Abstract
Background
While surveillance for Barrett’s esophagus and other gastrointestinal precancerous conditions is recommended, no analogous guidelines exist for gastric lesions. We sought to estimate the clinical benefits and cost-effectiveness of treatment and endoscopic surveillance to prevent gastric cancer.
Methods
We developed a state-transition decision model for a cohort of U.S. men with a recent incidental diagnosis of gastric precancerous lesions (dysplasia, intestinal metaplasia, or atrophy). Strategies included (1) no treatment or surveillance, and (2) referral for treatment and surveillance, and varied by treatment for dysplastic and cancerous lesions (surgery or endoscopic mucosal resection (EMR)) and surveillance frequency (none, every 10, 5, or 1 years). We restrict the term ‘post-treatment surveillance’ to surveillance in individuals after treatment. Data were based on published literature and databases. Outcomes included lifetime gastric cancer risk, quality-adjusted-life-expectancy, lifetime costs, and incremental cost-effectiveness ratios.
Results
For a 50-year-old cohort of men with dysplasia, lifetime gastric cancer risk was 5.9%. EMR with annual surveillance reduced lifetime cancer risk by 90% and cost $39,800 per quality-adjusted-life-year (QALY). Addition of post-treatment surveillance every 10 years provided little incremental benefit (~5%), but cost >$1 million per QALY. Results were most sensitive to surgical risks and proportion of lesions completely removed with EMR.
Conclusions
EMR with surveillance every 1 to 5 years for gastric dysplasia is promising for secondary cancer prevention, and has a cost-effectiveness ratio that would be considered attractive in the U.S. Endoscopic surveillance of less advanced lesions does not appear to be cost-effective, except possibly for immigrants from high-risk countries.
Keywords: gastric cancer, surveillance, secondary prevention, cost-effectiveness, outcomes research
INTRODUCTION
Although incidence has declined in recent years, gastric cancer remains the second most common cause of cancer-related deaths worldwide, with an estimated 700,000 deaths each year.1 Because of its high case fatality rate, poor prognosis, and limited treatment options, finding effective strategies for primary or secondary prevention of gastric cancer is a public health priority. Over the past thirty years, a better understanding of the development of gastric cancer through a series of precancerous stages and the role of Helicobacter pylori (H. pylori) has shifted the focus from palliative treatment to preventive strategies.2
With the rise in endoscopic utilization, driven in large part by individuals being screened for gastroesophageal reflux disease (GERD),3 the detection of precancerous gastric lesions has increased. These patients may be referred for a second endoscopy and biopsy to confirm the diagnosis, establish the scope of disease, and assess the entire stomach; the benefit of treatment and surveillance are uncertain however. Endoscopic surveillance of individuals with dysplasia, intestinal metaplasia and atrophy in the stomach may improve survival by detecting and removing advanced precancerous lesions before they progress to invasive cancer.4 But while routine surveillance for Barrett’s esophagus is recommended and guidelines on the optimal treatment and surveillance for other gastrointestinal premalignant conditions are available, they are lacking for gastric lesions.5-7 Currently, management of gastric precancerous lesions varies from surgery to annual surveillance for dysplasia8, 9 and from no treatment to surveillance every 3 to 5 years for less advanced lesions.10-14 For individuals with dysplasia, surgical removal of dysplastic lesions can reduce the risk of gastric cancer, but there is reluctance to expose patients to surgery and the associated mortality risk. Treatment with relatively new, less invasive endoscopic alternatives, such as endoscopic mucosal resection (EMR),2 can remove lesions with minimal mortality risk, but may have higher rates of recurrence and incomplete resections which require subsequent surgery.
Because the gastric precancerous process spans several decades, clinical trials on the effectiveness of treatment and surveillance strategies of varying frequency and ages on cancer mortality would require large sample sizes and long follow-up periods. No clinical study can evaluate all possible strategies, thus by synthesizing the best biologic, epidemiologic, and economic data, the use of modeling in a decision analytic framework can assist with decision-making, identify factors most likely to influence outcomes, and highlight where better data are needed.15 In addition, as costs associated with follow-up and the implementation of a surveillance program are important factors for decision makers evaluating alternative public health interventions to consider, the framework can provide insight on the potential cost-effectiveness of different strategies.
Detection and removal of advanced precancerous and cancerous lesions through endoscopic surveillance may reduce the risk of invasive gastric cancer. To contribute to the development of clinical guidelines, we sought to synthesize the best available data and comparatively assess the clinical benefits and cost-effectiveness of alternative strategies for management of individuals with precancerous gastric lesions.
METHODS
Analytic Overview
We used a previously developed natural history model of gastric cancer to estimate the benefits, costs and cost-effectiveness associated with treatment and routine endoscopic surveillance of precancerous lesions (i.e. dysplasia, intestinal metaplasia or atrophy) to reduce gastric cancer incidence and mortality.16 The model was calibrated using a likelihood-based approach to ensure multiple model outputs are consistent with U.S. epidemiologic data on the prevalence of precancerous lesions and incidence of gastric cancer. To reflect the uncertainty surrounding disease natural history, we used a randomly-selected subset of good-fitting parameter sets identified in our model calibration to project the mean (and range) of lifetime risk of cancer, life expectancy, quality-adjusted-life-expectancy and lifetime costs associated with different surveillance strategies. To assess the comparative performance of various strategies, we calculated incremental cost-effectiveness ratios, defined as the additional cost of a specific strategy divided by its additional clinical benefit, compared with the next least expensive strategy. We adopted a societal perspective and discounted all costs and clinical consequences at a rate of 3% per year as recommended by the U.S. Panel of Cost-Effectiveness in Health and Medicine.17 Costs are expressed in 2007 dollars. Sensitivity analyses assessed how key uncertain parameters and assumptions might influence results, including potential differences in underlying disease natural history by race and ethnicity. We also conducted a probabilistic sensitivity analysis using second-order Monte Carlo simulation.
Natural History Model
We have previously described a state-transition natural history model of noncardia intestinal gastric adenocarcinoma.16 After a recent incidental diagnosis of gastric precancerous lesions, (resulting from an upper gastrointestinal endoscopy for GERD, for example), a representative cohort of U.S. men enters the model into the health state corresponding to their diagnosis (Figure 1(a)); for analyses, cohorts consist of individuals with dysplasia only, intestinal metaplasia only or atrophy only, and are simulated separately. Individuals transition between health states at equal monthly intervals to reflect the natural history of disease over time, and are followed throughout their lifetime. While transition probabilities are generally constant, progression of dysplasia to asymptomatic cancer and background all-cause mortality are age-specific. We also developed models for specific race/ethnicity groups to reflect potential subgroup differences in natural history (see Sensitivity Analyses below).
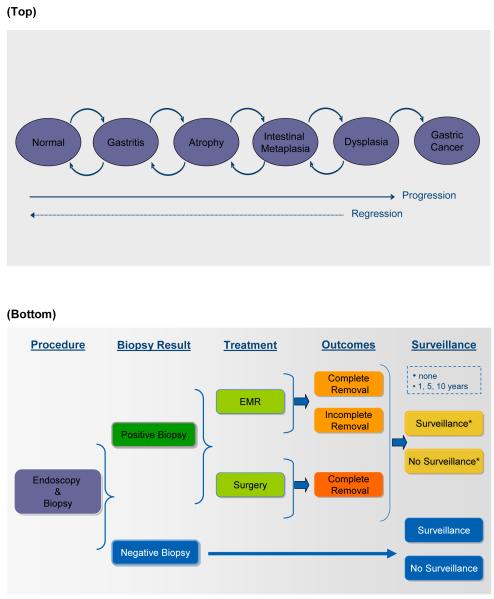
Figure 1.
(a) Gastric cancer natural history model. The model simulates the natural history of noncardia intestinal type gastric carcinogenesis through a series of health states. Each month, individuals can progress and regress among the health states. Individuals who develop cancer face disease-specific mortality rates, and all individuals face an age-dependent risk of dying from other causes. (b) Management options for individuals referred for treatment and surveillance. This figure depicts management options for individuals with a recent incidental diagnosis of gastric precancerous lesions (i.e. dysplasia, intestinal metaplasia, or atrophy), referred for treatment and surveillance. Individuals undergo endoscopy and biopsy to confirm and establish the scope of disease, with subsequent treatment and surveillance based on results. *Referred to as ‘post-treatment surveillance’.
We assumed that (1) H. pylori, acquired in childhood, increases the risk of developing gastritis and atrophy, but does not influence disease progression thereafter;18 (2) precancerous lesions may regress to less advanced lesions;19, 20 and (3) in the absence of other causes of death, gastric cancers become clinically symptomatic within 2 years.21
Model Calibration
To ensure the model is consistent with epidemiologic data, after identifying a plausible range for each natural history parameter in the published literature, we empirically calibrated the model to age-specific data on intestinal metaplasia prevalence and gastric cancer incidence in the U.S.22, 23 We first generated 100,000 unique parameter sets using the ranges defined for each parameter. For each parameter set, we then simulated the model and scored model outcomes produced according to their fit to calibration targets. Likelihood-based methods were used to identify a subset of good-fitting parameter sets, defined as those with goodness-of-fit scores statistically indistinguishable from the score of the best-fitting parameter set (α=0.05). To explicitly incorporate the effect of parameter uncertainty, analyses were conducted with 50 randomly selected good-fitting parameter sets. We reported the mean reduction in lifetime cancer risk as well as the projected range of reduction across the selected parameter sets. For cost-effectiveness analyses, incremental cost-effectiveness ratios were reported as the mean-costs divided by the mean-effects of the selected parameter sets.
Treatment and Surveillance Strategies
For individuals with a recent incidental diagnosis of precancerous gastric lesions, strategies included (1) no treatment or surveillance, and (2) referral for treatment and surveillance, which varied according to treatment modality for dysplastic and cancerous lesions and frequency of surveillance. Figure 1(b) depicts a schematic of management options for referrals. Individuals undergo endoscopy and biopsy to confirm the incidental diagnosis, establish the scope of disease, and assess the entire stomach. In accordance with the updated Sydney classification system,24 we assumed that 5 biopsy specimens from various locations of the stomach were taken, with additional biopsies for visible lesions, to detect and evaluate the presence dysplasia and asymptomatic cancerous lesions. Individuals with positive results for dysplastic or cancerous lesions receive treatment and undergo (1) post-treatment surveillance every 10, 5 or 1 years or (2) no surveillance. Individuals with negative results undergo (1) surveillance every 10, 5 or 1 years or (2) no surveillance. We restrict the term ‘post-treatment surveillance’ to surveillance in individuals who had a positive biopsy result and treatment. Treatment modalities for detected lesions included (1) surgery for all lesions and (2) EMR for lesions limited to the mucosa and surgery for those with submucosal invasion. Analyses were conducted separately for cohorts of men diagnosed with dysplasia, intestinal metaplasia and atrophy.
We made the following assumptions: (1) by clinical definition, all dysplastic lesions are limited to the mucosa and eligible for EMR; (2) a proportion of lesions treated with EMR results in incomplete resections and require additional EMR or surgery; (3) once detected, individuals with gastric cancer receive standard care and do not undergo additional post-treatment surveillance; (4) all individuals experiencing clinically significant endoscopic complications, including perforation and bleeding, require hospitalization and face surgical mortality risks; (5) given the high biopsy sensitivity and specificity for dysplastic and cancerous lesions, an individual’s initial precancerous lesion diagnosis – prior to referral – reflects his/her true disease state;25, 26 and (6) all H. pylori-positive individuals received antibiotic treatment when precancerous lesions were initially detected via endoscopy.
Clinical Data
Table 1 shows select model variables and their plausible ranges.25-50 As clinical data on the effectiveness of EMR to reduce the risk of gastric cancer are unavailable, we based treatment effectiveness on recurrence rates for early gastric cancers from prospective clinical EMR studies and gastric cancer screening programs in Japan.35, 38-44 Specifically, for dysplasia, we assumed that treatment reduced gastric cancer risk, including risk from metachronous gastric lesions (complete resections: RR=0.02; incomplete resections: RR=0.14); for asymptomatic cancer, treatment reduced mortality, from both recurrent tumors and metachronous lesions (complete resections: RR=0.00; incomplete resections: RR=0.53). For less advanced lesions, including gastritis, atrophy or intestinal metaplasia, incorrectly detected as dysplasia or asymptomatic gastric cancer (i.e. false positives), surveillance or treatment did not affect their progression to invasive cancer.
Table 1.
Select model variables: base case and plausible ranges
| Variables | Base Case | Range | Reference |
|---|---|---|---|
| Natural history* | |||
| Disease progression | |||
| Gastritis to atrophy | 0.014-0.091 | ‡ | |
| Atrophy to intestinal metaplasia | 0.018-0.102 | ‡ | |
| Intestinal metaplasia to dysplasia | 0.004-0.017 | ‡ | |
| Dysplasia to invasive cancer† | 0.000-0.012 | ‡ | |
| Disease regression | |||
| Atrophy to gastritis | 0.005-0.046 | ‡ | |
| Intestinal metaplasia to atrophy | 0.002-0.055 | ‡ | |
| Dysplasia to intestinal metaplasia | 0.025-0.072 | ‡ | |
| Five-year gastric cancer survival rate, % | 24.3 | 3.4-61.1 | 27 |
|
| |||
| Clinical, % | |||
| Endoscopic diagnosis for dysplasia and gastric cancer | |||
| Sensitivity | 0.81 | 0.78-0.95 | 25, 26 |
| Specificity | 1.00 | 0.98-1.00 | 25, 26 |
| EMR complications | |||
| Bleeding | 0.017 | 0.012-0.205 | 28 |
| Perforation | 0.002 | 0.001-0.052 | 28 |
| Endoscopy complications | |||
| Bleeding | 0.001 | 0.0002-0.006 | 29, 30 |
| Perforation | 0.001 | 0.0002-0.006 | 29, 30 |
| EMR-eligible lesions | |||
| Dysplasia | 1.00 | 0.95-1.00 | 31-33 |
| Asymptomatic gastric cancer | 0.20 | 0.11-0.29 | 34 |
| Incomplete resection among EMR-eligible lesions | 0.26 | 0.02-0.53 | 35 |
| Require surgery | 0.36 | 0.07-0.50 | 34-36 |
| Surgical mortality risk | 0.06-0.16 | § | 37 |
|
| |||
| Outcomes after treatment | |||
| Dysplasia | |||
| Relative risk of progressing to invasive cancer∥ | |||
| Surgery | 0.06 | 0.07-0.36 | 35, 38-42 |
| Complete EMR | 0.02 | 0.00-0.06 | 35, 38-42 |
| Incomplete EMR | 0.14 | 0.07-0.36 | 35, 38-42 |
| Asymptomatic gastric cancer | |||
| Relative risk of dying from gastric cancer | |||
| Surgery | 0.53 | 0.30-0.70 | 43, 44 |
| Complete EMR | 0.00 | 0.00-0.06 | 35, 38-42 |
| Incomplete EMR | 0.53 | 0.30-0.70 | 43, 44 |
|
| |||
| Direct medical costs, U.S. 2007$** | |||
| Endoscopy (CPT 43239, 89130) | 871 | 435-1740 | 45 |
| EMR (CPT 43236, 43251, 89130) | 1071 | 535-2140 | 45 |
| Bleeding/perforation complications (CPT 43501, DRG 568) | 19,040 | 9,520-38,080 | 45 |
| Surgery | |||
| Dysplasia (CPT 43610, DRG 568) | 18,720 | 9,360-37,440 | 45 |
| Asymptomatic gastric cancer (CPT 43611, DRG 567) | 28,763 | 14,380-57,530 | 45 |
| Gastric cancer treatment | 49,270 | 24,640-98,540 | 46 |
|
| |||
| Indirect costs, U.S. 2007$ | |||
| Median hourly wage | 15.10 | 10.06-23.87 | 47 |
| Lost time, hours | |||
| Endoscopy or EMR | 8 | ‡ | |
| Surgery | 80 | ‡ | |
| Gastric cancer treatment | 351 | 327-376 | 48 |
|
| |||
| Quality of life | |||
| Age-related quality weight, utility | 0.782-0.928 | --- | 49 |
| Utility reductions | |||
| Endoscopy or EMR | -1 day | ||
| Gastrectomy | -2 weeks | ||
| Cancer-related quality weight | |||
| Gastric cancer | 0.49 | 0.17-0.79 | 50 |
EMR = endoscopic mucosal resection; CPT = Current Procedural Terminology; DRG = Diagnosis Related Group
Constant yearly probabilities identified via empirical calibration to epidemiologic data unless otherwise noted.
Age-specific probability.
Base case indicates the range among 50 selected parameter sets used to reflect uncertainty in disease natural history.
Age-dependent. Varied ±50% in sensitivity analysis.
Based on clinical study data for EMR treatment for early gastric cancer.
For sensitivity analysis, we used 0.5-times and 2-times base case value.
Other clinical data, including biopsy test characteristics, risk of clinically significant endoscopic complications, and surgical mortality, were obtained from the published literature. While cancerous growths may be more visible than dysplasia, we conservatively assumed biopsy test characteristics were similar for both dysplastic and cancerous lesions. To estimate quality-adjusted life years (QALYs), age-specific quality of life weights derived from population-based data49 and for symptomatic gastric cancer50 were used, and endoscopic and surgical procedures were assumed to be associated with a 50% reduction in quality of life for 1 day and 2 weeks, respectively.
Cost Data
Direct medical costs associated with strategies were based on 2007 U.S. average Medicare reimbursement rates and the published literature. Costs included physician costs, pathologist costs (for evaluation of 5 biopsies), and facilities and/or hospitalization costs for endoscopic procedures, complications and surgery.45 Cancer treatment costs were based on a published analysis of Surveillance, Epidemiology and End Results (SEER) patients.46 Indirect patient costs were based on estimates of time lost from work and the 2007 median hourly wage from the U.S. Bureau of Labor Statistics.47 We assumed 1 day and 2 weeks of time lost from work for endoscopic procedures and surgery, respectively, and based time lost from work for gastric cancer treatment on an analysis of SEER patients.48
Sensitivity Analyses
We conducted extensive sensitivity analyses to evaluate the impact of alternative assumptions on results. To more fully account for uncertainty, we conducted a probabilistic sensitivity analysis using 1000 second-order Monte Carlo simulations in which each model parameter was simultaneously varied. We assigned distributions based on the nature of the data informing parameter estimates, using beta distributions for probabilities and normal distributions for resource use, indirect costs, and disutility weights. Because unit costs (e.g. cost of endoscopy) were based on Medicare reimbursement rates, we assumed these costs were deterministic and did not ascribe distributions.51
In addition to our base case analysis, we repeated the analysis for select race/ethnicity subgroups to assess the variability of results to potential underlying differences in natural history. For cohorts of non-Hispanic White and Hispanic men, we used natural history parameters identified through calibration of the model to subgroup-specific epidemiologic data.22, 23 As a proxy for immigrants from Asian countries with gastric cancer risk five- to six-fold greater than the U.S., parameters previously identified for a high-risk region of China were used.16
RESULTS
Model Validation
To assess model validity, we compared modeled output with data not used to parameterize or calibrate the model. Dysplasia prevalence (0.9% to 5.4%) approximated published estimates in Western countries (0.5 to 3.8%).31, 32, 52-54 The modeled 10-year gastric cancer risk for a cohort of 65-year olds was 3.6% (range=2.1-6.1%) which approximated a recent estimate in the Netherlands (5.9%).33
Reduction in Lifetime Risk of Gastric Cancer
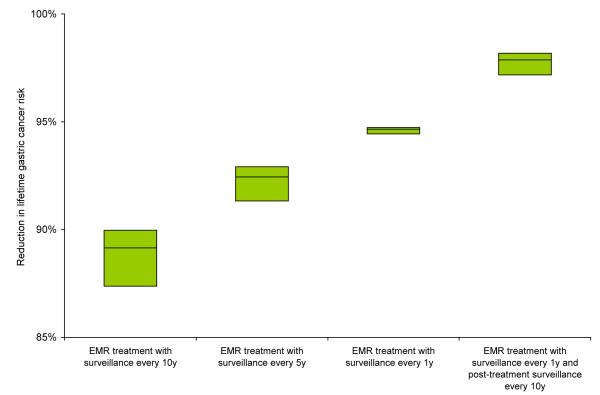
For a cohort of 50-year old men, in the absence of endoscopic surveillance, the lifetime gastric cancer risk was 5.9% for dysplasia, 1.0% for intestinal metaplasia and 0.3% for atrophy. Depending on the frequency of surveillance, strategies with EMR treatment reduced lifetime gastric cancer risk by 77% to 99% for individuals with dysplasia, 60% to 96% for intestinal metaplasia, and 53% to 93% for atrophy. Results were similar for strategies with surgery. Figure 2 depicts the reduction in lifetime gastric cancer risk for select strategies.
Figure 2. Reduction in lifetime gastric cancer risk for select strategies.
The range, indicated by the top and bottom edges of the shaded boxes, represents the minimum and maximum reductions achieved for each strategy for the selected parameter sets. The horizontal line within each box represented the mean reduction.
Cost-Effectiveness of Surveillance of Precancerous Lesions
Cost-effectiveness results for select strategies are shown in Table 2. Compared to no surveillance or treatment, all surgery-based strategies resulted in a loss of life expectancy as a result of the mortality risks associated with surgery. Strategies that included post-treatment surveillance were generally more costly and less effective than strategies with more frequent surveillance. For dysplasia, strategies with EMR and surveillance every 10, 5, or 1 years had incremental cost-effectiveness ratios (ICER) less than $50,000/QALY. For EMR and annual surveillance, the addition of post-treatment surveillance every 10 years increased quality-adjusted life expectancy by 0.5 days (~5%) at a cost of $1,048,000/QALY. All other strategies were either both more costly and less effective (i.e. strongly dominated) or less costly and less cost-effective (i.e. weakly dominated). For intestinal metaplasia, non-dominated strategies included EMR with surveillance every 10 years, with or without post-treatment surveillance every 10 years; both strategies had ICERs that exceeded $500,000/QALY. For atrophy, all strategies were more costly and less effective than no treatment or surveillance as a result of endoscopic and surgical mortality risks.
Table 2.
Cost-effectiveness results for select strategies for 50-year old men with dysplasia or intestinal metaplasia*
| Strategy† | Reduction in lifetime gastric cancer risk‡, % |
Undiscounted life expectancy, years |
Discounted QALE, years |
Incremental discounted QALE, days |
Discounted lifetime costs§, $ |
Incremental discounted costs, $ |
ICER, $ per QALY |
|
|---|---|---|---|---|---|---|---|---|
| Dysplasia | No treatment or surveillance | -- | 28.0839 | 15.1663 | -- | 1,930 | -- | -- |
| EMR with surveillance every 10 y | 89.2 (87.4-90.0) |
28.4888 | 15.3273 | 58.7 | 4,924 | 2995 | 18,600 | |
| EMR with surveillance every 5 y | 92.4 (91.3-92.9) |
28.5093 | 15.3358 | 3.1 | 5,102 | 177 | 20,900 | |
| EMR with surveillance every 1 y | 94.7 (94.4-94.7) |
28.5238 | 15.3416 | 2.1 | 5,333 | 231 | 39,800 | |
| EMR with surveillance every 1 y and post-treatment surveillance every 10 y |
97.9 (97.2-98.2) |
28.5314 | 15.3429 | 0.5 | 6,754 | 1,422 | 1,048,000 | |
|
| ||||||||
|
| ||||||||
| Intestinal metaplasia |
No treatment or surveillance | -- | 28.7110 | 15.4531 | -- | 262.04 | -- | -- |
| EMR with surveillance every 10 y | 59.8 (52.0-63.5) |
28.7303 | 15.4577 | 1.7 | 2756.78 | 2495 | 544,500 | |
| EMR with surveillance every 10 y and post-treatment surveillance every 10 y |
60.8 (54.4-66.5) |
28.7305 | 15.4577 | 0.0 | 2808.64 | 52 | 25,930,000 | |
Strategies shown are those that remained after excluding strategies that were more costly and less effective (i.e. strongly dominated) or less costly and less cost-effective (i.e. weakly dominated) than an alternative strategy. QALE = quality-adjusted life expectancy; ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life year; y = years.
No surveillance assumes cases identified only via symptoms.
Range represents reduction among selected parameter sets.
Costs are expressed in discounted 2007 U.S. dollars.
Sensitivity Analyses
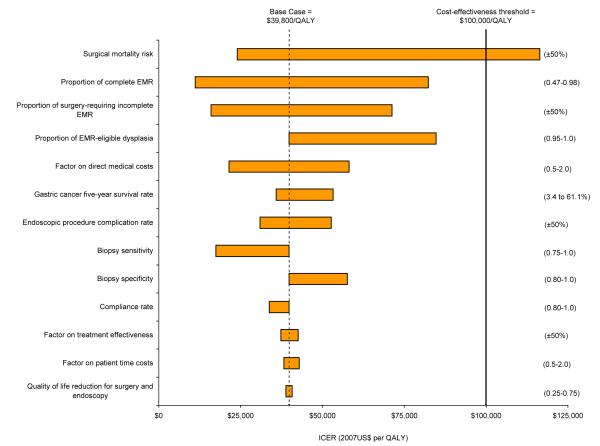
Univariate sensitivity analyses showed that for dysplasia, results for EMR with surveillance every 1 year were most sensitive to the risk of surgical mortality, probability of complete removal of lesions with EMR, and proportion of lesions with incomplete EMR treatment requiring surgery (Figure 3). Results were stable despite varying treatment effectiveness, biopsy test characteristics (including higher sensitivity for cancer), endoscopic complication rates, and indirect costs. For individuals with dysplasia, even if removal of dysplastic lesions only reduced gastric cancer risk by 50% (with no treatment effect on asymptomatic cancerous lesions), EMR with surveillance every 5 years was potentially attractive at a cost of $118,000 per QALY. The strategy was more costly and less effective (i.e. strongly dominated) than EMR with surveillance every 1 year. Surgery-based strategies were consistently dominated.
Figure 3. Sensitivity analysis on select variables for gastric dysplasia.
Graph depicts univariate sensitivity analyses for EMR with surveillance every 1 year. Values in parentheses indicate upper and lower bounds for each variable. The vertical dashed line indicates the incremental cost-effectiveness ratio for the base case. Bold line represents the commonly used $100,000 per QALY cost-effectiveness threshold.
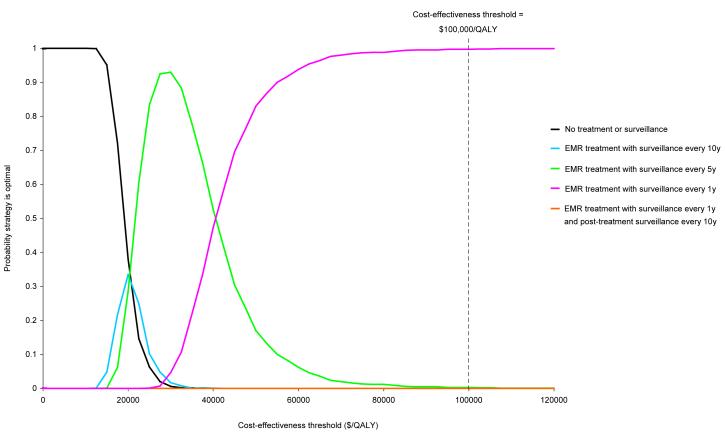
Probabilistic sensitivity analysis estimated that for dysplasia, at a cost-effectiveness threshold of $50,000 per QALY, the probability that EMR with surveillance every 1 year was the optimal strategy (i.e. most cost-effective strategy) was 83.0% (Figure 4). At the $100,000 per QALY threshold, the probability increased to 99.7%. For intestinal metaplasia, the probability that any treatment or surveillance strategy was optimal was zero for both thresholds.
Figure 4. Cost-effectiveness acceptability curves for select strategies for gastric dysplasia.
Results are based on 1000 second-order Monte Carlo simulations in which model variables were simultaneously varied. Dotted line indicates the $100,000 per QALY cost-effectiveness threshold often used as a benchmark in the U.S.
To determine whether results varied by subgroup, we repeated analyses for cohorts of varying age and race/ethnicity (Table 3). For dysplasia, EMR with less frequent surveillance every 10 years was optimal for younger individuals (ICER=$39,800/QALY) while the addition of post-treatment surveillance every year remained unattractive for older individuals (ICER=$1,200,000/QALY). Results were similar for race/ethnicity subgroups, although the ICER for Hispanics was higher (ICER=$70,100/QALY), reflecting the greater uncertainty surrounding disease natural history parameters. For intestinal metaplasia, strategies were consistently unattractive, except among immigrants from the high-risk region of China, for which EMR surveillance every 5 years was potentially attractive (ICER=$80,600/QALY). If 5% of the cohort had dysplasia undetected during their initial diagnosis, the strategy became more attractive for Chinese immigrants (ICER=$54,200/QALY), but all strategies remained unattractive for the other subgroups.
Table 3.
Cost-effectiveness results for select subgroups with dysplasia or intestinal metaplasia
| ICER (2007 U.S.$ per QALY)*† |
|||||||
|---|---|---|---|---|---|---|---|
| Strategy | Base case | Age |
Race/ethnicity |
||||
| 40 | 60 | Non-Hispanic White |
Hispanic | Asian immigrant |
|||
| Dysplasia | EMR with surveillance every 10 y | 18,600 | 39,800 | ‡ | 18,400 | 27,700 | 19,700 |
| EMR with surveillance every 5 y | 20,900 | 110,100 | 12,200 | 20,200 | 32,200 | 20,700 | |
| EMR with surveillance every 1 y | 39,800 | ‡ | 13,500 | 38,400 | 70,100 | 36,200 | |
| EMR with surveillance every 1 y and post-treatment surveillance every 10 y |
1,048,000 | ‡ | 1,203,000 | 986,800 | 15,397,000 | 1,614,400 | |
| EMR with surveillance every 1 y and post-treatment surveillance every 5 y |
‡ | ‡ | 92,610,000 | ‡ | ‡ | ‡ | |
|
| |||||||
|
| |||||||
| Intestinal metaplasia |
EMR with surveillance every 10 y | 544,500 | 615,600 | 1,757,300 | 409,900 | 1,374,500 | 60,400 |
| EMR with surveillance every 10 y and post-treatment surveillance every 10 y |
25,930,000 | 4,295,000 | ‡ | ‡ | ‡ | ‡ | |
| EMR with surveillance every 5 y | ‡ | ‡ | 2,493,800 | ‡ | ‡ | 80,600 | |
| EMR with surveillance every 5 y and post-treatment surveillance every 10 y |
‡ | ‡ | ‡ | 14,502,000 | ‡ | 2,342,800 | |
ICER = incremental cost-effectiveness ratio; y = years.
Strategies in bold indicate optimal strategy given a cost-effectiveness threshold of $100,000/QALY.
Strategy was more costly and less effective (i.e. strongly dominated) or less costly and less cost-effective (i.e. weekly dominated) than an alternative strategy.
DISCUSSION
Endoscopic mucosal resection and routine surveillance of advanced precancerous lesions has the potential to significantly reduce the mortality and morbidity associated with gastric cancer. Using a simulation model of gastric cancer natural history, we estimate that among 50-year old men with dysplasia, approximately one in every twenty will develop gastric cancer in their lifetime, which is similar to the risk of colorectal cancer in the like-aged general U.S. population27 or individuals with Barrett’s esophagus.55 By removing dysplastic and asymptomatic cancerous lesions, EMR with surveillance every 1 to 5 years can reduce gastric cancer risk by 90%, and would be considered cost-effective in the U.S. given its comparability to other interventions society has elected to adopt and considers to be good value for resources invested.56 While surgical removal of detected dysplastic and cancerous lesions can also significantly reduce gastric cancer risk, the associated mortality risks outweigh the benefits on cancer outcomes, and results in a loss in life expectancy compared to no surveillance. Post-treatment surveillance provides little added benefit, with costs exceeding $1,000,000 per QALY.
Surveillance and treatment of less advanced lesions can also potentially reduce cancer risk by 60%, but because of the lower risk of progressing to gastric cancer, does not appear to be cost-effective. This finding is consistent with results from the Netherlands histopathology registry which concluded that gastric cancer risk among individuals with atrophy or intestinal metaplasia does not warrant routine surveillance.33 Our results were insensitive to assumptions on treatment effectiveness. For example, if EMR removed all risk of progressing from intestinal metaplasia to invasive cancer, EMR with surveillance for individuals every 10 years would still be unattractive at a cost of $450,000 per QALY. Similarly, if EMR reduced cancer risk by only 50% for individuals with dysplasia, treatment with surveillance every 5 years would still be considered cost-effective with a cost of $100,000 per QALY. Although EMR is commonly used to treat gastric cancer in Japan, utilization in the U.S. is currently limited. With the effectiveness and cost-effectiveness of surveillance most sensitive to the risk of surgical mortality and likelihood of complete removal of dysplastic lesions, EMR with surveillance every 5 years may be the preferred strategy while expertise for the procedure is developed.
As the risk of gastric cancer varies by race and ethnicity group, we used natural history parameter sets estimated through calibration to disease data for select subgroups to determine whether risk-specific surveillance protocols are warranted. For individuals with dysplasia, we found that EMR with annual surveillance was the optimal strategy across all subgroups, although less frequent surveillance was warranted for younger individuals. While the prevalence of dysplasia is higher among Hispanic men, the risk of progressing to gastric cancer is similar to non-Hispanic White men. This is consistent with epidemiologic studies that suggest exposures early on in life, such as H. pylori infection, are responsible for the majority of disease risk variation by influencing initiation of the precancerous process. One exception, however, was for individuals with intestinal metaplasia from a high risk country, such as China, where gastric cancer risk is considerably higher.1 For these higher-risk individuals, we found that EMR with surveillance every 5 to 10 years could potentially be cost-effective.
Our study has several limitations. First, we used data from multiple sources with varied study designs, and many variables and assumptions are uncertain. We based the effectiveness of EMR to reduce gastric cancer risk among dysplastic individuals on Japanese data for early gastric cancers. Given the diagnostic similarities between the Western definition of dysplasia and the Japanese definition of early gastric cancer,57 these data likely provide reasonable estimates, although additional data are needed to better reflect underlying natural history and etiologic differences.58 The effectiveness of EMR and surgical treatment to reduce the risk of gastric cancer was also based on different studies, and differences may be due to variations in study design. If we assumed that EMR was only as effective as surgery for both complete and incomplete lesions, results remained largely unchanged and all surgery-based strategies were still dominated by less costly and more effective EMR-based strategies. Second, we did not include the benefits of detecting diffuse type gastric cancers during surveillance. We allowed precancerous lesions to regress to less advanced lesions, although data from epidemiologic studies are conflicting. Similarly, we based the risk of mortality from gastric cancer on the overall five-year survival rates for gastric cancers, but surveillance may detect cancers at earlier stages which have more favorable rates and lower treatment costs. Gastric cancers may also remain asymptomatic for longer periods of time.59 With all of these assumptions, we biased our results against surveillance, and may therefore have underestimated the benefits of treatment and surveillance on long-term cancer outcomes. Third, we assumed that treatment had no effect on disease natural history for individuals with precancerous lesions, yet a recent clinical study showed that H. pylori treatment after EMR for gastric cancer may significantly reduce the risk of metachronous cancer.60 If treatment increased the relative risk of regression from atrophy to gastritis by two-fold,61 for individuals with atrophy, all strategies were still dominated; results also remained largely unchanged for individuals with intestinal metaplasia or dysplasia. Fourth, we were unable to stratify intestinal metaplasia and dysplasia by subtype in our model as subtype-specific prevalence data for model calibration were unavailable. Lesion prevalence may also have fallen in recent years, although absolute changes in gastric cancer incidence have been small.62 As better data become available, our model can be refined and updated to reflect these data and provide more accurate estimates. Our findings are also based on a randomly selected subset of good-fitting natural history parameters identified via calibration; model outcomes may differ with other subsets. Lastly, we used natural history parameters calibrated to data for one high-risk region of China to provide insight for Asian immigrants. Since the risk of disease progression may be lower among immigrants compared to individuals in China due to changes in diet and other behavioral or environmental factors, we may have overestimated the benefit of surveillance in this population. As disease risk varies widely in Asia, our findings are not generalizable to all immigrants, but suggest that additional surveillance may be warranted for those from high-risk countries.
EMR treatment with endoscopic surveillance every 1 to 5 years for individuals with gastric dysplasia is promising for secondary cancer prevention, and has a cost-effectiveness ratio that would be considered attractive in the U.S. While more data on the effectiveness of endoscopic treatments are needed, individuals with dysplasia face considerable risk of progressing to invasive cancer and should be closely monitored while additional data are awaited. Although endoscopic surveillance of less advanced metaplastic lesions may substantially reduce gastric cancer risk, it does not appear to be cost-effective, except possibly for immigrants from high-risk countries.
Acknowledgments
Support: Dr. Yeh was funded by the National Cancer Institute (R25-CA057711).
Footnotes
Financial disclosures: None
REFERENCES
- 1.Ferlay J, Bray F, Pisani P, Parkin DM. Cancer Incidence, Mortality and Prevalence Worldwide. IARC Cancer Base No. 5. Version 2.0. IARCPress; Lyon: 2004. GLOBOCAN 2002. [Google Scholar]
- 2.de Vries AC, Haringsma J, Kuipers EJ. The detection, surveillance and treatment of premalignant gastric lesions related to Helicobacter pylori infection. Helicobacter. 2007;12(1):1–15. doi: 10.1111/j.1523-5378.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 3.Richter JE. Gastrooesophageal reflux disease. Best Pract Res Clin Gastroenterol. 2007;21(4):609–31. doi: 10.1016/j.bpg.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute US Department of Health and Human Services, National Institutes of Health; Report of the Stomach/Esophageal Cancer Progress Review Group. 2002
- 5.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103(3):788–97. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 6.Genta RM, Rugge M. Review article: pre-neoplastic states of the gastric mucosa--a practical approach for the perplexed clinician. Aliment Pharmacol Ther. 2001;15(Suppl 1):43–50. doi: 10.1046/j.1365-2036.2001.00110.x. [DOI] [PubMed] [Google Scholar]
- 7.Hirota WK, Zuckerman MJ, Adler DG, Davila RE, Egan J, Leighton JA, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63(4):570–80. doi: 10.1016/j.gie.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Rugge M, Farinati F, Baffa R, Sonego F, Di Mario F, Leandro G, et al. Gastric epithelial dysplasia in the natural history of gastric cancer: a multicenter prospective follow-up study. Interdisciplinary Group on Gastric Epithelial Dysplasia. Gastroenterology. 1994;107(5):1288–96. doi: 10.1016/0016-5085(94)90529-0. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein WM, Goldstein NS. Gastric dysplasia and its management. Gastroenterology. 1994;107(5):1543–5. doi: 10.1016/0016-5085(94)90561-4. [DOI] [PubMed] [Google Scholar]
- 10.Rokkas T, Filipe MI, Sladen GE. Detection of an increased incidence of early gastric cancer in patients with intestinal metaplasia type III who are closely followed up. Gut. 1991;32(10):1110–3. doi: 10.1136/gut.32.10.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whiting JL, Sigurdsson A, Rowlands DC, Hallissey MT, Fielding JW. The long term results of endoscopic surveillance of premalignant gastric lesions. Gut. 2002;50(3):378–81. doi: 10.1136/gut.50.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fennerty MB. Gastric intestinal metaplasia on routine endoscopic biopsy. Gastroenterology. 2003;125(2):586–90. doi: 10.1016/s0016-5085(03)00957-0. [DOI] [PubMed] [Google Scholar]
- 13.West AB, Fischer R, Kapadia C, Genta RM, Wang TC, Lauwers GY. What and whom to treat with metaplasia. J Clin Gastroenterol. 2003;36(Suppl1):S61–62. [Google Scholar]
- 14.Dinis-Ribeiro M, Lopes C, da Costa-Pereira A, Moreira-Dias L. We would welcome guidelines for surveillance of patients with gastric atrophic chronic and intestinal metaplasia! Helicobacter. 2008;13(1):75–6. doi: 10.1111/j.1523-5378.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- 15.Goldie SJ, Goldhaber-Fiebert JD, Garnett GP. Chapter 18: Public health policy for cervical cancer prevention: The role of decision science, economic evaluation, and mathematical modeling. Vaccine. 2006;24(Suppl 3):S155–63. doi: 10.1016/j.vaccine.2006.05.112. [DOI] [PubMed] [Google Scholar]
- 16.Yeh JM, Kuntz KM, Ezzati M, Hur C, Kong CY, Goldie SJ. Development of an empirically calibrated model of gastric cancer in two high-risk countries. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1179–87. doi: 10.1158/1055-9965.EPI-07-2539. [DOI] [PubMed] [Google Scholar]
- 17.Gold MR, Siegel JE, Russel LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. Oxford University Press; New York: 1996. [Google Scholar]
- 18.Kuipers EJ, Uyterlinde AM, Pena AS, Roosendaal R, Pals G, Nelis GF, et al. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345(8964):1525–8. doi: 10.1016/s0140-6736(95)91084-0. [DOI] [PubMed] [Google Scholar]
- 19.Correa P, Haenszel W, Cuello C, Zavala D, Fontham E, Zarama G, et al. Gastric precancerous process in a high risk population: cohort follow-up. Cancer Res. 1990;50(15):4737–40. [PubMed] [Google Scholar]
- 20.You WC, Li JY, Blot WJ, Chang YS, Jin ML, Gail MH, et al. Evolution of precancerous lesions in a rural Chinese population at high risk of gastric cancer. Int J Cancer. 1999;83(5):615–9. doi: 10.1002/(sici)1097-0215(19991126)83:5<615::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Craanen ME, Dekker W, Ferwerda J, Blok P, Tytgat GN. Early gastric cancer: a clinicopathologic study. J Clin Gastroenterol. 1991;13(3):274–83. doi: 10.1097/00004836-199106000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Fennerty MB, Emerson JC, Sampliner RE, McGee DL, Hixson LJ, Garewal HS. Gastric intestinal metaplasia in ethnic groups in the southwestern United States. Cancer Epidemiol Biomarkers Prev. 1992;1(4):293–6. [PubMed] [Google Scholar]
- 23.U.S. Cancer Statistics Working Group U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; Atlanta: United States Cancer Statistics: 1999–2004 Incidence and Mortality Web-based Report. 2007
- 24.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20(10):1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Hosokawa O, Tsuda S, Kidani E, Watanabe K, Tanigawa Y, Shirasaki S, et al. Diagnosis of gastric cancer up to three years after negative upper gastrointestinal endoscopy. Endoscopy. 1998;30(8):669–74. doi: 10.1055/s-2007-1001386. [DOI] [PubMed] [Google Scholar]
- 26.Guarner J, Herrera-Goepfert R, Mohar A, Smith C, Schofield A, Halperin D, et al. Diagnostic yield of gastric biopsy specimens when screening for preneoplastic lesions. Hum Pathol. 2003;34(1):28–31. doi: 10.1053/hupa.2003.3. [DOI] [PubMed] [Google Scholar]
- 27.Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, et al. SEER Cancer Statistics Review, 1975-2000. National Cancer Institute; Bethesda: 2003. [Google Scholar]
- 28.Wang YP, Bennett C, Pan T. Endoscopic mucosal resection for early gastric cancer. Cochrane Database Syst Rev. 2006;(1) doi: 10.1002/14651858.CD004276.pub2. CD004276. [DOI] [PubMed] [Google Scholar]
- 29.Schauer PR, Schwesinger WH, Page CP, Stewart RM, Levine BA, Sirinek KR. Complications of surgical endoscopy. A decade of experience from a surgical residency training program. Surg Endosc. 1997;11(1):8–11. doi: 10.1007/s004649900284. [DOI] [PubMed] [Google Scholar]
- 30.Silvis SE, Nebel O, Rogers G, Sugawa C, Mandelstam P. Endoscopic complications. Results of the 1974 American Society for Gastrointestinal Endoscopy Survey. JAMA. 1976;235(9):928–30. doi: 10.1001/jama.235.9.928. [DOI] [PubMed] [Google Scholar]
- 31.Bearzi I, Brancorsini D, Santinelli A, Rezai B, Mannello B, Ranaldi R. Gastric dysplasia: a ten-year follow-up study. Pathol Res Pract. 1994;190(1):61–8. doi: 10.1016/s0344-0338(11)80497-8. [DOI] [PubMed] [Google Scholar]
- 32.Koch HK, Oehlert M, Oehlert W. An evaluation of gastric dysplasia in the years 1986 and 1987. Pathol Res Pract. 1990;186(1):80–4. doi: 10.1016/S0344-0338(11)81013-7. [DOI] [PubMed] [Google Scholar]
- 33.de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134(4):945–52. doi: 10.1053/j.gastro.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 34.Takekoshi T, Baba Y, Ota H, Kato Y, Yanagisawa A, Takagi K, et al. Endoscopic resection of early gastric carcinoma: results of a retrospective analysis of 308 cases. Endoscopy. 1994;26(4):352–8. doi: 10.1055/s-2007-1008990. [DOI] [PubMed] [Google Scholar]
- 35.Kojima T, Parra-Blanco A, Takahashi H, Fujita R. Outcome of endoscopic mucosal resection for early gastric cancer: review of the Japanese literature. Gastrointest Endosc. 1998;48(5):550–4. doi: 10.1016/s0016-5107(98)70108-7. discussion 54-5. [DOI] [PubMed] [Google Scholar]
- 36.Nagano H, Ohyama S, Fukunaga T, Seto Y, Fujisaki J, Yamaguchi T, et al. Indications for gastrectomy after incomplete EMR for early gastric cancer. Gastric Cancer. 2005;8(3):149–54. doi: 10.1007/s10120-005-0328-5. [DOI] [PubMed] [Google Scholar]
- 37.Finlayson EV, Birkmeyer JD. Operative mortality with elective surgery in older adults. Eff Clin Pract. 2001;4(4):172–7. [PubMed] [Google Scholar]
- 38.Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48(2):225–9. doi: 10.1136/gut.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ida K, Nakazawa S, Yoshino J, Hiki Y, Akamatsu T, Asaki S, et al. Multicentre collaborative prospective study of endoscopic treatment of early gastric cancer. Digestive Endoscopy. 2004;16(4):295–302. [Google Scholar]
- 40.Tanabe S, Koizumi W, Mitomi H, Nakai H, Murakami S, Nagaba S, et al. Clinical outcome of endoscopic aspiration mucosectomy for early stage gastric cancer. Gastrointest Endosc. 2002;56(5):708–13. doi: 10.1067/mge.2002.129085. [DOI] [PubMed] [Google Scholar]
- 41.Makuuchi H, Kise Y, Shimada H, Chino O, Tanaka H. Endoscopic mucosal resection for early gastric cancer. Semin Surg Oncol. 1999;17(2):108–16. doi: 10.1002/(sici)1098-2388(199909)17:2<108::aid-ssu5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 42.Lauwers GY, Ban S, Mino M, Ota S, Matsumoto T, Arai S, et al. Endoscopic mucosal resection for gastric epithelial neoplasms: a study of 39 cases with emphasis on the evaluation of specimens and recommendations for optimal pathologic analysis. Mod Pathol. 2004;17(1):2–8. doi: 10.1038/modpathol.3800012. [DOI] [PubMed] [Google Scholar]
- 43.Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S. Gastric cancer screening and subsequent risk of gastric cancer: a large-scale population-based cohort study, with a 13-year follow-up in Japan. Int J Cancer. 2006;118(9):2315–21. doi: 10.1002/ijc.21664. [DOI] [PubMed] [Google Scholar]
- 44.Miyamoto A, Kuriyama S, Nishino Y, Tsubono Y, Nakaya N, Ohmori K, et al. Lower risk of death from gastric cancer among participants of gastric cancer screening in Japan: a population-based cohort study. Prev Med. 2007;44(1):12–9. doi: 10.1016/j.ypmed.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 45.U.S. Department of Health and Human Services, Center for Medicare & Medicaid Services U.S. Department of Health and Human Services, Center for Medicare & Medicaid Services; Washington, D.C.: Fee Schedule. 2007
- 46.Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100(9):630–41. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 47.Bureau of Labor Statistics, U.S. Department of Labor U.S. Government Printing Office; Washington DC: Occupational Employment and Wages. 2007
- 48.Yabroff KR, Davis WW, Lamont EB, Fahey A, Topor M, Brown ML, et al. Patient time costs associated with cancer care. J Natl Cancer Inst. 2007;99(1):14–23. doi: 10.1093/jnci/djk001. [DOI] [PubMed] [Google Scholar]
- 49.Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making. 2006;26(4):391–400. doi: 10.1177/0272989X06290497. [DOI] [PubMed] [Google Scholar]
- 50.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care. 1998;36(6):778–92. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Briggs AH, Goeree R, Blackhouse G, O’Brien BJ. Probabilistic analysis of cost-effectiveness models: choosing between treatment strategies for gastroesophageal reflux disease. Med Decis Making. 2002;22(4):290–308. doi: 10.1177/0272989X0202200408. [DOI] [PubMed] [Google Scholar]
- 52.Lauwers GY, Srivastava A. Gastric preneoplastic lesions and epithelial dysplasia. Gastroenterol Clin North Am. 2007;36(4):813–29. vi. doi: 10.1016/j.gtc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Camilleri JP, Potet F, Amat C, Molas G. Gastric muscosal dysplasia: Preliminary results of a prospective study of patients followed for periods of up to six years. In: Ming SC, editor. Precursors of Gastric Cancer. Praeger; New York: 1984. [Google Scholar]
- 54.Farinati F, Rugge M, Di Mario F, Valiante F, Baffa R. Early and advanced gastric cancer in the follow-up of moderate and severe gastric dysplasia patients. A prospective study. I.G.G.E.D.--Interdisciplinary Group on Gastric Epithelial Dysplasia. Endoscopy. 1993;25(4):261–4. doi: 10.1055/s-2007-1010310. [DOI] [PubMed] [Google Scholar]
- 55.Sharma P, Falk GW, Sampliner R, Jon Spechler S, Wang K. Management of nondysplastic Barrett’s esophagus: where are we now? Am J Gastroenterol. 2009;104(4):805–8. doi: 10.1038/ajg.2008.75. [DOI] [PubMed] [Google Scholar]
- 56.Neumann PJ, Sandberg EA, Bell CM, Stone PW, Chapman RH. Are pharmaceuticals cost-effective? A review of the evidence. Health Aff (Millwood) 2000;19(2):92–109. doi: 10.1377/hlthaff.19.2.92. [DOI] [PubMed] [Google Scholar]
- 57.Jass JR. Discrepancies between East and West. Cancer. 2000;88(5):969–70. doi: 10.1002/(sici)1097-0142(20000301)88:5<969::aid-cncr2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 58.Naylor GM, Gotoda T, Dixon M, Shimoda T, Gatta L, Owen R, et al. Why does Japan have a high incidence of gastric cancer? Comparison of gastritis between UK and Japanese patients. Gut. 2006;55(11):1545–52. doi: 10.1136/gut.2005.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Everett SM, Axon AT. Early gastric cancer: disease or pseudo-disease? Lancet. 1998;351(9112):1350–2. doi: 10.1016/s0140-6736(98)04365-7. [DOI] [PubMed] [Google Scholar]
- 60.Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372(9636):392–7. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 61.You WC, Brown LM, Zhang L, Li JY, Jin ML, Chang YS, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst. 2006;98(14):974–83. doi: 10.1093/jnci/djj264. [DOI] [PubMed] [Google Scholar]
- 62.de Vries AC, Meijer GA, Looman CW, Casparie MK, Hansen BE, van Grieken NC, et al. Epidemiological trends of pre-malignant gastric lesions: a long-term nationwide study in the Netherlands. Gut. 2007;56(12):1665–70. doi: 10.1136/gut.2007.127167. [DOI] [PMC free article] [PubMed] [Google Scholar]