Abstract
Botulinum neurotoxins (BoNTs) function by delivering a protease to neuronal cells that cleave SNARE proteins and inactivate neurotransmitter exocytosis. Small (14 kDa) binding domains specific for the protease of BoNT serotypes A or B were selected from libraries of heavy chain only antibody domains (VHHs or nanobodies) cloned from immunized alpacas. Several VHHs bind the BoNT proteases with high affinity (KD near 1 nM) and include potent inhibitors of BoNT/A protease activity (Ki near 1 nM). The VHHs retain their binding specificity and inhibitory functions when expressed within mammalian neuronal cells as intrabodies. A VHH inhibitor of BoNT/A protease was able to protect neuronal cell SNAP25 protein from cleavage following intoxication with BoNT/A holotoxin. These results demonstrate that VHH domains have potential as components of therapeutic agents for reversal of botulism intoxication.
Keywords: VHH, Nanobody, Intrabody, Botulinum, Neurotoxin, BoNT, Metalloproteinase
1. Introduction
Botulinum neurotoxins (BoNT) act on the peripheral nervous system to inhibit the release of acetylcholine from pre-synaptic nerve terminals at the neuromuscular junction, causing flaccid paralysis. The holotoxins are about 150 kDa consisting of three domains that are separately responsible for neuron receptor binding, translocation and catalysis. Once internalized into motor neurons, the mode of action of these toxins involves proteolytic cleavage of SNARE proteins that play key roles in neurotransmitter release. SNARE proteolysis is mediated by the 50 kDa metalloproteinase domain of the toxin, also called the light chain (Lc). The proteases for several of the seven known botulinum serotypes, notably BoNT/A and BoNT/B, are remarkably stable once in nerve cell cytosol and intoxication can last for several months before normal function returns. Because of its extreme potency, persistence and relative ease of production, BoNTs are considered among the most serious (CDC Category A) of the current bioterrorism threats.
Antitoxin agents are available that can prevent BoNT intoxication if administered prior to the development of major symptoms. Currently there is no antidote that can reverse the symptoms of intoxication once they have occurred. As a result, botulism patients must be maintained on respirators, often for many months, before motor function eventually returns. Reversal of nerve intoxication must involve inhibition and/or elimination of the protease from intoxicated neurons. Several research teams are working to develop small molecule drugs that inhibit BoNT Lc proteases and reverse intoxication. Biomolecules that bind BoNT proteases with high affinity, particularly those that inhibit its enzyme activity, could also have value in the development of botulism therapeutic agents.
Camelids such as camels, llamas and alpacas produce a class of heavy chain only antibodies (HcAbs) that lack a light chain and thus bind antigens entirely through their VH domain. The VH domain from HcAbs is called the VHH domain. Recombinant VHHs (also called nanobodies) generally express to high levels in a soluble and functional form within microbial host systems (Arbabi Ghahroudi et al., 1997), probably because the domain naturally folds and functions independent of VL interactions. VHHs also generally have improved hydrodynamic properties and stability as compared to conventional recombinant antibodies (Dumoulin et al., 2002; van der Linden et al., 1999). Furthermore, VHHs appear to have an improved ability to bind enzyme active site pockets leading to biomolecular inhibitors of catalytic function (Lauwereys et al., 1998). Evidence is growing that VHHs are often functional as intracellular antibodies termed “intrabodies” when expressed in the reducing environment of eukaryotic cytosol (Jobling et al., 2003; Verheesen et al., 2006). The unique features of VHHs have begun to be exploited for therapeutic and other possible commercial applications (Gibbs, 2005). Here we report the identification and characterization of recombinant VHHs, prepared from alpacas immunized with BoNT/A and BoNT/B proteases, which bind the BoNT proteases with high affinity. Some of the BoNT VHHs potently inhibit BoNT protease function and remain functional when expressed within neurons.
2. Materials and methods
2.1. Preparation of recombinant BoNT/A and BoNT/B proteases
The coding sequences of BoNT/A protease (A-Lc), encoding amino acids 1–448 of BoNT/A holotoxin, and BoNT/B protease (B-Lc), amino acids 1–442 of BoNT/B holotoxin, were synthesized employing codons optimized for expression within Escherichia coli (Midland Certified Reagent Company, Inc.). These DNAs were cloned into pET14b (Novagen) in frame with the hexahistidine coding sequence at the amino terminus. The plasmids were transformed into Rosetta-gami E. coli cells (Novagen) and induced for expression as recommended by the manufacturer. The yield of soluble protein was significantly improved by culturing the cells overnight at 15 °C following induction with IPTG. Recombinant proteases were purified from the soluble cell extract by immobilized metal affinity chromatography using standard procedures. Fusions of A-Lc and B-Lc were also produced to glutathione-S-transferase (GST). For this, the Lc coding regions were introduced into a derivative of the pGEX vector (GE Healthcare) in frame with GST coding DNA. The GST fusion proteins were purified by standard glutathione affinity methods. The recombinant BoNT Lc proteins were >95% pure as estimated by SDS-PAGE and remained soluble and enzymatically active for several months at 4 °C or for at least several years when stored at >1 mg/ml in 50% glycerol at −20 °C.
2.2. Immunization of alpacas with BoNT/A and BoNT/B proteases
Purified recombinant A-Lc was used to immunize two alpacas essentially as previously described (Maass et al., 2007). Two alpacas were purchased locally and maintained in pasture. Alpacas were given four immunizations of recombinant A-Lc at two-week intervals. Following the final boost, B cells were harvested (see below). Twelve months later, the same alpacas were similarly immunized with recombinant B-Lc. The final immunization prior to B cell harvest contained both B-Lc and A-Lc in an effort to boost cross-reactive epitopes.
2.3. Identification of VHHs that bind to BoNT proteases
Two VHH-display libraries were produced for this work. The first library was prepared from B cells obtained from the alpacas following immunization with A-Lc. Procedures for alpaca VHH identification from this library were virtually identical to those we previously reported for another antigen using the HQ2-2 vector (Maass et al., 2007).
The second VHH-display library was prepared from B cells obtained from the same alpacas after immunization with B-Lc and boosting for A-Lc. This library was prepared in the JSC vector generally as described by Sepulveda and Shoemaker (2008). PCR amplification employed the improved primer design as we reported in Maass et al. (2007). The single forward primer used was AlpVh-F1 (GATCGCCGGCCAGKTGCAGCTCGTGGAGTCNGGNGG) and the two reverse primers were AlpVHH-R1 (GATCACTAGTGGGGTCTTCGCTGTGGTGCG) and AlpVHH-R2 (GATCACTAGTTTGTGGTTTTGGTGTCTTGGG). The reverse primers prime mRNA for the short and long hinge VHH coding regions, respectively. Amplification was typically performed with 35 cycles of 95 °C, 1 min; 50 °C, 1 min; 72 °C, 1 min. Amplified VHH cDNA (0.4 kb) was purified, digested with NgoMIV and SpeI and ligated into the similarly digested JSC vector. Using high efficiency E. coli transformation methods, more than 106 independent clones were obtained and pooled to make both VHH-display libraries. At least 18 random clones were picked and characterized by DNA fingerprinting and >90% had inserts of the proper size.
Panning for VHH-displayed phage that binds to A-Lc or B-Lc was done mostly as described previously (Maass et al., 2007) using target protein coated onto single wells of a 12-well plate. Diminishing concentrations of target protein (from 20 to 0.01 μg/ml), reduced incubation times and longer washing times were employed in subsequent panning cycles in an effort to select for phage with higher affinity to the target protein. Bound phage was recovered from wells in two steps. First, 500 μl of a fresh overnight culture of ER2738 E. coli cells were added to the well for 15 min at 37 °C and removed. In the second step, phage remaining on the plastic following the E. coli infection was subjected to an additional elution in 0.2 M glycine pH 2.2 for 10 min. Finally, the phage recovered by low pH elution was neutralized and used to infect the bacteria recovered from the same well in the previous step (15 min at 37 °C). The E. coli were then plated onto ampicillin and tetracycline plates. Phage clones were screened for binding to A-Lc and/or B-Lc by phage ELISA and positive clones were analyzed by BstN1 fingerprinting of the phagemids (Maass et al., 2007) to identify unique clones.
An effort was made to identify phage that bound to both A-Lc and B-Lc. Using the B-Lc library, alternative panning cycles for A-Lc and B-Lc were performed with GST fusion proteins of A-Lc (GST/A-Lc) and B-Lc (GST/B-Lc) as the target and glutathione magnetic beads (Promega) to purify phage bound to the target. Eppendorf tubes and beads were pre-blocked for 30 min at 20 °C with 4% non-fat dry milk in PBS (mPBS). The GST/B-Lc or GST/A-Lc (varying from 10 to 0.01 μg/ml) were mixed with phage and incubated in mPBS for 1 h at 20 °C. The beads (∼5 μl settled) were added and incubated a further 30 min. After ten washes with 1 ml of PBS/0.1% Tween 20, the bound phage was eluted for 15 min at 20 °C with 50 mM glutathione and 50 mM Tris pH 8. The elution was repeated and combined with the first eluate. The eluate pool was added to ER2738 E. coli, incubated 15 min at 37 °C and plated as above. Eluate titers were obtained and compared with titers of phage eluted from beads lacking target protein. Hundreds of individual phage clones were screened by ELISA and positive clones that derived from panning cycles using lower concentrations of target protein or showing evidence of binding to both A-Lc and B-Lc were preferentially selected for further characterization as above.
2.4. Microbial expression of VHHs
Selected VHH coding DNAs were cloned into bacterial expression vectors for expression of soluble recombinant protein mostly as previously described (Maass et al., 2007; Sepulveda and Shoemaker, 2008). Some VHH coding DNAs were also cloned into the pET32b expression vector (Novagen) for cytosolic expression in E. coli Rosetta-gami 2 (DE3)pLacI (Novagen) as a fusion to thioredoxin. All VHHs contained a carboxyl terminal epitope tag for detection, either E-tag or myc tag, and hexahistidine to facilitate purification. In one instance (VHH-B8), an amber codon, TAG, present within the VHH coding region was modified to a glutamine codon, CAG, by site-directed mutagenesis. This was done using the QuikChange site-directed mutagenesis kit (Stratagene) as directed by the manufacturer. The introduction of the desired mutation, without other changes, was confirmed by DNA sequencing.
2.5. FRET-based enzyme assay
The proteolytic activity of recombinant BoNT/A Lc (2 nM) was assayed with the BoTest™ reporter from BioSentinel Pharmaceuticals, consisting of amino acids 141–206 from mouse SNAP25 protein fused to cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP), at a concentration of 0.3 mM. Reaction volumes were 200 ml containing 50 mM HEPES pH 7.1, 2 mM DTT, 0.5 mg/ml BSA, 0.1% Tween 20 and 10 mM ZnCl2. Reaction temperature was 37 °C. A Photon Technology International instrument was used for the assay at excitation wavelength of 437 nm and emission wavelength at 527 and 475 nm. Readings were collected at one point per second during 3 s, 8 s intervals for 15 min. Enzyme activity was calculated from the initial D ratio 527:475 per second, and was between 0.00198 and 0.00204 for the uninhibited reaction.
2.6. BIACore analysis
Solution affinity of VHH-B8 to BoNT/A Lc was measured by a surface plasmon resonance technique using a Biacore (Bia2000) as previously described (Hu et al., 2009). A CM5 sensor chip was immobilized with purified VHH protein via covalent conjugation of the amine groups in VHH to the carboxyl groups on the chip using an amine coupling kit (Biacore). Then BoNT/A Lc was injected at a series of 2-fold dilutions starting from 50 nM over the chip at room temperature in 20 mM Tris–HCl (pH 8.0) and 150 mM NaCl at a flow rate of 10 ml/min. After each injection, the chip surface was regenerated with 10 mM glycine–HCl, pH 2.5. The equilibrium affinity and kinetics of VHH binding to Lc were determined by fitting the sensogram to the 1:1 Langmuir binding model.
2.7. Mammalian cell expression and intoxication
VHH coding DNAs were amplified by PCR and ligated into the mammalian expression vector, pcDNA3.1 (Invitrogen) such that they were fused in frame to an amino terminal yellow fluorescent protein (YFP) coding region. The expression plasmids were also engineered to contain coding DNA for a streptavidin binding peptide (SBP) (Keefe et al., 2001) at the amino terminus, upstream of the YFP. BoNT/A protease was cloned into pcDNA3.1 fused to an amino terminal green fluorescent protein (GFP) domain (GFP/A-Lc) or CFP domain (CFP/A-Lc). A mammalian expression vector encoding an “indicator” protein for monitoring BoNT/A protease function was prepared. The indicator protein was a fusion protein, YFP/SNAP25/CFP, containing an amino terminal YFP domain followed by SNAP25 (amino acids 140-206) and a carboxyl terminal CFP. The coding DNA is cloned into the pcDNA3.1. Plasmid DNAs were prepared and used for transfection of the neuroblastoma cell lines, murine Neuro2a or human M17, using FuGene HD reagent as recommended by the manufacturer (Roche).
M17 (ATCC# CRL-2267) cells were maintained in Dulbecco's Modified Eagle Medium (DMEM) (Gibco) containing 10% fetal bovine serum (FBS) (Gibco). Neuro2a (ATCC# CCL-131) cells were maintained in Minimum Essential Medium Eagle (MEME) (Gibco) plus 10% FBS. For experiments, 2 × 105 cells were seeded onto each well of a 24-well plate and maintained at 37 °C. After 24 h, culture medium was replaced with fresh medium before experimental treatments. For transfection, 0.5 μg of GFP-Lc A or B and 0.5 μg of YFP/B8 or YFP/B10 were mixed in 50 μl of serum free medium. Transfection reagent FuGene HD was added into the plasmid mixture at a ratio of 1:3 (DNA [μg]:Fugene [μl]) and incubated at room temperature for 15 min before the transfection mixture was applied to cells for 24 h. After transfection, cells were collected following trypsin treatment and washed once with 1 ml Dulbecco's phosphate buffered saline (DPBS) for cell extract preparation. Protein extracts were made with 50 μl of lysis buffer (DPBS containing 1× protease inhibitors, 1 mg/ml BSA, 0.1% Triton-X100) and incubated on ice for 30 min.
Prior to cell intoxication, a 50 μl solution of serum-free DMEM was prepared containing BoNT/A (Metabiologics). The BoNT/A mixture was applied to cultured cells containing 0.5 ml fresh culture medium in a well of a 24-well plate (final BoNT/A concentration 10 nM). At various times later, cell pellets were collected and dissolved in 50 μl of sample buffer and boiled for 10 min prior to gel electrophoresis.
2.8. Streptavidin affinity purification
Cell debris and protein extracts from transfected cells were separated by centrifugation at 13,000 rpm for 15 min at 4 °C prior to pull-down experiments. 25 μl of streptavidin beads (Dynabeads® M-280 Streptavidin, Invitrogen) were washed twice with 50 μl of DPBS and once with 50 μl of cell lysis buffer. Washed beads were re-suspended with 50 μl of protein extract and incubated at 4 °C for 16 h with rotation. Protein-bead complexes were washed four times with 100 μl of ice-cold DPBS. Bound proteins were eluted from beads by adding 25 μl of sample buffer [62.5 mM Tris–HCl, pH 6.8, 2% SDS, 10% glycerol and 0.002% bromophenol blue plus 5% beta-mercaptoethanol] and boiling for 5 min prior to gel electrophoresis. Protein eluates were resolved through SDS-PAGE (4–15% gradient gel, 160 × 160 × 1 mm, BioRad).
2.9. Microscopy and co-localization of CFP-BoNT/A-Lc and YFP-VHH
For fluorescent microscopy and studies on co-localization of the CFP/BoNT/A-Lc and YFP/VHH, the proteins were expressed in M17 cells by transfection of the plasmids as described above. Cells were imaged by fluorescent microscopy using a Nikon TE2000 and the images were captured digitally. For CFP fluorescence a 440/30 nm band base emission filter was used and for YFP fluorescence a 530/20 emission filter was used.
3. Results and discussion
3.1. Identification of alpaca VHHs with affinity for BoNT/A and BoNT/B proteases
Two alpacas were immunized with recombinant BoNT/A light chain (A-Lc) protease achieving titers exceeding 106. A VHH-display library was produced from B cells of the immunized alpacas in which the VHH coding DNA was amplified by PCR and ligated into an M13 phage display vector. Several hundred thousand independent clones were obtained, amplified and the phage was panned through multiple cycles for binding to A-Lc. After characterization of more than 100 clones recognizing A-Lc by ELISA, 36 clones with apparently unique BstN1 fingerprints were selected for sequencing. Alignment of these clones together with 17 random alpaca VHHs (not shown) revealed that all A-Lc binding VHHs fell into two clearly distinct homology groups. Four representatives of the first homology group (ALc-A6, D4, E3 and G6, Genbank accession numbers FJ643071-4) were selected for expression based on sequence divergence and tested for A-Lc binding by dilution ELISA. Binding to A-Lc was detected for all four VHHs when diluted to 10 nM or less (not shown). VHH ALc-D4 had the highest apparent affinity and A-Lc could be detected by ELISA when diluted to 0.5 nM. Most of the A-Lc binding VHHs isolated were members of the second VHH homology group. Surprisingly, members of this group always contained a single amber codon within the VHH coding region that was not always at the same amino acid position. Functional VHH display of these VHHs would occur, probably at a lower level, since the phage was produced within an amber suppressor strain of E. coli. A representative of the most frequently obtained VHH sequence in this homology group (ALc-B8, Genbank accession number FJ643070) was selected for further study.
The same two alpacas were immunized about a year later with recombinant BoNT/B Lc (B-Lc) and developed anti-B-Lc titers near 106. Prior to B cell preparation, the animals received a final boost with both recombinant A-Lc and B-Lc. A phage display library was prepared as above and panned for B-Lc binding. Characterization of more than 100 clones found to be positive for B-Lc binding identified 15 unique clones and the VHH coding regions were sequenced. Homology analysis revealed that all clones were contained within two closely related groups. Within each homology group, all unique family members encode VHHs differing by only 1–5 amino acids involving conservative amino acid variations. One representative of each group (BLc-B10 and BLc-C3, Genbank accession numbers GU168771-2) was selected for further study.
In a separate effort, panning cycles were alternately performed with B-Lc and A-Lc to select for clones expressing VHHs that bind to both A-Lc and B-Lc. Following extensive screening, only a single clone was found that expressed phage binding to both A-Lc and B-Lc. Bacteria from this clone were found to harbor two plasmids. One of the plasmids was a typical phagemid encoding a VHH similar to BLc-B10. The second plasmid was also a phagemid but it was unstable in the bacteria and contained a significant deletion that included part of the gene III coding region. This plasmid contained a new VHH coding DNA (ALc-H7, Genbank accession numbers FJ643075) that was specific for A-Lc (see below). Thus, although the panning process selected for a bacterial clone expressing VHH with binding specificity for both A-Lc and B-Lc, the dual specificity was the result of a very unusual clone harboring two phagemids, each encoding a different VHH, each specific for a different Lc serotype. This indicates that single VHHs able to bind to both Lc serotypes were likely not present in our library and are either rare or non-existent within the alpacas.
3.2. Soluble, recombinant VHHs bind A-Lc or B-Lc
DNA encoding the three selected VHHs shown to bind A-Lc (ALc-B8, ALc-D4 and ALc-H7) and the two selected VHHs shown to bind B-Lc (BLc-B10 and BLc-C3) were each cloned into an E. coli expression vector. The amino acid sequences of the VHHs are shown in Fig. 1a. An amber codon present in ALc-B8 at position 7 (Fig. 1a) was changed to a glutamine codon, the amino acid almost always found in VHHs at this position. Each VHH was expressed in E. coli and purified.
Fig. 1.
Characterization of the five selected VHH anti-BoNT Lc binding proteins. (A) Amino acid sequences of the five selected anti-BoNT Lc VHH domains aligned for maximum homology. (B) Dilution ELISA to measure binding of five VHHs to plates coated by 5 ug/ml of either BoNT/A-Lc (left) or B-Lc (right). VHH-B8 (▲), VHH-H7 (●), VHH-D4 (■), VHH-B10 (□), VHH-C3 (○). The x axis represents the VHH concentration and the y axis represents the ELISA signal.
The five selected VHHs were titered by ELISA for their recognition of BoNT A-Lc or B-Lc. As shown in Fig. 1b, VHHs ALc-B8, ALc-D4 and ALc-H7 were entirely specific for A-Lc. ALc-B8 and ALc-H7 have the highest apparent affinity for A-Lc and binding to A-Lc can easily be detected when these VHHs have been diluted to sub-nanomolar concentrations. VHH BLc-B10 and BLc-C3 were entirely specific for B-Lc and BLc-B10 had significantly higher apparent affinity than BLc-C3. Based on this ELISA, BLc-B10 displayed a high apparent affinity for B-Lc that was equivalent or better to that of ALc-B8 and ALc-H7 for A-Lc.
3.3. Two anti-BoNT/A-Lc VHHs potently inhibit the protease activity
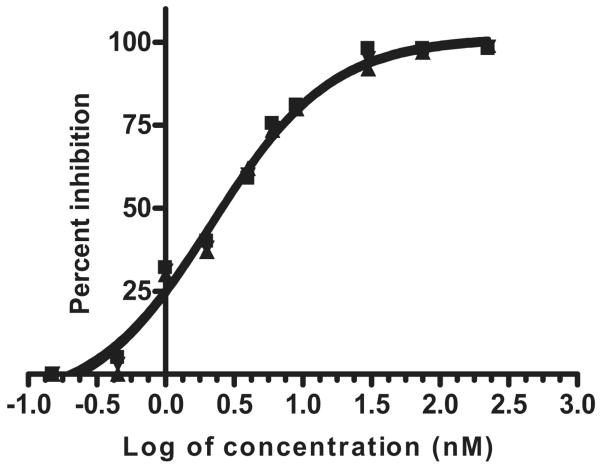
Each of the five anti-BoNT Lc VHHs, purified from recombinant E. coli, was tested for its effect on the proteolytic function of the protease to which it binds. The two B-Lc binding VHHs had no detectable inhibitory effect on B-Lc proteolytic activity (not shown). VHHs ALc-B8 and ALc-H7 were potent inhibitors of A-Lc protease. As shown in Fig. 2, both VHHs inhibited A-Lc protease in a FRET-based SNAP25 cleavage assay. Both VHHs inhibited 2 nM BoNT/A Lc with IC50 values in the range of 1.6–2.5 nM providing evidence for a near stoichiometric inhibition of the protease. VHH ALc-D4 did not significantly inhibit the A-Lc protease activity in the FRET based assay.
Fig. 2.
Two VHHs potently inhibit BoNT/A-Lc protease activity as measured by the FRET assay. Inhibition dose–response curve obtained with Prism 4™ software for VHH ALc-B8 (■) and ALc-H7 (▲) inhibition of BoNT/A-Lc. Values shown are averages of three independent determinations for each Lc. Enzyme activity calculated from the initial Δ ratio 527:475 per second, was 0.00201 ± 0.00003 for the uninhibited reaction. The recombinant VHHs and Lcs were produced in E. coli.
The two VHHs showing strong inhibition of A-Lc protease activity, ALc-B8 and ALc-H7, differ at 31 amino acid positions and the CDR3 differs in size by 4 amino acids. Despite this, B8 and H7 cluster together when aligned with random VHH sequences indicating they may be related (not shown). A competition ELISA was performed in which VHHs having a myc tag were tested for binding to A-Lc in the presence of 10× or 100× excess of VHHs having an E-tag, or vice versa. The data showed that a 100-fold excess of ALc-B8 or ALc-H7 reduced binding of the other to A-Lc by >80% (not shown), demonstrating that these VHHs share the same, or closely apposed, epitopes on A-Lc. VHH ALc-D4 did not compete for the binding of ALc-B8 or ALc-H7 to A-Lc, or vice versa. Similar competition experiments with BLc-B10 and BLc-C3 found that these VHHs do not compete with the other for binding to B-Lc.
3.4. Surface plasmon resonance confirmed high affinity of ALc-B8 and ALc-H7
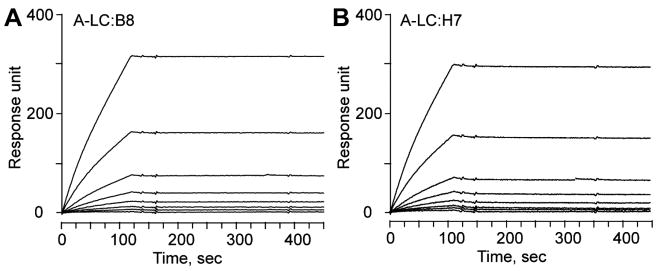
To examine the basis of the potent inhibition of BoNT/A-Lc protease activity by VHH ALc-B8 and ALc-H7, we measured the solution affinity of Lc protease for VHH-immobilized surface-by-surface plasmon resonance. For these experiments, VHHs were purified following expression and secretion from the E. coli periplasm or expression in the cytosol without noteworthy differences in affinity. As shown in Fig. 3, the affinity (KD) of ALc-B8 to BoNT/A Lc was estimated to be 1.06 nM with an association rate, kon = 6.95 × 104 M−1 s−1 and a dissociation rate, koff = 7.4 × 10−5 s−1. The kinetics of the ALc-H7 binding to A-Lc was comparable as ALc-B8 (KD = 0.66 nM, kon = 7.84 × 104 M−1 s−1, koff = 5.22 × 10−5). The binding of these VHHs to BoNT/A-Lc appeared specific as there was no binding to BoNT/B-Lc. The extremely slow dissociation rate of ALc-B8 and ALc-H7 from BoNT/A found through the affinity studies likely explains the near stoichiometric inhibition of BoNT/A protease by these VHHs.
Fig. 3.
Ultra-high affinity binding of VHH-B8 (A) and VHH-H7 (B) to A-Lc measured by surface plasmon resonance. A-Lc was injected over a B8 or H7 coated surface at a series of 2-fold dilutions beginning at 50 nM with the lowest response obtained at 0 nM. The VHHs used in this experiment were expressed as thioredoxin fusion proteins and purified from E. coli cytosol.
3.5. Anti-BoNT Lc VHHs are functional expressed in neuronal cells
The coding DNA for VHH ALc-B8, ALc-D4 and BLc-B10 were inserted into a mammalian expression plasmid, fused in frame to an amino terminal YFP. The ALc-B8 expression plasmid (YFP/B8) or a control plasmid (pcDNA3.1) was co-transfected into Neuro2a neuroblastoma cells together with an expression plasmid for A-Lc fused to CFP (CFP/A-Lc). A day later the cells were lysed and extracts incubated with a FRET-based assay substrate for BoNT/A protease. Cell extracts containing ALc-B8 were strongly inhibitory of A-Lc proteolytic activity as compared to the control extracts (not shown).
YFP/B8 and YFP/B10 were next tested for their BoNT Lc binding function following expression within Neuro2a neuroblastoma cells. Each of the VHH fusion proteins also contains a streptavidin binding peptide at the amino terminus to permit their rapid purification from cell extracts. The YFP/B8 or YFP/B10 fusion proteins were co-expressed in Neuro2a cells with either CFP/A-Lc or CFP/B-Lc. The next day, the VHHs were purified by streptavidin affinity and the co-purification of BoNT Lc was assessed by Western blot (Fig. 4). CFP/A-Lc was observed in the streptavidin bound fraction when co-expressed with YFP/B8 but not when co-expressed with YFP/B10 indicating that the YFP/B8 was associated with A-Lc in the neuronal cell extracts. In a similar streptavidin pull-down experiment, CFP/B-Lc was found associated with YFP/B10 in the streptavidin bound fraction, but not when co-expressed with YFP/B8, showing that YFP/B10 retains B-Lc binding function when expressed in neuronal cells. The amount of Lc that co-purifies with each VHH appears not to exceed the amount of VHH as expected for a 1:1 stoichiometric interaction. For this reason, some of the targeted Lc remains in the unbound fraction.
Fig. 4.
VHH ALc-B8 and BLc-B10 specifically bind to their target Lc expressed in neuronal cell extracts. Neuro2a cells were co-transfected with expression plasmids either for ALc-B8 or BLc-B10 and for BoNT/A-Lc or BoNT/B-Lc as indicated. The Lcs were expressed as fusions to GFP and the VHHs were fused to YFP and a streptavidin binding peptide (see Section 2). After 24 h post-transfection, cell extracts were prepared and the ALc-B8 or BLc-B10 VHHs were incubated with streptavidin beads. The bound or unbound fractions following streptavidin affinity purification were obtained and the samples were resolved by SDS-PAGE and a Western blot was performed with anti-GFP antibody (Santa Cruz) that recognizes both GFP and YFP.
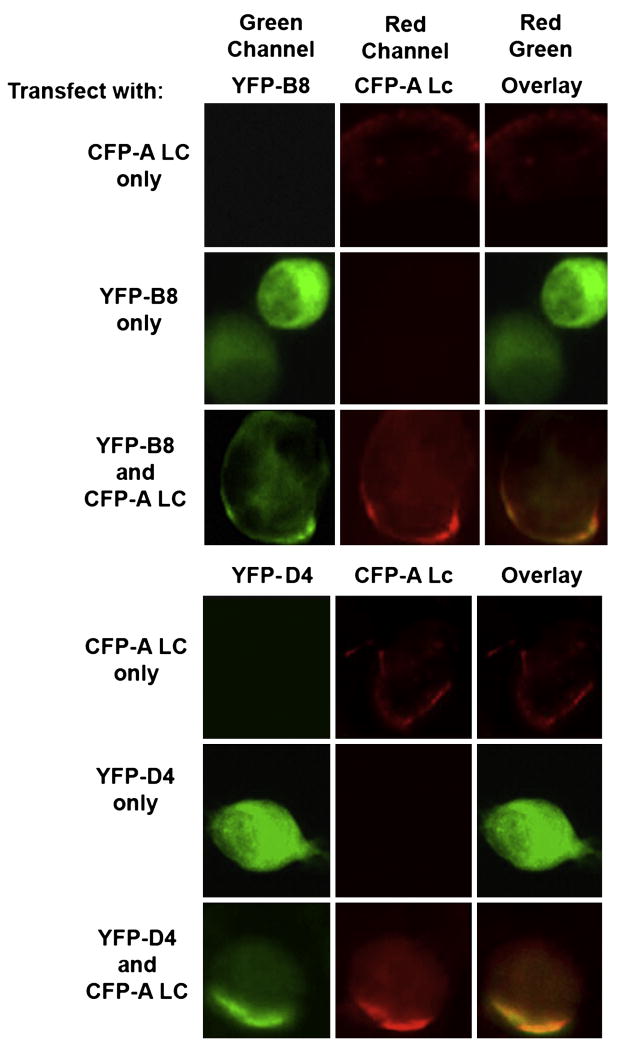
We next tested whether the BoNT/A-Lc binding VHHs would co-localize with the A-Lc within living neuronal cells using a co-localization strategy. It has been reported that BoNT/A Lc, but not BoNT/B Lc, localizes to plasma membranes when expressed within neuronal cells (Fernandez-Salas et al., 2004a,b). As expected, several days following transfection of Neuro2a cells with an expression vector for CFP/A-Lc, CFP fluorescence becomes localized primarily to the plasma membrane (Fig. 5). Transfection of Neuro2a cells with expression plasmids for VHH ALc-B8 (YFP/B8) or ALc-D4 (YFP/D4) in the absence of BoNT/A-Lc results in YFP fluorescence throughout the cell suggesting cytosolic localization. In contrast, when both the A-Lc and the VHHs were co-expressed in Neuro2a, both the CFP and the YFP fluorescence became localized to the plasma membrane after about four days indicating that the VHHs became bound to the A-Lc and co-localized with this membrane-associated protein. These results indicate that the A-Lc binding property remains functional for both A-Lc targeting VHHs, ALc-B8 and ALc-D4, even when they are expressed in the cytosol of neuronal cells.
Fig. 5.
VHHs ALc-B8 and ALc-D4 co-localize with BoNT/A protease when co-expressed within cultured neuronal cells. All images are of M17 neuroblastoma cells transfected with one or two expression plasmids as indicated. After four days post-transfection the cells were viewed by fluorescence microscopy for YFP-VHH (green channel) or CFP-BoNT/A Lc (red channel) to determine the distribution of the fusion proteins. An overlay is shown that represents areas of green and red color co-localization as orange. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.6. VHH ALc-B8 inhibits SNAP25 proteolysis following BoNT intoxication of neuronal cells
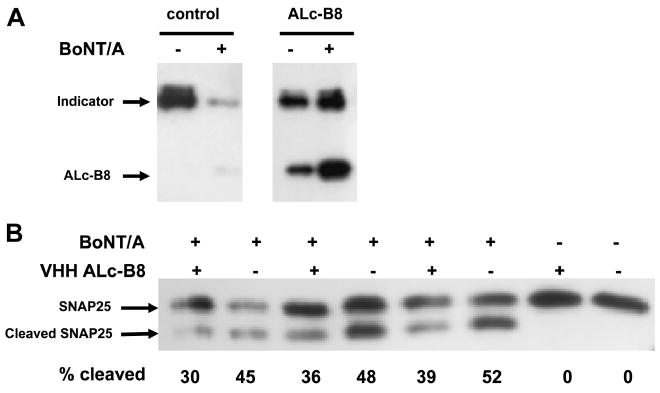
VHH ALc-B8 is a potent inhibitor of A-Lc and remains fully functional as a binding protein expressed within neuronal cells (see above). To determine whether the VHH functions as an inhibitor of A-Lc when the protease is delivered to neurons by BoNT/A holotoxin intoxication, M17 neuroblastoma cells were transfected with an “indicator” expression plasmid for SNAP25 flanked by an amino terminal YFP and a carboxyl terminal CFP. When these cells were intoxicated with BoNT/A, the indicator protein was cleaved and the level of full-size protein substantially diminished (Fig. 6a). When an expression plasmid for VHH ALc-B8 was co-transfected with the plasmid expressing the indicator protein, BoNT/A cleavage of the indicator protein was not detectable, indicating that the A-Lc was inhibited by the VHH.
Fig. 6.
VHH ALc-B8 inhibits BoNT/A protease when co-expressed within cultured neuronal cells. (A) M17 cells were co-transfected with an expression vector encoding the indicator protein, YFP/SNAP25/CFP, and either a control plasmid (−) or an expression plasmid for YFP/ALc-B8 (+) as indicated. After 24 h post-transfection, the cells were exposed to 10 nM BoNT/A (+) or media (−). Cell extracts were prepared 24 h later and the cleavage of indicator by BoNT/A was detected by Western blot with anti-GFP antibody which recognizes both the indicator and the YFP/ALc-B8. The weak band co-migrating with ALc-B8 in the control cells is a BoNT/A cleavage product of the indicator protein. (B) M17 cells were transfected with either a control plasmid (−) or an expression plasmid for YFP/ALc-B8 (+). 24 h later, transfected cells were exposed to 10 nM of BoNT/A (+) or media (−). Cell extracts were prepared 24 h later and BoNT/A cleavage of endogenous SNAP25 was detected by Western blot with anti-SNAP25 antibody (Sigma).
We next tested whether VHH ALc-B8 could protect the endogenous SNAP25 substrate of A-Lc from cleavage by BoNT/A. This is complicated by the fact that only a portion of cells become transfected with the expression plasmid or become intoxicated by BoNT/A. As such, we can expect to see inhibition of BoNT/A only in the overlapping portion of cells that become both intoxicated and transfected. Thus, these studies have substantial background and multiple replicates were performed. As seen in Fig. 6b, cell populations transfected with ALc-B8 expression plasmid have reduced levels of SNAP25 cleavage following intoxication with BoNT/A as compared to cells transfected controls. In this experiment, which included additional replicates, the reduction in cleavage was highly significant (P < 0.01) compared to cells transfected with a control plasmid. Other experiments performed similarly with ALc-B8 transfection into neuroblastoma cells have consistently reproduced this finding (not shown).
4. Conclusions
Botulinum neurotoxin is a NIAID Category A Biodefense Priority Pathogen because of its extreme toxicity and the lack of available therapeutic agents. Biomolecule therapies for botulism can be envisioned that include protein agents delivered to, or expressed within intoxicated neurons that inactivate or accelerate degradation of the cytosolic BoNT protease. Such agents will require simple, high affinity, specific binding agents that recognize the intraneuronal protease responsible for the toxicity. The value of these binding agents increases if they are also capable of potent protease inhibition. VHHs are inherently small (∼ 14 kDa), soluble and stable binding molecules that express well as recombinant fusion proteins (Dumoulin et al., 2002; van der Linden et al., 1999).
Here we show that camelid VHH domains can be identified that bind to BoNT proteases with high affinity, sometimes with potent inhibitory properties, and retain these functions when expressed in mammalian neuronal cell cytosol. In several cases, VHHs that bind to BoNT proteases with KD near 1 nM and with almost undetectable off-rates were found. Two of the highest affinity VHHs inhibit BoNT/A protease with Ki approximately the same as the KD, near 1 nM. It was expected that high affinity VHH inhibitors would be necessary to inhibit BoNT proteases within cells because the protease is present at very low levels within an intoxicated cell. Our results show that VHH ALc-B8 is clearly able to inhibit BoNT protease within intoxicated neuronal cells. If targeted delivery systems can be developed to promote entry of VHHs to intoxicated neurons, anti-BoNT protease VHHs would have promise as components of therapeutic biomolecular agents for reversing the symptoms of botulism.
Acknowledgments
We are grateful to Dr. David Wilson for preparing some of the A-Lc and to Dr. Randall Kincaid for providing the GST fusion proteins to A-Lc and B-Lc. We greatly appreciate the assistance of Tony Pernthaner and Sally Cole for immunizing the alpacas, titering alpaca sera and preparing alpaca cDNA, and Dr. David Maass for preparing the A-Lc VHH-display library. We thank Nick Salzameda for testing the VHHs for inhibition of B-Lc activity. Finally we thank Dr. Patrick Skelly and Dr. Saul Tzipori for helpful discussions. This project was funded in part with Federal funds from the NIAID, NIH, DHHS, under Contract No. N01-AI-30050 and Award Number U54 AI057159. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Abbreviations
- HcAbs
heavy chain only antibodies
- VHH
heavy chain only VH domain
- BoNT
botulinum neurotoxin
- Lc
light chain
- SNARE
soluble NSF attachment protein
- GST
glutathione-S-transferase
- IPTG
isopropyl β-d-1-thiogalactopyranoside
- GFP
green fluorescent protein
- CFP
cyan fluorescent protein
- YFP
yellow fluorescent protein
- SBP
streptavidin binding protein
- CMV
cytomegalovirus
Footnotes
Conflict of interest statement
None declared.
References
- Arbabi Ghahroudi M, Desmyter A, Wyns L, Hamers R, Muyldermans S. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 1997;414:521–526. doi: 10.1016/s0014-5793(97)01062-4. [DOI] [PubMed] [Google Scholar]
- Dumoulin M, Conrath K, Van Meirhaeghe A, Meersman F, Heremans K, Frenken LG, Muyldermans S, Wyns L, Matagne A. Single-domain antibody fragments with high conformational stability. Protein Sci. 2002;11:500–515. doi: 10.1110/ps.34602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Salas E, Ho H, Garay P, Steward LE, Aoki KR. Is the light chain subcellular localization an important factor in botulinum toxin duration of action? Mov Disord. 2004a;19(Suppl 8):S23–S34. doi: 10.1002/mds.20006. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salas E, Steward LE, Ho H, Garay PE, Sun SW, Gilmore MA, Ordas JV, Wang J, Francis J, Aoki KR. Plasma membrane localization signals in the light chain of botulinum neurotoxin. Proc Natl Acad Sci U S A. 2004b;101:3208–3213. doi: 10.1073/pnas.0400229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs WW. Nanobodies. Sci Am. 2005;293:78–83. doi: 10.1038/scientificamerican0805-78. [DOI] [PubMed] [Google Scholar]
- Hu X, Kang S, Chen X, Shoemaker CB, Jin MM. Yeast surface two-hybrid for quantitative in vivo detection of protein–protein interactions via the secretary pathway. J Biol Chem. 2009;284:16369–16376. doi: 10.1074/jbc.M109.001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling SA, Jarman C, Teh MM, Holmberg N, Blake C, Verhoeyen ME. Immunomodulation of enzyme function in plants by single-domain antibody fragments. Nat Biotechnol. 2003;21:77–80. doi: 10.1038/nbt772. [DOI] [PubMed] [Google Scholar]
- Keefe AD, Wilson DS, Seelig B, Szostak JW. One-step purification of recombinant proteins using a nanomolar-affinity streptavidin-binding peptide, the SBP-Tag. Protein Expr Purif. 2001;23:440–446. doi: 10.1006/prep.2001.1515. [DOI] [PubMed] [Google Scholar]
- Lauwereys M, Arbabi Ghahroudi M, Desmyter A, Kinne J, Holzer W, De Genst E, Wyns L, Muyldermans S. Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J. 1998;17:3512–3520. doi: 10.1093/emboj/17.13.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden RH, Frenken LG, de Geus B, Harmsen MM, Ruuls RC, Stok W, de Ron L, Wilson S, Davis P, Verrips CT. Comparison of physical chemical properties of llama VHH antibody fragments and mouse monoclonal antibodies. Biochim Biophys Acta. 1999;1431:37–46. doi: 10.1016/s0167-4838(99)00030-8. [DOI] [PubMed] [Google Scholar]
- Maass DR, Sepulveda J, Pernthaner A, Shoemaker CB. Alpaca (Lama pacos) as a convenient source of recombinant camelid heavy chain antibodies (VHHs) J Immunol Methods. 2007;324:13–25. doi: 10.1016/j.jim.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda J, Shoemaker CB. Design and testing of PCR primers for the construction of scFv libraries representing the immunoglobulin repertoire of rats. J Immunol Methods. 2008;332:92–102. doi: 10.1016/j.jim.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheesen P, deKluijver A, vanKoningsbruggen S, deBrij M, deHaard HJ, van Ommen GJ, van der Maarel SM, Verrips CT. Prevention of oculopharyngeal muscular dystrophy-associated aggregation of nuclear polyA-binding protein with a single-domain intracellular antibody. Hum Mol Genet. 2006;15:105–111. doi: 10.1093/hmg/ddi432. [DOI] [PubMed] [Google Scholar]