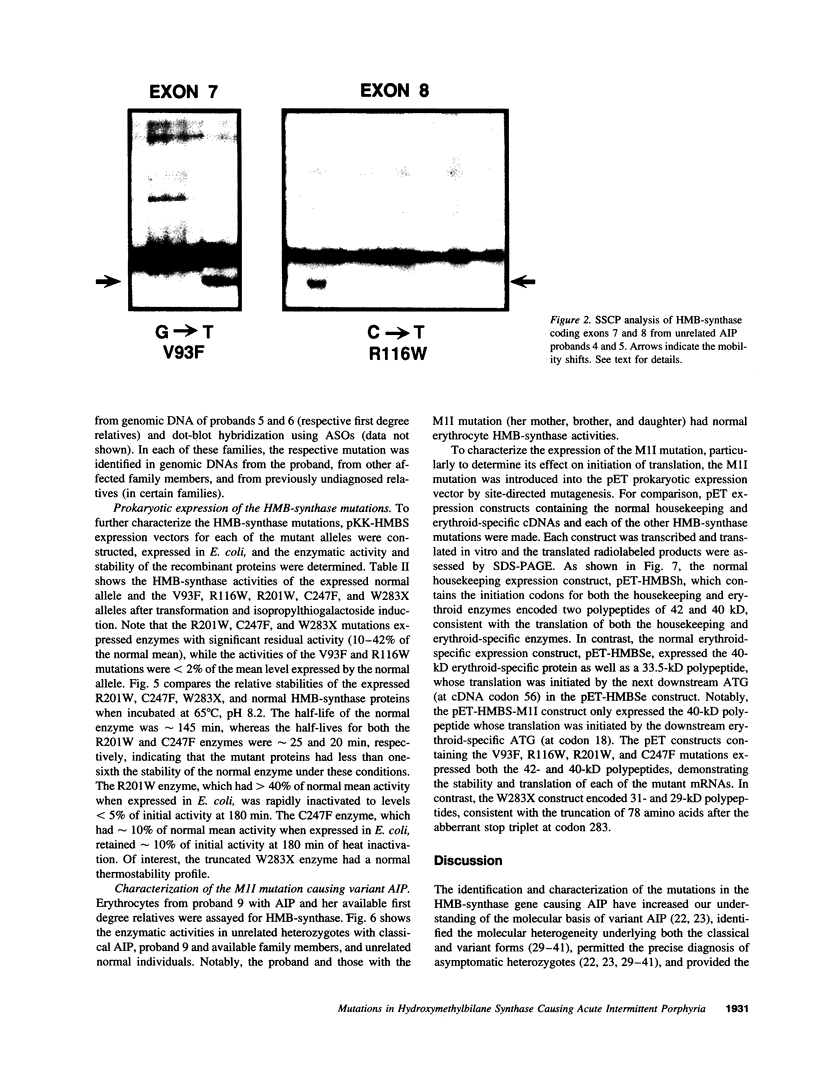
Abstract
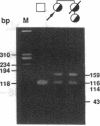
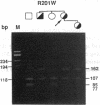
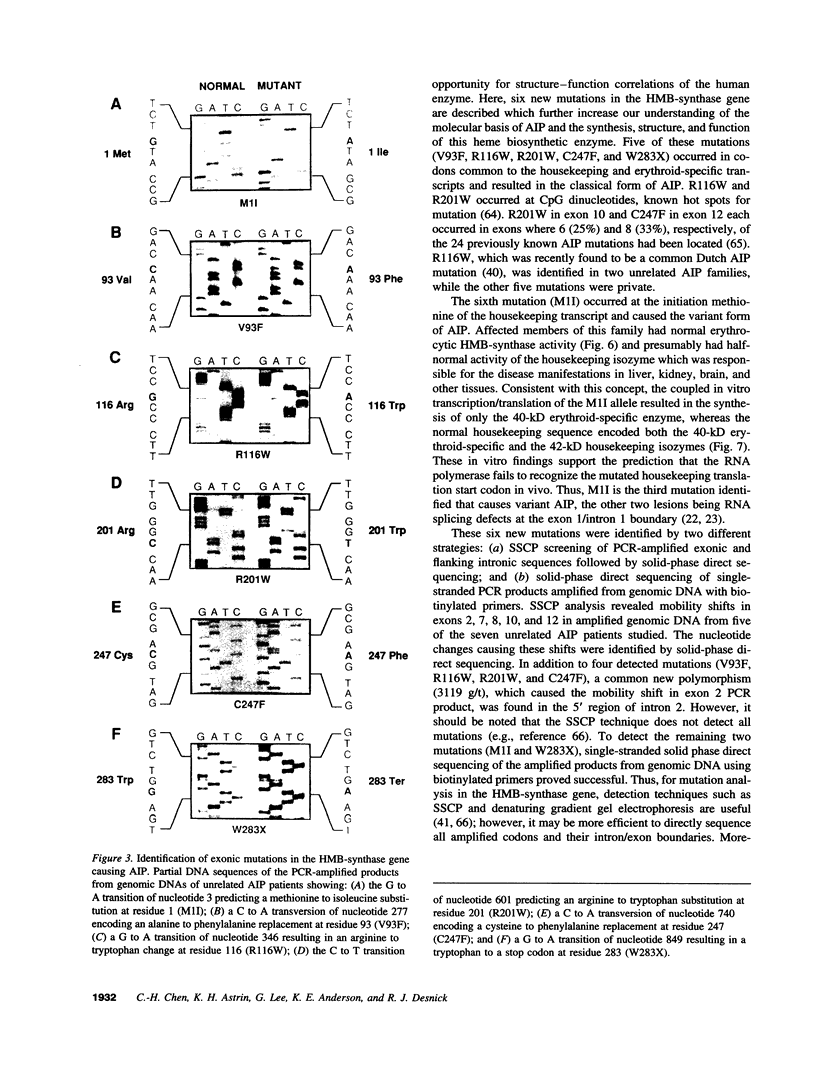
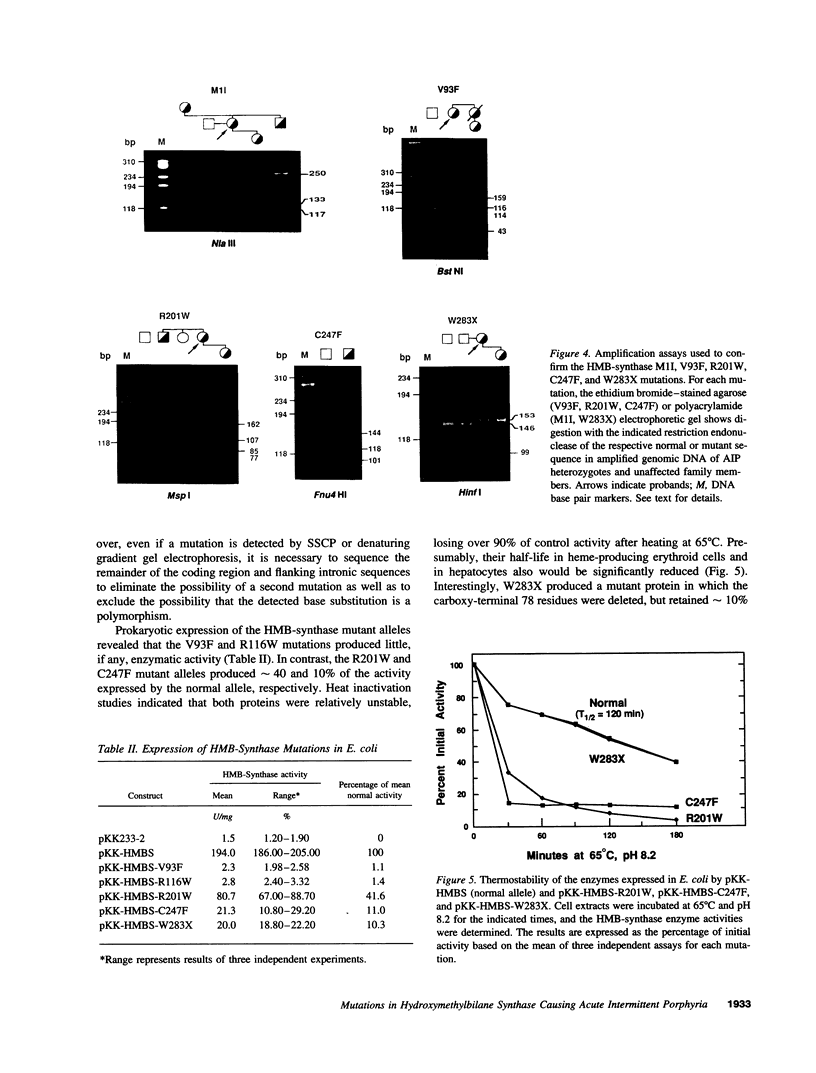
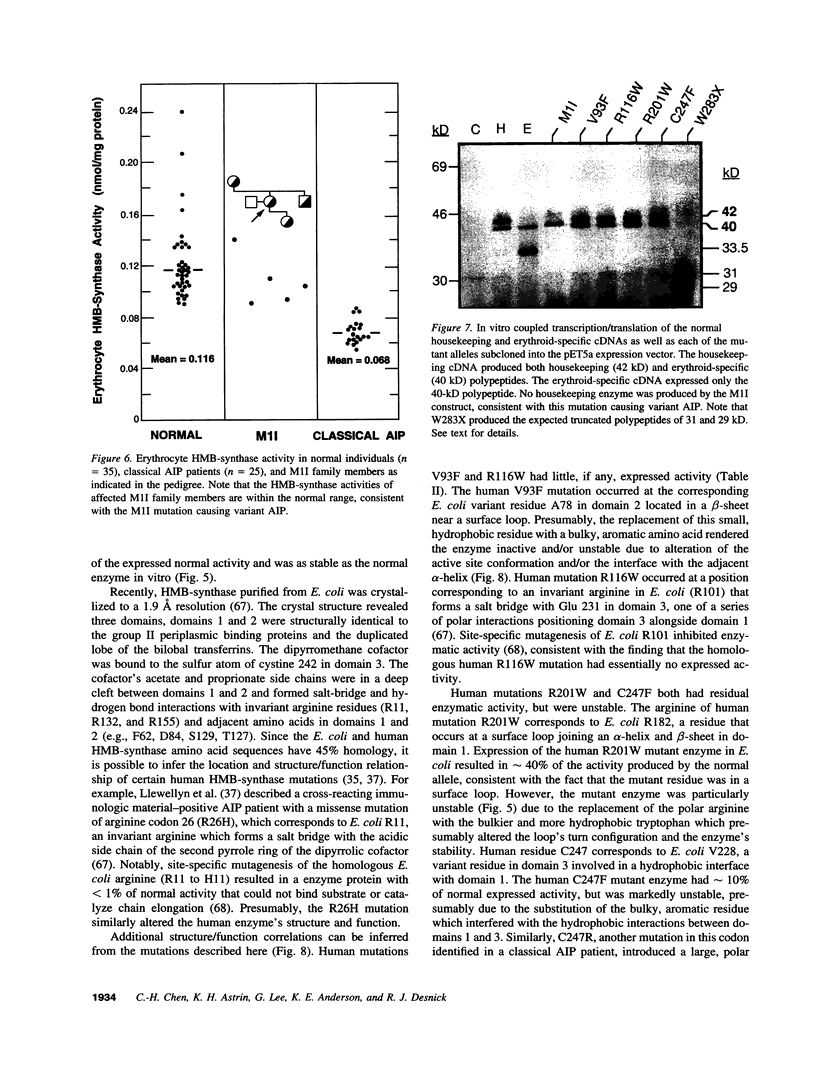
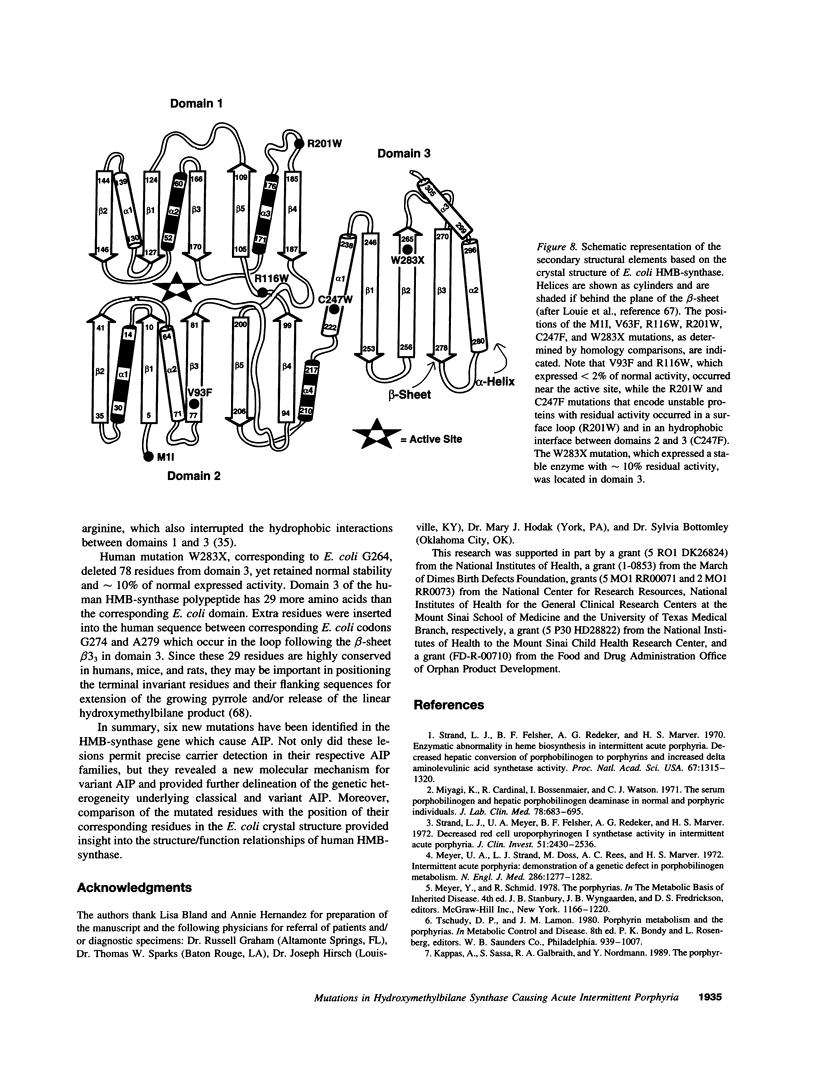
Acute intermittent porphyria (AIP), an autosomal dominant inborn error, results from the half-normal activity of the heme biosynthetic enzyme, hydroxymethylbilane synthase (EC 4.3.1.8). Diagnosis of AIP heterozygotes is essential to prevent acute, life-threatening neurologic attacks by avoiding various precipitating factors. Since biochemical diagnosis is problematic, the identification of hydroxymethylbilane synthase mutations has facilitated the detection of AIP heterozygotes. Molecular analyses of unrelated AIP patients revealed six exonic mutations: an initiating methionine to isoleucine substitution (M1I) in a patient with variant AIP, which precluded translation of the housekeeping, but not the erythroid-specific isozyme; four missense mutations in classical AIP patients, V93F, R116W, R201W, C247F; and a nonsense mutation W283X in a classical AIP patient, which truncated the housekeeping and erythroid-specific isozymes. Each mutation was confirmed in genomic DNA from family members. The W283X lesion was found in another unrelated AIP family. Expression of each mutation in Escherichia coli revealed that R201W, C247F, and W283X had residual activity. In vitro transcription/translation studies indicated that the M1I allele produced only the erythroid-specific enzyme, while the other mutant alleles encoded both isozymes. These mutations provide insight into the molecular pathology of classic and variant AIP and facilitate molecular diagnosis in AIP families.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. A., Gusella J. F. Use of cyclosporin A in establishing Epstein-Barr virus-transformed human lymphoblastoid cell lines. In Vitro. 1984 Nov;20(11):856–858. doi: 10.1007/BF02619631. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Desnick R. J. Purification and properties of uroporphyrinogen I synthase from human erythrocytes. Identification of stable enzyme-substrate intermediates. J Biol Chem. 1980 Mar 10;255(5):1993–1999. [PubMed] [Google Scholar]
- Anderson P. M., Reddy R. M., Anderson K. E., Desnick R. J. Characterization of the porphobilinogen deaminase deficiency in acute intermittent porphyria. Immunologic evidence for heterogeneity of the genetic defect. J Clin Invest. 1981 Jul;68(1):1–12. doi: 10.1172/JCI110223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D., Schafer M., White R. Restriction sites containing CpG show a higher frequency of polymorphism in human DNA. Cell. 1984 Jan;36(1):131–138. doi: 10.1016/0092-8674(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Bonaïti-Pellié C., Phung L., Nordmann Y. Recurrence risk estimation of acute intermittent porphyria based on analysis of porphobilinogen deaminase activity: a Bayesian approach. Am J Med Genet. 1984 Dec;19(4):755–762. doi: 10.1002/ajmg.1320190415. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
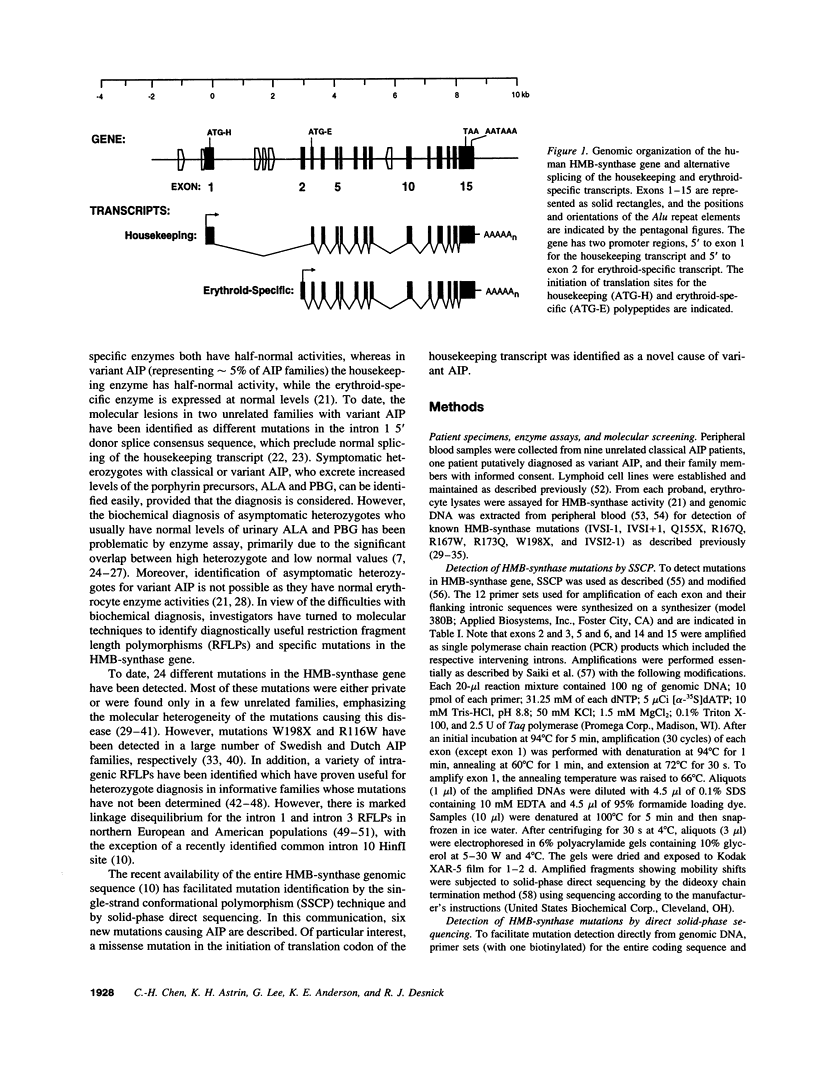
- Chretien S., Dubart A., Beaupain D., Raich N., Grandchamp B., Rosa J., Goossens M., Romeo P. H. Alternative transcription and splicing of the human porphobilinogen deaminase gene result either in tissue-specific or in housekeeping expression. Proc Natl Acad Sci U S A. 1988 Jan;85(1):6–10. doi: 10.1073/pnas.85.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daimon M., Morita Y., Yamatani K., Igarashi M., Fukase N., Ohnuma H., Sugiyama K., Ogawa A., Manaka H., Tominaga M. Two new polymorphisms in introns 2 and 3 of the human porphobilinogen deaminase gene. Hum Genet. 1993 Sep;92(2):115–116. doi: 10.1007/BF00219676. [DOI] [PubMed] [Google Scholar]
- Daimon M., Yamatani K., Igarashi M., Fukase N., Ogawa A., Tominaga M., Sasaki H. Acute intermittent porphyria caused by a G to C mutation in exon 12 of the porphobilinogen deaminase gene that results in exon skipping. Hum Genet. 1993 Dec;92(6):549–553. doi: 10.1007/BF00420937. [DOI] [PubMed] [Google Scholar]
- Delfau M. H., Picat C., De Rooij F., Voortman G., Deybach J. C., Nordmann Y., Grandchamp B. Molecular heterogeneity of acute intermittent porphyria: identification of four additional mutations resulting in the CRIM-negative subtype of the disease. Am J Hum Genet. 1991 Aug;49(2):421–428. [PMC free article] [PubMed] [Google Scholar]
- Delfau M. H., Picat C., de Rooij F. W., Hamer K., Bogard M., Wilson J. H., Deybach J. C., Nordmann Y., Grandchamp B. Two different point G to A mutations in exon 10 of the porphobilinogen deaminase gene are responsible for acute intermittent porphyria. J Clin Invest. 1990 Nov;86(5):1511–1516. doi: 10.1172/JCI114869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnick R. J., Ostasiewicz L. T., Tishler P. A., Mustajoki P. Acute intermittent porphyria: characterization of a novel mutation in the structural gene for porphobilinogen deaminase. Demonstration of noncatalytic enzyme intermediates stabilized by bound substrate. J Clin Invest. 1985 Aug;76(2):865–874. doi: 10.1172/JCI112044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandchamp B., De Verneuil H., Beaumont C., Chretien S., Walter O., Nordmann Y. Tissue-specific expression of porphobilinogen deaminase. Two isoenzymes from a single gene. Eur J Biochem. 1987 Jan 2;162(1):105–110. doi: 10.1111/j.1432-1033.1987.tb10548.x. [DOI] [PubMed] [Google Scholar]
- Grandchamp B., Picat C., Kauppinen R., Mignotte V., Peltonen L., Mustajoki P., Roméo P. H., Goossens M., Nordmann Y. Molecular analysis of acute intermittent porphyria in a Finnish family with normal erythrocyte porphobilinogen deaminase. Eur J Clin Invest. 1989 Oct;19(5):415–418. doi: 10.1111/j.1365-2362.1989.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Grandchamp B., Picat C., Mignotte V., Wilson J. H., Te Velde K., Sandkuyl L., Roméo P. H., Goossens M., Nordmann Y. Tissue-specific splicing mutation in acute intermittent porphyria. Proc Natl Acad Sci U S A. 1989 Jan;86(2):661–664. doi: 10.1073/pnas.86.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandchamp B., Picat C., de Rooij F., Beaumont C., Wilson P., Deybach J. C., Nordmann Y. A point mutation G----A in exon 12 of the porphobilinogen deaminase gene results in exon skipping and is responsible for acute intermittent porphyria. Nucleic Acids Res. 1989 Aug 25;17(16):6637–6649. doi: 10.1093/nar/17.16.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X. F., Lee J. S., Delfau M. H., Grandchamp B. PCR detection of a G/T polymorphism at exon 10 of the porphobilinogen deaminase gene (PBG-D). Nucleic Acids Res. 1991 Apr 25;19(8):1966–1966. doi: 10.1093/nar/19.8.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X. F., de Rooij F., Lee J. S., Te Velde K., Deybach J. C., Nordmann Y., Grandchamp B. High prevalence of a point mutation in the porphobilinogen deaminase gene in Dutch patients with acute intermittent porphyria. Hum Genet. 1993 Mar;91(2):128–130. doi: 10.1007/BF00222712. [DOI] [PubMed] [Google Scholar]
- Gu X. F., de Rooij F., Voortman G., Te Velde K., Deybach J. C., Nordmann Y., Grandchamp B. Detection of eleven mutations causing acute intermittent porphyria using denaturing gradient gel electrophoresis. Hum Genet. 1994 Jan;93(1):47–52. doi: 10.1007/BF00218912. [DOI] [PubMed] [Google Scholar]
- Gu X. F., de Rooij F., Voortman G., Te Velde K., Nordmann Y., Grandchamp B. High frequency of mutations in exon 10 of the porphobilinogen deaminase gene in patients with a CRIM-positive subtype of acute intermittent porphyria. Am J Hum Genet. 1992 Sep;51(3):660–665. [PMC free article] [PubMed] [Google Scholar]
- Gu X. F., de Rooij F., de Baar E., Bruyland M., Lissens W., Nordmann Y., Grandchamp B. Two novel mutations of the porphobilinogen deaminase gene in acute intermittent porphyria. Hum Mol Genet. 1993 Oct;2(10):1735–1736. doi: 10.1093/hmg/2.10.1735. [DOI] [PubMed] [Google Scholar]
- Hultman T., Ståhl S., Hornes E., Uhlén M. Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res. 1989 Jul 11;17(13):4937–4946. doi: 10.1093/nar/17.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. M., Woodcock S. C. Mutagenesis of arginine residues in the catalytic cleft of Escherichia coli porphobilinogen deaminase that affects dipyrromethane cofactor assembly and tetrapyrrole chain initiation and elongation. Biochem J. 1991 Dec 1;280(Pt 2):445–449. doi: 10.1042/bj2800445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppinen R., Peltonen L., Palotie A., Mustajoki P. RFLP analysis of three different types of acute intermittent porphyria. Hum Genet. 1990 Jul;85(2):160–164. doi: 10.1007/BF00193189. [DOI] [PubMed] [Google Scholar]
- Lamon J. M., Frykholm B. C., Tschudy D. P. Family evaluations in acute intermittent porphyria using red cell uroporphyrinogen I synthetase. J Med Genet. 1979 Apr;16(2):134–139. doi: 10.1136/jmg.16.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Anvret M. A PstI polymorphism for the human porphobilinogen deaminase gene (PBG). Nucleic Acids Res. 1987 Aug 11;15(15):6307–6307. doi: 10.1093/nar/15.15.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Anvret M. Identification of the most common mutation within the porphobilinogen deaminase gene in Swedish patients with acute intermittent porphyria. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10912–10915. doi: 10.1073/pnas.88.23.10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Anvret M., Lindsten J., Lannfelt L., Gellerfors P., Wetterberg L., Floderus Y., Thunell S. DNA polymorphisms within the porphobilinogen deaminase gene in two Swedish families with acute intermittent porphyria. Hum Genet. 1988 Aug;79(4):379–381. doi: 10.1007/BF00282182. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Lindsten J., Anvret M. Haplotyping of the human porphobilinogen deaminase gene in acute intermittent porphyria by polymerase chain reaction. Hum Genet. 1990 Feb;84(3):241–243. doi: 10.1007/BF00200567. [DOI] [PubMed] [Google Scholar]
- Levran O., Desnick R. J., Schuchman E. H. Niemann-Pick disease: a frequent missense mutation in the acid sphingomyelinase gene of Ashkenazi Jewish type A and B patients. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3748–3752. doi: 10.1073/pnas.88.9.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn D. H., Kalsheker N. A., Elder G. H., Harrison P. R., Chretien S., Goossens M. A MspI polymorphism for the human porphobilinogen deaminase gene. Nucleic Acids Res. 1987 Feb 11;15(3):1349–1349. doi: 10.1093/nar/15.3.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn D. H., Whatley S., Elder G. H. Acute intermittent porphyria caused by an arginine to histidine substitution (R26H) in the cofactor-binding cleft of porphobilinogen deaminase. Hum Mol Genet. 1993 Aug;2(8):1315–1316. doi: 10.1093/hmg/2.8.1315. [DOI] [PubMed] [Google Scholar]
- Louie G. V., Brownlie P. D., Lambert R., Cooper J. B., Blundell T. L., Wood S. P., Warren M. J., Woodcock S. C., Jordan P. M. Structure of porphobilinogen deaminase reveals a flexible multidomain polymerase with a single catalytic site. Nature. 1992 Sep 3;359(6390):33–39. doi: 10.1038/359033a0. [DOI] [PubMed] [Google Scholar]
- McColl K. E., Moore M. R., Thompson G. G., Goldberg A. Screening for latent acute intermittent porphyria: the value of measuring both leucocyte delta-aminolaevulinic acid synthase and erythrocyte uroporphyrinogen-1-synthase activities. J Med Genet. 1982 Aug;19(4):271–276. doi: 10.1136/jmg.19.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U. A., Strand L. J., Doss M., Rees A. C., Marver H. S. Intermittent acute porphyria--demonstration of a genetic defect in porphobilinogen metabolism. N Engl J Med. 1972 Jun 15;286(24):1277–1282. doi: 10.1056/NEJM197206152862401. [DOI] [PubMed] [Google Scholar]
- Mgone C. S., Lanyon W. G., Moore M. R., Connor J. M. Detection of seven point mutations in the porphobilinogen deaminase gene in patients with acute intermittent porphyria, by direct sequencing of in vitro amplified cDNA. Hum Genet. 1992 Sep-Oct;90(1-2):12–16. doi: 10.1007/BF00210738. [DOI] [PubMed] [Google Scholar]
- Mgone C. S., Lanyon W. G., Moore M. R., Louie G. V., Connor J. M. Detection of a high mutation frequency in exon 12 of the porphobilinogen deaminase gene in patients with acute intermittent porphyria. Hum Genet. 1993 Dec;92(6):619–622. doi: 10.1007/BF00420949. [DOI] [PubMed] [Google Scholar]
- Michaud J., Brody L. C., Steel G., Fontaine G., Martin L. S., Valle D., Mitchell G. Strand-separating conformational polymorphism analysis: efficacy of detection of point mutations in the human ornithine delta-aminotransferase gene. Genomics. 1992 Jun;13(2):389–394. doi: 10.1016/0888-7543(92)90258-t. [DOI] [PubMed] [Google Scholar]
- Mignotte V., Eleouet J. F., Raich N., Romeo P. H. Cis- and trans-acting elements involved in the regulation of the erythroid promoter of the human porphobilinogen deaminase gene. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6548–6552. doi: 10.1073/pnas.86.17.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi K., Cardinal R., Bossenmaier I., Watson C. J. The serum porphobilinogen and hepatic porphobilinogen deaminase in normal and porphyric individuals. J Lab Clin Med. 1971 Nov;78(5):683–695. [PubMed] [Google Scholar]
- Mustajoki P., Desnick R. J. Genetic heterogeneity in acute intermittent porphyria: characterisation and frequency of porphobilinogen deaminase mutations in Finland. Br Med J (Clin Res Ed) 1985 Aug 24;291(6494):505–509. doi: 10.1136/bmj.291.6494.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustajoki P. Normal erythrocyte uroporphyrinogen I synthase in a kindred with acute intermittent porphyria. Ann Intern Med. 1981 Aug;95(2):162–166. doi: 10.7326/0003-4819-95-2-162. [DOI] [PubMed] [Google Scholar]
- Mustajoki P., Tenhunen R. Variant of acute intermittent porphyria with normal erythrocyte uroporphyrinogen-I-synthase activity. Eur J Clin Invest. 1985 Oct;15(5):281–284. doi: 10.1111/j.1365-2362.1985.tb00185.x. [DOI] [PubMed] [Google Scholar]
- Namba H., Narahara K., Tsuji K., Yokoyama Y., Seino Y. Assignment of human porphobilinogen deaminase to 11q24.1----q24.2 by in situ hybridization and gene dosage studies. Cytogenet Cell Genet. 1991;57(2-3):105–108. doi: 10.1159/000133123. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Picat C., Bourgeois F., Grandchamp B. PCR detection of a C/T polymorphism in exon 1 of the porphobilinogen deaminase gene (PBGD). Nucleic Acids Res. 1991 Sep 25;19(18):5099–5099. doi: 10.1093/nar/19.18.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierach C. A., Weimer M. K., Cardinal R. A., Bossenmaier I. C., Bloomer J. R., Blommer J. R. Red blood cell porphobilinogen deaminase in the evaluation of acute intermittent porphyria. JAMA. 1987 Jan 2;257(1):60–61. [PubMed] [Google Scholar]
- Raich N., Mignotte V., Dubart A., Beaupain D., Leboulch P., Romana M., Chabret C., Charnay P., Papayannopoulou T., Goossens M. Regulated expression of the overlapping ubiquitous and erythroid transcription units of the human porphobilinogen deaminase (PBG-D) gene introduced into non-erythroid and erythroid cells. J Biol Chem. 1989 Jun 15;264(17):10186–10192. [PubMed] [Google Scholar]
- Raich N., Romeo P. H., Dubart A., Beaupain D., Cohen-Solal M., Goossens M. Molecular cloning and complete primary sequence of human erythrocyte porphobilinogen deaminase. Nucleic Acids Res. 1986 Aug 11;14(15):5955–5968. doi: 10.1093/nar/14.15.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. The "megaprimer" method of site-directed mutagenesis. Biotechniques. 1990 Apr;8(4):404–407. [PubMed] [Google Scholar]
- Schreiber W. E., Jamani A., Ritchie B. Detection of a T/C polymorphism in the porphobilinogen deaminase gene by polymerase chain reaction amplification of specific alleles. Clin Chem. 1992 Oct;38(10):2153–2155. [PubMed] [Google Scholar]
- Scobie G. A., Llewellyn D. H., Urquhart A. J., Smyth S. J., Kalsheker N. A., Harrison P. R., Elder G. H. Acute intermittent porphyria caused by a C----T mutation that produces a stop codon in the porphobilinogen deaminase gene. Hum Genet. 1990 Oct;85(6):631–634. doi: 10.1007/BF00193588. [DOI] [PubMed] [Google Scholar]
- Scobie G. A., Urquhart A. J., Elder G. H., Kalsheker N. A., Llewellyn D. H., Smyth J., Harrison P. R. Linkage disequilibrium between DNA polymorphisms within the porphobilinogen deaminase gene. Hum Genet. 1990 Jul;85(2):157–159. doi: 10.1007/BF00193188. [DOI] [PubMed] [Google Scholar]
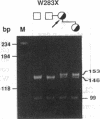
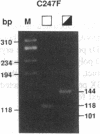
- Sheffield V. C., Beck J. S., Kwitek A. E., Sandstrom D. W., Stone E. M. The sensitivity of single-strand conformation polymorphism analysis for the detection of single base substitutions. Genomics. 1993 May;16(2):325–332. doi: 10.1006/geno.1993.1193. [DOI] [PubMed] [Google Scholar]
- Strand L. J., Felsher B. F., Redeker A. G., Marver H. S. Heme biosynthesis in intermittent acute prophyria: decreased hepatic conversion of porphobilinogen to porphyrins and increased delta aminolevulinic acid synthetase activity. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1315–1320. doi: 10.1073/pnas.67.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand L. J., Meyer U. A., Felsher B. F., Redeker A. G., Marver H. S. Decreased red cell uroporphyrinogen I synthetase activity in intermittent acute porphyria. J Clin Invest. 1972 Oct;51(10):2530–2536. doi: 10.1172/JCI107068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. F., Bishop D. F., Desnick R. J. Human uroporphyrinogen III synthase: molecular cloning, nucleotide sequence, and expression of a full-length cDNA. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7049–7053. doi: 10.1073/pnas.85.19.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. L., Arredondo-Vega F. X., Giampietro P. F., Smith M., Anderson W. F., Desnick R. J. Regional gene assignment of human porphobilinogen deaminase and esterase A4 to chromosome 11q23 leads to 11qter. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5734–5738. doi: 10.1073/pnas.78.9.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner C. A., Yoo H. W., Roberts A. G., Desnick R. J. Congenital erythropoietic porphyria: identification and expression of exonic mutations in the uroporphyrinogen III synthase gene. J Clin Invest. 1992 Feb;89(2):693–700. doi: 10.1172/JCI115637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H. W., Warner C. A., Chen C. H., Desnick R. J. Hydroxymethylbilane synthase: complete genomic sequence and amplifiable polymorphisms in the human gene. Genomics. 1993 Jan;15(1):21–29. doi: 10.1006/geno.1993.1005. [DOI] [PubMed] [Google Scholar]