tissue engineering to the bedside, a patient’s distal thumb was reconstructed using autologous periosteal cells.72 However, these autologous cells were grown in culture for nine weeks before they could be expanded enough to reimplant into the patient. In order to make a significant clinical impact, the ex vivo expansion of cells must be accelerated. While proliferation rates are cell-specific, the speed of autologous cell expansion will likely continue to be a rate-limiting step in the time to their therapeutic application. One potential means of avoiding the time-consuming process of in vitro cell expansion is to use ASCs for cell-based constructs. A small volume of lipoaspirate allows for the harvest of a large number of these cells thus negating the need for expansion in culture prior to implantation. Additionally, the automation of cell culture may provide a means to ensure the safety and efficiency of cell expansion for commercial production of bioengineered constructs.73,74
Bioactive Molecules-Orchestrating the Regeneration of Intricate Tissues
Organ level tissue engineering requires a microenvironment that not only provides substrates for cell proliferation but also affords signaling cues directing morphogenesis and cell differentiation into tissue-specific structures. Growth factors play a key role in tissue regeneration and are expressed during different phases of tissue development.
As discussed in the previous section, vascularization is a critical bottleneck for tissue engineered constructs. Vascular endothelial growth factor (VEGF) has been widely incorporated as a component of biomaterials to promote vessel growth in various tissue engineering strategies.75–77 Several other growth factors have been used to promote the proliferation of vascular structures in vitro, such as fibroblast growth factor-1 (FGF-1), insulin-like growth factor-1 (IGF-1), hepatocye growth factor (HGF), angiopoetin-1 and platelet-derived growth factor (PDGF).78–80
Organ-level morphogenesis also requires the presence of tissue-specific signals particularly growth factors to induce the formation of new tissue. Recent research efforts have focused on biomatrices incorporating growth factors to restore and regenerate multiple tissues.81–83 In bone tissue engineering, growth factors including members of the bone morphogenic protein (BMP) family have been used to enhance and facilitate bone formation. Our laboratory has utilized several bioactive factors for bone engineering including bone morphogenic protein 2 (BMP-2) and retinoic acid and have demonstrated that these molecules enhance bone formation in critical-size calvarial defects.47 Using similar approaches in the bioengineering of cartilage, nerve and skin, multiple groups have used potent growth factors such as transforming growth factor-beta (TGFβ), FGF and epidermal growth factor (EGF) to successfully induce tissue-specific growth after injury.83–85
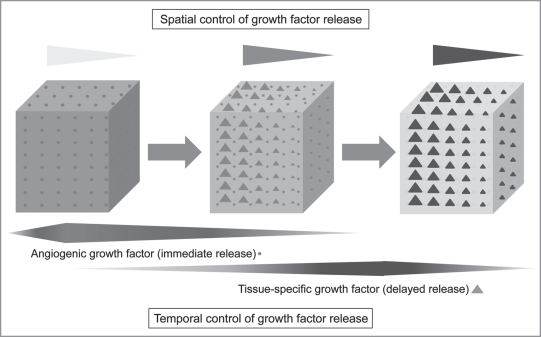
Bioengineering efforts have typically focused on the delivery of single factors for tissue regeneration. However, it is likely that a complex organ, which comprises multiple microstructural elements, requires the orchestrated interplay of multiple molecules to generate a functional substitute (Fig. 4). Several studies have shown that the combination of multiple growth factors in a polymeric scaffold reveals superior results than those seen with single factor treatment. Dual delivery of BMP-2 and TGFβ3 incorporated in a biodegradable hydrogel lead to significant in vivo bone formation by bone marrow stromal cells, whereas these growth factors applied individually produced no osteogenic effect.86 Using a similar approach to engineer muscle tissue, sustained IGF-1 delivery alone was found to enhance muscle fiber regeneration, while the combined delivery of both VEGF and IGF-1 led to parallel angiogenesis, reinnervation and myogenesis.87
Figure 4.
Controlled growth factor release from a biomaterial scaffold. A time-dependant release of multiple growth factors is necessary to facilitate the regeneration of a complex organ. Here the delivery of an angiogenic growth factor (circle) is followed by the delayed release of a tissue specific growth factor (triangle). The spatial control of growth factor concentration can also be incorporated into the biomaterial to allow for guided cell migration and tissue regeneration.
The effects of growth factors on cell differentiation, proliferation and migration are also strongly dependent upon the timing, release kinetics and spatial distribution of expression. The ability to control timing and concentration of multiple growth factors within the same scaffold would allow fine tuning of the complexity within a bioengineered tissue. Intelligent hydrogel matrices that comprise these features have been developed to facilitate regeneration.88,89 In a recent study, an alginate/PDLLA scaffold incorporating VEGF and BMP-2 was seeded with human bone marrow MSCs and used to study bone regeneration in a critical-size bone defect.90 The encapsulation of VEGF into alginate fibers lead to an accelerated release rate at the beginning whereas the PDLLA encapsulated BMP-2 showed a slower and prolonged release rate. This approach showed a significant increase in the quantity of regenerated bone compared to individual growth factor application.90
As investigators begin to explore the clinical application of growth factors in tissue engineered constructs, the ease of production and stability of these biofactors are important considerations. The engineering of biomaterials incorporating growth factors will be limited if recombinant growth factors cannot be easily mass produced as active proteins. This issue can be addressed through protein modifications that not only allow for easier production but also improve functionality.91,92 Another challenging issue is the ability to retain the active growth factor at the site of implantation. Collagen-binding domains (CBD) can be fused with growth factors and have been shown to increase retention and prolong growth factor release. A CBD-FGF-1 chimera significantly increased the proliferation of smooth muscle cells in a collagen matrix as compared to the native growth factor.93 The heparin-binding capacity of growth factors can also be utilized for incorporation into microparticles and biomatrices to ensure a prolonged and controlled release over time.94
Conclusions and Perspectives for the Future
Progress in tissue engineering as a field of basic scientific inquiry continues to surpass the development of tissue engineering as an industry. While the concept of scaling up tissues in terms of size and complexity is appreciated by most tissue engineers, the practical challenges involved are familiar only to those who have attempted to transition a tissue engineered product to the commercial sector. As tissue engineering companies continue to rebound from previous financial challenges, a focus on low-cost production strategies will be critical for the successful mass production of effective tissue engineered products. Tissue engineering requires a multidisciplinary approach—materials science, cell biology, chemistry and clinical medicine. More importantly, the translation of tissue engineering to the patient in need of organ replacement also requires integration of the industry perspective at every step of research and development to allow this exciting and highly promising field to reach its full therapeutic potential.
Abbreviations
- ECM
extracellular matrix
- EMB
explanted microcirculatory bed
- iPS
induced pluripotent stem cells
- ASC
adipose-derived stromal cells
- SVF
stromal-vascular fraction
- BM-MSC
bone marrow-derived mesenchymal stem cells
- VEGF
vascular endothelial growth factor
- FGF-1
fibroblast growth factor-1
- IGF-1
insulin-like growth factor-1
- HGF
hepatocyte growth factor
- PDGF
platelet-derived growth factor
- BMP-2
bone morphogenic protein 2
- TGFβ
transforming growth factorbeta
- EGF
epidermal growth factor
- CBD
collagen-binding domains
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/12139
References
- 1.The organ procurement and transplant network/scientific registry of transplant recipients annual report: U.S. Department of Health & Human Services. 2008.
- 2.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 3.Ingber DE, Mow VC, Butler D, Niklason L, Huard J, Mao J, et al. Tissue engineering and developmental biology: going biomimetic. Tissue Eng. 2006;12:3265–3283. doi: 10.1089/ten.2006.12.3265. [DOI] [PubMed] [Google Scholar]
- 4.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 5.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissueengineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 6.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 7.Yanaga H, Imai K, Fujimoto T, Yanaga K. Generating ears from cultured autologous auricular chondrocytes by using two-stage implantation in treatment of microtia. Plast Reconstr Surg. 2009;124:817–825. doi: 10.1097/PRS.0b013e3181b17c0e. [DOI] [PubMed] [Google Scholar]
- 8.Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 9.Warnke PH, Springer IN, Wiltfang J, Acil Y, Eufinger H, Wehmoller M, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364:766–770. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 10.Hench LL. Biomaterials. Science. 1980;208:826–831. doi: 10.1126/science.6246576. [DOI] [PubMed] [Google Scholar]
- 11.Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002;295:1014–1017. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 12.Stevens MM, George JH. Exploring and engineering the cell surface interface. Science. 2005;310:1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 13.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 14.Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol. 2007;213:565–573. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- 15.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boland T, Xu T, Damon B, Cui X. Application of inkjet printing to tissue engineering. Biotechnol J. 2006;1:910–917. doi: 10.1002/biot.200600081. [DOI] [PubMed] [Google Scholar]
- 17.Atala A. Engineering organs. Curr Opin Biotechnol. 2009;20:575–592. doi: 10.1016/j.copbio.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Roth EA, Xu T, Das M, Gregory C, Hickman JJ, Boland T. Inkjet printing for high-throughput cell patterning. Biomaterials. 2004;25:3707–3715. doi: 10.1016/j.biomaterials.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura M, Kobayashi A, Takagi F, Watanabe A, Hiruma Y, Ohuchi K, et al. Biocompatible inkjet printing technique for designed seeding of individual living cells. Tissue Eng. 2005;11:1658–1666. doi: 10.1089/ten.2005.11.1658. [DOI] [PubMed] [Google Scholar]
- 20.Ilkhanizadeh S, Teixeira AI, Hermanson O. Inkjet printing of macromolecules on hydrogels to steer neural stem cell differentiation. Biomaterials. 2007;28:3936–3943. doi: 10.1016/j.biomaterials.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 21.Bartolovic K, Mongkoldhumrongkul N, Waddington SN, Jayasinghe SN, Howe SJ. The differentiation and engraftment potential of mouse hematopoietic stem cells is maintained after bio-electrospray. Analyst. 2010;135:157–164. doi: 10.1039/b917813a. [DOI] [PubMed] [Google Scholar]
- 22.Jayasinghe SN, Irvine S, McEwan JR. Cell electrospinning highly concentrated cellular suspensions containing primary living organisms into cell-bearing threads and scaffolds. Nanomedicine. 2007;2:555–567. doi: 10.2217/17435889.2.4.555. [DOI] [PubMed] [Google Scholar]
- 23.Sahoo S, Lee WC, Goh JC, Toh SL. Bio-electrospraying: a potentially safe technique for delivering progenitor cells. Biotechnol Bioeng. 2010. [DOI] [PubMed]
- 24.Visconti RP, Kasyanov V, Gentile C, Zhang J, Markwald RR, Mironov V. Towards organ printing: engineering an intra-organ branched vascular tree. Expert Opin Biol Ther. 2010;10:409–420. doi: 10.1517/14712590903563352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30:2164–2174. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Deng Z, Wang H, Yang Z, Guo W, Li Y, et al. Expansion and delivery of human fibroblasts on micronized acellular dermal matrix for skin regeneration. Biomaterials. 2009;30:2666–2674. doi: 10.1016/j.biomaterials.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 27.Roessner ED, Thier S, Hohenberger P, Schwarz M, Pott P, Dinter D, et al. Acellular dermal matrix seeded with autologous fibroblasts improves wound breaking strength in a rodent soft tissue damage model in neoadjuvant settings. J Biomater Appl. 2009. In press. [DOI] [PubMed]
- 28.Sutherland RS, Baskin LS, Hayward SW, Cunha GR. Regeneration of bladder urothelium, smooth muscle, blood vessels and nerves into an acellular tissue matrix. J Urol. 1996;156:571–577. doi: 10.1097/00005392-199608001-00002. [DOI] [PubMed] [Google Scholar]
- 29.Yoo JJ, Meng J, Oberpenning F, Atala A. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology. 1998;51:221–225. doi: 10.1016/s0090-4295(97)00644-4. [DOI] [PubMed] [Google Scholar]
- 30.Probst M, Dahiya R, Carrier S, Tanagho EA. Reproduction of functional smooth muscle tissue and partial bladder replacement. Br J Urol. 1997;79:505–515. doi: 10.1046/j.1464-410x.1997.00103.x. [DOI] [PubMed] [Google Scholar]
- 31.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 32.Baptista PM, Orlando G, Mirmalek-Sani SH, Siddiqui M, Atala A, Soker S. Whole organ decellularization-a tool for bioscaffold fabrication and organ bioengineering. Conf Proc IEEE Eng Med Biol Soc. 2009;1:6526–6529. doi: 10.1109/IEMBS.2009.5333145. [DOI] [PubMed] [Google Scholar]
- 33.Asakawa N, Shimizu T, Tsuda Y, Sekiya S, Sasagawa T, Yamato M, et al. Pre-vascularization of in vitro threedimensional tissues created by cell sheet engineering. Biomaterials. 2010;31:3903–3909. doi: 10.1016/j.biomaterials.2010.01.105. [DOI] [PubMed] [Google Scholar]
- 34.Chang EI, Bonillas RG, El-ftesi S, Ceradini DJ, Vial IN, Chan DA, et al. Tissue engineering using autologous microcirculatory beds as vascularized bioscaffolds. FASEB J. 2009;23:906–915. doi: 10.1096/fj.08-114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutherland DE, Numata M, Matas AJ, Simmons RL, Najarian JS. Hepatocellular transplantation in acute liver failure. Surgery. 1977;82:124–132. [PubMed] [Google Scholar]
- 36.Russell PS. Selective transplantation. An emerging concept. Ann Surg. 1985;201:255–262. doi: 10.1097/00000658-198503000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vacanti JP, Morse MA, Saltzman WM, Domb AJ, Perez-Atayde A, Langer R. Selective cell transplantation using bioabsorbable artificial polymers as matrices. J Pediatr Surg. 1988;23:3–9. doi: 10.1016/s0022-3468(88)80529-3. [DOI] [PubMed] [Google Scholar]
- 38.Puelacher WC, Mooney D, Langer R, Upton J, Vacanti JP, Vacanti CA. Design of nasoseptal cartilage replacements synthesized from biodegradable polymers and chondrocytes. Biomaterials. 1994;15:774–778. doi: 10.1016/0142-9612(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 39.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci USA. 2003;100:12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guenou H, Nissan X, Larcher F, Feteira J, Lemaitre G, Saidani M, et al. Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: a preclinical study. Lancet. 2009;374:1745–1753. doi: 10.1016/S0140-6736(09)61496-3. [DOI] [PubMed] [Google Scholar]
- 41.Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 42.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Fernandez A, Nelson TJ, Yamada S, Reyes S, Alekseev AE, Perez-Terzic C, et al. iPS programmed without c-MYC yield proficient cardiogenesis for functional heart chimerism. Circ Res. 2009;105:648–656. doi: 10.1161/CIRCRESAHA.109.203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W, Wang D, Qin J, Liu C, Zhang Q, Zhang X, et al. Generation of functional hepatocytes from mouse induced pluripotent stem cells. J Cell Physiol. 2010;222:492–501. doi: 10.1002/jcp.22000. [DOI] [PubMed] [Google Scholar]
- 45.Xu Y, Balooch G, Chiou M, Bekerman E, Ritchie RO, Longaker MT. Analysis of the material properties of early chondrogenic differentiated adipose-derived stromal cells (ASC) using an in vitro three-dimensional micromass culture system. Biochem Biophys Res Commun. 2007;359:311–316. doi: 10.1016/j.bbrc.2007.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol. 2006;290:1139–1146. doi: 10.1152/ajpcell.00415.2005. [DOI] [PubMed] [Google Scholar]
- 47.Cowan CM, Aalami OO, Shi YY, Chou YF, Mari C, Thomas R, et al. Bone morphogenetic protein 2 and retinoic acid accelerate in vivo bone formation, osteoclast recruitment and bone turnover. Tissue Eng. 2005;11:645–658. doi: 10.1089/ten.2005.11.645. [DOI] [PubMed] [Google Scholar]
- 48.Quarto N, Longaker MT. Differential expression of specific FGF ligands and receptor isoforms during osteogenic differentiation of mouse Adipose-derived Stem Cells (mASCs) recapitulates the in vivo osteogenic pattern. Gene. 2008;424:130–140. doi: 10.1016/j.gene.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 49.Hammerick KE, James AW, Huang Z, Prinz FB, Longaker MT. Pulsed direct current electric fields enhance osteogenesis in adipose-derived stromal cells. Tissue Eng Part A. 2010;16:917–931. doi: 10.1089/ten.tea.2009.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thangarajah H, Vial IN, Chang E, El-Ftesi S, Januszyk M, Chang EI, et al. IFATS collection: Adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells. 2009;27:266–274. doi: 10.1634/stemcells.2008-0276. [DOI] [PubMed] [Google Scholar]
- 51.Rodbell M. Metabolism of isolated fat cells I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- 52.Hauner H, Entenmann G, Wabitsch M, Gaillard D, Ailhaud G, Negrel R, et al. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J Clin Invest. 1989;84:1663–1670. doi: 10.1172/JCI114345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 54.Guilak F, Lott KE, Awad HA, Cao Q, Hicok KC, Fermor B, et al. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206:229–237. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 55.Yang M, Ma QJ, Dang GT, Ma K, Chen P, Zhou CY. In vitro and in vivo induction of bone formation based on ex vivo gene therapy using rat adipose-derived adult stem cells expressing BMP-7. Cytotherapy. 2005;7:273–281. doi: 10.1080/14653240510027244. [DOI] [PubMed] [Google Scholar]
- 56.Seo MJ, Suh SY, Bae YC, Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258–264. doi: 10.1016/j.bbrc.2004.12.158. [DOI] [PubMed] [Google Scholar]
- 57.Ogawa R, Mizuno H, Hyakusoku H, Watanabe A, Migita M, Shimada T. Chondrogenic and osteogenic differentiation of adipose-derived stem cells isolated from GFP transgenic mice. J Nippon Med Sch. 2004;71:240–241. doi: 10.1272/jnms.71.240. [DOI] [PubMed] [Google Scholar]
- 58.Huang JI, Zuk PA, Jones NF, Zhu M, Lorenz HP, Hedrick MH, et al. Chondrogenic potential of multipotential cells from human adipose tissue. Plast Reconstr Surg. 2004;113:585–594. doi: 10.1097/01.PRS.0000101063.27008.E1. [DOI] [PubMed] [Google Scholar]
- 59.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 60.Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875–884. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 61.Gronthos S, Graves SE, Ohta S, Simmons PJ. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994;84:4164–4173. [PubMed] [Google Scholar]
- 62.Halvorsen YC, Wilkison WO, Gimble JM. Adiposederived stromal cells—their utility and potential in bone formation. Int J Obes Relat Metab Disord. 2000;24:41–44. doi: 10.1038/sj.ijo.0801503. [DOI] [PubMed] [Google Scholar]
- 63.Lendeckel S, Jodicke A, Christophis P, Heidinger K, Wolff J, Fraser JK, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32:370–373. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 64.Mesimaki K, Lindroos B, Tornwall J, Mauno J, Lindqvist C, Kontio R, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–209. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 65.Kulakov AA, Goldshtein DV, Grigoryan AS, Rzhaninova AA, Alekseeva IS, Arutyunyan IV, et al. Clinical study of the efficiency of combined cell transplant on the basis of multipotent mesenchymal stromal adipose tissue cells in patients with pronounced deficit of the maxillary and mandibulary bone tissue. Bull Exp Biol Med. 2008;146:522–525. doi: 10.1007/s10517-009-0322-8. [DOI] [PubMed] [Google Scholar]
- 66.Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, et al. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966–979. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levi B, James AW, Nelson ER, Vistnes D, Wu B, Lee M, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS ONE. 2010;5:11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 69.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 70.Pera MF. Stem cells. A new year and a new era. Nature. 2008;451:135–136. doi: 10.1038/451135a. [DOI] [PubMed] [Google Scholar]
- 71.Koh CJ, Atala A. Tissue engineering, stem cells and cloning: opportunities for regenerative medicine. J Am Soc Nephrol. 2004;15:1113–1125. doi: 10.1097/01.asn.0000119683.59068.f0. [DOI] [PubMed] [Google Scholar]
- 72.Vacanti CA, Bonassar LJ, Vacanti MP, Shufflebarger J. Replacement of an avulsed phalanx with tissueengineered bone. N Engl J Med. 2001;344:1511–1514. doi: 10.1056/NEJM200105173442004. [DOI] [PubMed] [Google Scholar]
- 73.Kato R, Iejima D, Agata H, Asahina I, Okada K, Ueda M, et al. A compact, automated cell culture system for clinical scale cell expansion from primary tissues. Tissue Eng Part C Methods. 2010. In press. [DOI] [PubMed]
- 74.Pramatarov I. Investigation of the incidence of silicosis and silicotuberculosis in BCG-vaccinated underground workers of the Rodopsk Coal Basin. Gig Tr Prof Zabol. 1965;9:41–43. [PubMed] [Google Scholar]
- 75.Dai J, Rabie AB. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res. 2007;86:937–950. doi: 10.1177/154405910708601006. [DOI] [PubMed] [Google Scholar]
- 76.Chiu LL, Radisic M. Scaffolds with covalently immobilized VEGF and Angiopoietin-1 for vascularization of engineered tissues. Biomaterials. 2010;31:226–241. doi: 10.1016/j.biomaterials.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 77.Hao X, Silva EA, Mansson-Broberg A, Grinnemo KH, Siddiqui AJ, Dellgren G, et al. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc Res. 2007;75:178–185. doi: 10.1016/j.cardiores.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 78.Murakami M, Simons M. Fibroblast growth factor regulation of neovascularization. Curr Opin Hematol. 2008;15:215–220. doi: 10.1097/MOH.0b013e3282f97d98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moya ML, Lucas S, Francis-Sedlak M, Liu X, Garfinkel MR, Huang JJ, et al. Sustained delivery of FGF-1 increases vascular density in comparison to bolus administration. Microvasc Res. 2009;78:142–147. doi: 10.1016/j.mvr.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 80.Freeman I, Cohen S. The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization. Biomaterials. 2009;30:2122–2131. doi: 10.1016/j.biomaterials.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 81.Dodla MC, Bellamkonda RV. Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials. 2008;29:33–46. doi: 10.1016/j.biomaterials.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levenberg S, Burdick JA, Kraehenbuehl T, Langer R. Neurotrophin-induced differentiation of human embryonic stem cells on three-dimensional polymeric scaffolds. Tissue Eng. 2005;11:506–512. doi: 10.1089/ten.2005.11.506. [DOI] [PubMed] [Google Scholar]
- 83.Midha R, Munro CA, Dalton PD, Tator CH, Shoichet MS. Growth factor enhancement of peripheral nerve regeneration through a novel synthetic hydrogel tube. J Neurosurg. 2003;99:555–565. doi: 10.3171/jns.2003.99.3.0555. [DOI] [PubMed] [Google Scholar]
- 84.Hardwicke J, Schmaljohann D, Boyce D, Thomas D. Epidermal growth factor therapy and wound healing-past, present and future perspectives. Surgeon. 2008;6:172–177. doi: 10.1016/s1479-666x(08)80114-x. [DOI] [PubMed] [Google Scholar]
- 85.Roberts AB. Transforming growth factor-beta: activity and efficacy in animal models of wound healing. Wound Repair Regen. 1995;3:408–418. doi: 10.1046/j.1524-475X.1995.30405.x. [DOI] [PubMed] [Google Scholar]
- 86.Simmons CA, Alsberg E, Hsiong S, Kim WJ, Mooney DJ. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone. 2004;35:562–569. doi: 10.1016/j.bone.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 87.Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, et al. Regenerative Medicine Special Feature: Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci USA. 2010;107:3287–3292. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tessmar JK, Gopferich AM. Matrices and scaffolds for protein delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:274–291. doi: 10.1016/j.addr.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 89.Zhang S, Uludag H. Nanoparticulate systems for growth factor delivery. Pharm Res. 2009;26:1561–1580. doi: 10.1007/s11095-009-9897-z. [DOI] [PubMed] [Google Scholar]
- 90.Kanczler JM, Ginty PJ, White L, Clarke NM, Howdle SM, Shakesheff KM, et al. The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials. 2010;31:1242–1250. doi: 10.1016/j.biomaterials.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 91.Nagaoka M, Jiang HL, Hoshiba T, Akaike T, Cho CS. Application of recombinant fusion proteins for tissue engineering. Ann Biomed Eng. 2010;38:683–693. doi: 10.1007/s10439-010-9935-3. [DOI] [PubMed] [Google Scholar]
- 92.Geutjes PJ, Nillesen ST, Lammers G, Daamen WF, van Kuppevelt TH. Cloning, large-scale production and purification of active dimeric rat vascular endothelial growth factor (rrVEGF-164) Protein Expr Purif. 2010;69:76–82. doi: 10.1016/j.pep.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 93.Pang Y, Wang X, Ucuzian AA, Brey EM, Burgess WH, Jones KJ, et al. Local delivery of a collagen-binding FGF-1 chimera to smooth muscle cells in collagen scaffolds for vascular tissue engineering. Biomaterials. 2010;31:878–885. doi: 10.1016/j.biomaterials.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Joung YK, Bae JW, Park KD. Controlled release of heparin-binding growth factors using heparin-containing particulate systems for tissue regeneration. Expert Opin Drug Deliv. 2008;5:1173–1184. doi: 10.1517/17425240802431811. [DOI] [PubMed] [Google Scholar]