Abstract
We tested whether at-risk older adults receiving memory training showed better memory self-efficacy, metamemory, memory performance, and function in instrumental activities of daily living than participants receiving a health promotion training comparison condition. We followed participants for 26 months. The sample was mostly female (79%) and Caucasian (71%), with 17% Hispanics, and 12% African Americans; average age was 75 years and average education was 13 years. The memory training group made greater gains on global cognition and had fewer memory complaints, but both groups generally maintained their performance on the other cognitive measures and IADLs throughout the 24-month study period. Black and Hispanic participants made greater gains than Whites did on some memory performance measures but not on memory self-efficacy. The unexpected finding that minority elders made the largest gains merits further study. This study contributed to the knowledge base of geropsychiatric nursing by providing evidence for an effective psychosocial intervention that could be delivered by advanced practice nurses.
Trial Registration ClinicalTrials.gov NCT00094731
Keywords: Memory Training, Psychosocial Intervention, Memory Performance, Instrumental Activities of Daily Living, Older Adults, Health Training, Memory Self-Efficacy
Older adults are often aware, or are made aware, of cognitive slips, euphemistically called senior moments (Carey, 2006). Such memory complaints have been identified as predictors of mild cognitive impairment (MCI), and they can set off a diagnostic cascade, leading ultimately to a conversion to dementia for some individuals (Comijs, Deeg, Dik, Twisk, & Jonker, 2002; Pearman & Storandt, 2004; Winblad et al., 2004; Zandi, 2004; Zeintl, Kliegel, Rast, & Zimprich, 2006). A recent U.S. study concluded that 22% of adults over the age of 71 might have MCI (Plassman et al., 2008). Since the number of new cases of Alzheimer’s Disease (AD) is expected to increase 3-fold to 13.2 million by 2050, the development of preventive interventions is urgent (Hebert, Scherr, Bienias, Bennett, & Evans, 2003; Morgan et al., 2007).
Individuals’ subjective evaluations of their memories may be cause for concern but numerous studies suggest their perceptions may be modifiable since memory complaints are often associated with anxiety and depression (Cockburn & Smith, 1994; Dux et al., 2008; Fiocco, Wan, Weekes, Pim, & Lupien, 2006; McDougall, Strauss, Holston, & Martin, 1999). Adults at risk for impaired episodic memory and executive function are often aged 65 years and older and have depressive symptoms (Butters et al., 2008; Herrmann, Goodwin, & Ebmeler, 2007; Carlson, Xue, Zhou, & Fried, 2009); Federal Interagency Forum on Aging Related Statistics, 2004. Additional risk factors include living alone and multiple comorbidities, which may lead to social isolation (Finkel, Reynolds, McArdle, & Pedersen, 2007; Michael, Berkman, Colditz, Holmes & Kawachi, 2002; Mendes de Leon, Gold, Glass, Kaplan, & George, 2001; Salthouse, 2003; Wilson et al., 2002).
Memory performance varies as a function of individuals’ self-efficacy beliefs (Artistico, Cervone, & Pezzuti, 2003; Bandura, 1989; Berry, 1989; Berry, West, & Dennehey, 1989; Jennings, & Darwin, 2003; Lachman, Steinberg, & Trotter, 1987; McDougall, 2004). A recent study of older adults found consistent decline in memory self-efficacy with age (McDougall, 2009); chronological age in years was significantly inversely related to memory self-efficacy. Memory self-efficacy may mediate memory performance, and interventions designed to improve self-efficacy may be as important as teaching compensatory mnemonic strategies (Berry & West, 1993; Lachman, Weaver, Bandura, Elliott, & Lewkowicz, 1992; Rebok & Balcerak, 1989; West, Bagwell, & Dark-Freudeman, 2008).
Though not uniformly accepted, mental stimulation, or “use it or lose it” emphasizes that engagement in novelty and innovation may help reverse decline in cognitive function and help an individual maintain cognition (Light, 1991; Salthouse, 1991). This disuse hypothesis is driving the interest in whether brain exercises can prevent, or delay what is considered “normal” decline in memory (Boron, Willis, & Schaie, 2007; Salthouse, 2006; Salthouse, Berish, & Miles, 2002; Verghese et al., 2003). Interventions have included the plasticity-based adaptive cognitive training of the IMPACT study and computer training (Slegers, van Boxtel, & Jolles, 2009; Smith et al., 2009). A recent metanalytic review found no evidence that cognitive intervention programs slow or delay the progression of AD (Papp, Walsh, & Snyder, 2009).
Nevertheless, psychosocial interventions developed to prevent memory decline have also targeted functional ability, loneliness, isolation, physical activity, and the social and/or physical environment (Cattan, White, Bond, & Learmouth, 2005; McDougall, 2002; Oswald, Rupprecht, Gunzelmann, & Tritt, 1996; Phelan, Williams, Penninx, LoGerfo, & Leveille, 2004). The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) trials (Ball et al., 2002; Jobe et al., 2001) found that participants in each intervention group improved their targeted cognitive abilities and maintained these gains for 5 years (Willis et al., 2006). The ACTIVE trials, however did not address the subjective aspects of memory self-efficacy and metamemory (Lachman, Andreoletti, & Pearman, 2006; Scogin, 1997; West et al., 2008).
The ability to perform independent living skills is dependent on intact executive function (Dodge, Du, Saxton, & Ganguli, 2006; Johnson, Lui, & Yaffe, 2007). As age and memory loss increase, cognitively-demanding instrumental activities such as remembering to take medications, managing money, or using the telephone, may become difficult to perform (Royall, Palmer, Chiodo, & Polk, 2005). The focus of the current study was everyday memory—the day-to-day operations of memory in real-world ordinary situations (West & Sinnott, 1991; West, Crook, & Barron, 1992). The goal was to improve everyday memory and ultimately performance of IADLs.
The Cognitive Behavioral Model of Everyday Memory (CBMEM) tested here was derived from Bandura’s Self-Efficacy Theory (Bandura & Locke, 2003; Dellefield & McDougall, 1996; McDougall, 1998; McDougall et al., 1999; McDougall, 2009). We tested the hypothesis that older adults who received the CBMEM-based memory training intervention would show significantly better memory self-efficacy, memory strategies, and memory performance, and better function in IADLs than participants in a health promotion training condition at post-class (2 months after baseline) and over the remaining three assessment points: post-booster (6 months), post-class follow-up (14 months), and end of study (26 months). This paper reports the findings from baseline to post-class and from baseline to end of study.
Methods
Settings and Sample
Independent adults were recruited from a metropolitan area in Central Texas via print and TV media, as well as directly from city-run senior activity centers, churches, health fairs, and festivals. The trainings were implemented at seven sites in the community: four senior centers, a university-based wellness clinic, and two apartment complexes for low-income older adults.
Eligibility criteria included age ≥65 years, ability to speak and understand English, no sensory loss or cognitive impairment, and willingness to participate in the study for 24 months. Sensory loss was determined over the telephone or in person by self-report evaluation of hearing and vision. Visual and hearing acuity were further evaluated at the “in-person” eligibility screening by evaluator observation and by a self-report checklist developed for the study.
Ability to communication in English was assessed using a checklist designed for the study and completed by a team member at the initial screening. We asked seven questions to determine eligibility in this order: Are you able to hear conversations on the phone? Are you able to comprehend English conversations? Are you able to articulate enough to be understood? Are you able to participate in two-sided conversations? Can you decipher concrete and abstract conversational content? Are you able to make an appointment for follow-up testing? Can you repeat back the appointment time and place? If any question was answered incorrectly, the individual was deemed ineligible.
The Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, 1975) was used to screen for cognitive impairment and monitor cognitive decline throughout the study. Only those with scores ≥23 were eligible to participate in the study. However, the eligibility score was reduced to ≥20 at one senior center in order to recruit Hispanics with low education. Four females and one male were recruited using this adjusted screening protocol. In addition, the Controlled Oral Word Association Test (COWAT) from the Multilingual Aphasia Examination (Sumerall, Timmons, James, Ewing, & Oehlert, 1997) and The Trail-Making Test were administered during screening (Reitan, 1958), and participants were required to pass Trails A and/or Trails B at or above the 10th percentile for their age group for inclusion in the study.
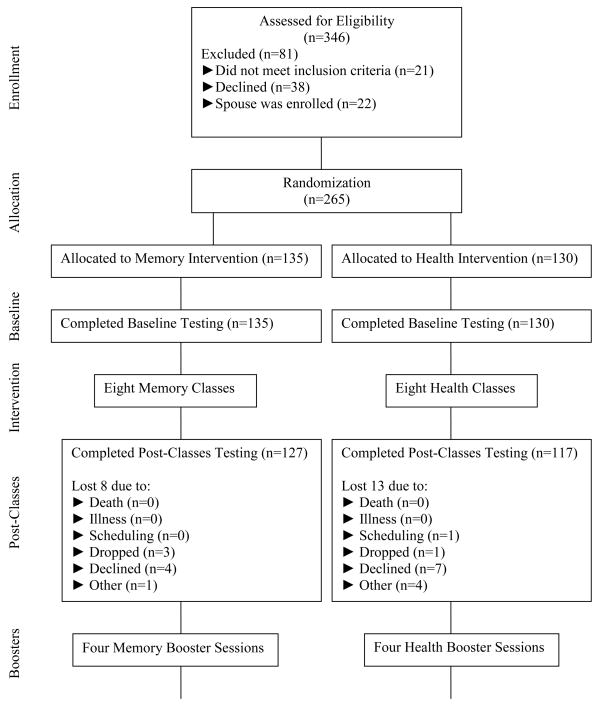
Those with AD or other dementia, Hodgkin’s disease, neuroblastoma, or cancer of the liver, lung, or brain were excluded from the study. Medical eligibility was assessed verbally using a 4-item health status checklist designed for the project. Three hundred forty-six adults were recruited, but 81 did not participate: 21 did not meet inclusion criteria; 38 declined; and 22 were female spouses in a couple, who were excluded so that adequate numbers of males would be represented in the sample (Figure 1). The final sample (N=265) was 72% non-Hispanic White, 17% Hispanic, and 11% African American. The average age was 75 years. The majority of participants were female (77%). The university’s Institutional Review Board approved the study.
Figure 1.
Flow chart
Once participants were screened and consented, they were randomly assigned numeric codes corresponding to either the memory or health promotion training interventions. Randomization was separate for each of the sites. One hundred thirty-five individuals were assigned to memory training and 130 were assigned to health training. Eleven groups met at seven sites in the community. Both training groups participated in 12 sessions.
Memory Training
The CBMEM-based intervention consisted of eight classes and four booster sessions (McDougall, 1998, 1999, 2000, 2002). The memory training was based on the four components of self-efficacy theory: enactive mastery experience, vicarious experience, verbal persuasion, and physiologic arousal. A female septuagenarian role model taught the memory training classes. In each memory class, the first 20 minutes were dedicated to guided practice of stress inoculation that included a progressive muscle relaxation exercise with visualization. Next, homework from an earlier session was discussed. Class topics included memory and health; memory functions and mechanisms; factors affecting memory for people of all ages; memory beliefs and aging; and use of internal and external memory strategies (McDougall, 2009). During the 8-session classroom component of the memory training, 30 minutes of practice with memory strategies were allocated during each class to strengthen enactive mastery experience, the strongest component of self-efficacy. After each memory training class, participants wrote down some aspect of their learning, or an essential point from the class, such as a memory strategy. Each participant was given a plus or minus (0, 1) for the class session. Between the 8-session CBMEM, and the 4 booster sessions, a participant would have a possible score between 0 and 12. On completion of the study, participants received a memory improvement book for older adults entitled Improving Your Memory (Fogler & Stern, 1994).
Health Promotion Training
The comparison treatment included 18 health promotion topics that emphasized successful aging. The classes were interactive health discussions; no memory topics were presented or discussed, no homework was assigned, and no content retention tests were used. The topics were developed based on three focus groups conducted in the community with diverse older adults before the clinical trial (Austin-Wells, Zimmerman, & McDougall, 2003). The topics included alternative medicine, exercise, spirituality and health, weight management, getting the most from your doctor visit, caring for the caretaker, healing foods, drug interactions, osteoporosis, maintaining relationships, health myths, consumer fraud, nutrition, leisure activities, writing family stories, health monitoring tests for home use, and buying drugs in foreign countries. A middle-aged mature male nursing student with 20 years of experience taught the classes. Learning was not assessed in the health training classes.
Booster Sessions
In the booster sessions an additional 8 hours of classroom time were delivered to both the memory and health groups over the 3 months following the initial training. Four 2-hour sessions met weekly for 1 month. For the intervention group, the booster training sessions provided a transitional experience between classroom work on memory improvement and practical applications. The booster sessions were designed to boost learning, enhance retention, and assist with the transfer of memory strategies to everyday life. The emphasis was on practical everyday memory strategies needed for IADLs. Individuals were provided opportunities to practice strategies that could be incorporated into their everyday activities and routines in order to maintain IADLs. The health training group received training in new areas of health promotion not previously presented in their earlier 8 sessions.
Measures
All cognitive performance and self-report measures were administered at baseline, post-class (2 months after baseline), post-booster (6 months), post-classroom follow-up (14 months), and end of study (26 months). Cognitive performance was tested with the MMSE (Folstein et al., 1975), which was used as a global measure to monitor potential cognitive decline throughout the study.
Verbal memory performance was tested with the Hopkins Verbal Learning Test-Revised (HVLT-R), which assesses immediate recall, delayed recall, and recognition memory (Brandt, 1991). Test/retest correlation was .66 for the Delayed Recall Subscale, which was used in these data analyses. Visual memory performance was measured with the Brief Visuospatial Memory Test-Revised (BVMT-R), which asks the individual to reproduce a series of geometric designs (Benedict, 1997). Test/retest correlation was .72.
The Rivermead Behavioural Memory Test (RBMT), which was used to test everyday memory bridges laboratory-based measures of memory and assessments obtained by self-report and observation. The standardized profile score (SPS) has a possible range from 0 to 24 and is sometimes interpreted with cut-off points for four groups of memory function: normal (22–24), poor (17–21), moderately impaired (10–16), and severely impaired (0–9). Alpha reliability was .73 (Cockburn & Smith, 1989; Wilson, Cockburn, Baddeley, & Hiorns, 1989).
The Direct Assessment of Functional Status (DAFS) was used to assess ability to function with IADLs. The DAFS measures performance in test time orientation, communication abilities, transportation, and financial, shopping, eating, and dressing/grooming skills (Loewenstein et al., 1989, 1992). The DAFS demonstrated high interrater and test-retest reliabilities for patients at a memory disorder clinic (English- and Spanish-speaking) and normal controls. Alpha reliability was .79.
Memory self-efficacy was measured by the Memory Self Efficacy Questionnaire (MSEQ), which asks respondents to predict their performance level and estimate their strength and confidence in performing 10 everyday tasks (Berry, et al., 1989). The 35-item version of the MSEQ was used; alpha reliability was .95.
The Spielberger State-Trait Anxiety Inventory (STAI) was used to measure anxiety. The STAI was developed to differentiate the temporary condition of “state anxiety” and the chronic condition of “trait anxiety” in adults (Spielberger, Gorsuch, & Lushene, 1970). Alpha reliabilities were .85 and .89.
The Center for Epidemiologic Studies Scale (CES-D), which emphasizes somatic complaints (rated on a 4-point Likert scale), was used to evaluate depressive symptoms (Radloff & Teri, 1986). In older adults, reliability coefficients were from .85 to .91. Alpha reliability in this study was .78.
The Metamemory in Adulthood Questionnaire (MIA) was used to measure aspects of memory knowledge (Dixon, Hultsch, & Hertzog, 1988). The MIA consists of 108 statements in seven subscales, and responses are rated on a 5-point Likert scale. Within the Strategy subscale (18 items), we differentiated how often participants used both internal memory strategies (elaboration, rehearsal, etc.), and external strategies (lists, notes, etc.) to enhance their everyday memory function. Alpha reliabilities were .78 and .77, respectively. Memory complaints were measured with a revised MIA based on a conceptual item-analysis and a factor analysis of 690 older adults. The revised MIA complaint subscale consists of 21 items and captures individuals’ perceptions of their memory abilities as generally stable or subject to long-term decline. A higher average item score (> 2.5) indicates greater stability and fewer complaints about failing memory (McDougall, Becker, & Arheart, 2006). Mean scores range from 0 to 5.. Alpha reliability was .91.
Statistical Analysis
Hierarchical linear models (HLM) were used to test the efficacy of the intervention (Bryk & Raudenbush, 1992). Age, education, and racial/ethnic group were included as covariates. Number of classes attended was also entered as a covariate, to control for dosage effects. Two HLM analyses were conducted for each outcome measure: one to evaluate treatment and covariate effects, including effects of ethnicity, from baseline to the end of classes, and a similar analysis to evaluate effects from baseline to end of study. The three-level analysis models included repeated measures at Level 1. At Level 2, participants were modeled within sites. Since time was included as a Level 1 explanatory variable and the treatment variable was entered in the model at Level 2 for the gains (as well as in the model for initial status), the model tested whether the “treatment” or “control” group made greater gains across time.
In the Level III model, between-site variables were identified. Because of the limited number of sites, we did not attempt to explain variation across sites, but controlled for it in the analyses. We examined relative gains from beginning to end of classes and from beginning to end of the 26-month study. Other analyses, including all five time points and allowing for a non-linear trajectory, yielded similar results. For each of the analyses in which the memory treatment effect was not statistically significant, we performed an additional HLM analysis that did not include the treatment effect or covariates. The purpose was to examine the direction and statistical significance of overall change over time from baseline to end of classes or baseline to end of study when the memory group did not differ significantly from the health group.
Results
Baseline Comparisons
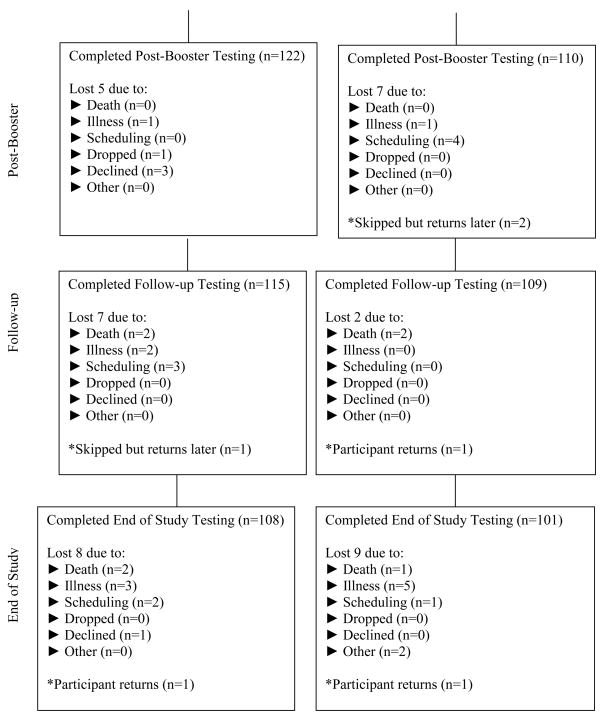
The analytic sample consisted of 265 randomized participants: 135 in the memory training and 130 in the health promotion training (Figure 1). Eight deaths occurred during the study unrelated to the protocol. Ninety-five percent of participants completed the memory training; 87% completed the health promotion training (six or more of the eight training sessions). At the final testing, 108 and 101 individuals were in the memory training and the health promotion groups, respectively. Eighty-four percent in the memory training and 78% in the health promotion completed three or more booster sessions. The memory and health groups did not differ significantly at baseline on either the outcome measures or demographics (t-test). When sex, education, age, MMSE score, health status, and memory vs. health training group were entered simultaneously into a logistic regression as predictors of finishing the study, being female (p = .03) and reporting better perceived health (p = .04) were the only significant predictors. Cronbach’s alphas ranged from .73 to .95. Means and standard deviations for outcome measures are in Table 2.
Table 2.
Descriptive Statistics from Baseline to End of Study
| Baseline (0 Months) | Post-Classes (2 Months) | Post-Boosters (6 Months) | Follow-up (14 Months) | End of Study (26 Months) | ||
|---|---|---|---|---|---|---|
| Score Range | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Brief Visuospatioal Memory Test | ||||||
| Intervention | 0–100 | 44.07± 13.50 | 43.97 ± 13.79 | 43.50 ± 13.02 | 42.21 ± 13.67 | 41.46 ± 14.05 |
| Comparison | 0–100 | 44.03 ± 15.04 | 43.15 ± 14.78 | 43.85 ± 14.44 | 40.83 ± 15.22 | 42.90 ± 16.57 |
| Direct Assessment of Functional Status | ||||||
| Intervention | 0–89 | 83.05 ± 4.13 | 83.81 ± 4.30 | 83.27 ± 4.98 | 83.43 ± 5.21 | 82.57 ± 5.50 |
| Comparison | 0–89 | 81.95 ± 6.30 | 83.11 ± 6.00 | 82.66 ± 6.23 | 83.13 ± 5.99 | 82.08 ± 6.93 |
| Hopkins Verbal Learning Test | ||||||
| Intervention | 0–100 | 49.64 ± 11.12 | 50.31 ± 10.08 | 51.49 ± 9.09 | 51.63 ± 10.11 | 50.48 ± 10.87 |
| Comparison | 0–100 | 47.45 ± 11.27 | 48.23 ± 11.98 | 48.41 ± 11.25 | 49.00 ± 12.15 | 48.28 ± 13.28 |
| Mini Mental State Exam | ||||||
| Intervention | 0–30 | 28.05 ± 2.06 | 28.31 ± 1.82 | 28.49 ± 1.86 | 28.33 ± 2.15 | 28.04 ± 2.27 |
| Comparison | 0–30 | 27.98 ± 2.22 | 27.77 ± 2.33 | 28.19 ± 1.83 | 27.89 ± 2.39 | 27.95 ± 2.15 |
| Rivermead Behavioural Memory | ||||||
| Intervention | 0–24 | 19.08 ± 3.44 | 20.08 ± 3.18 | 18.36 ± 4.00 | 20.20 ± 3.96 | 19.13 ± 3.93 |
| Comparison | 0–24 | 18.31 ± 4.60 | 19.07 ± 4.57 | 18.18 ± 4.71 | 19.77 ± 3.95 | 18.47 ± 4.78 |
| Memory Complaints | ||||||
| Intervention | 1–5 | 2.72 ± .62 | 2.93 ± .60 | 2.94 ± .61 | 2.90 ± .66 | 2.89 ± .64 |
| Comparison | 1–5 | 2.76 ± .54 | 2.80 ± .53 | 2.81 ± .57 | 2.76 ± .63 | 2.75 ± .58 |
| Memory Self Efficacy | ||||||
| Intervention | 0–100 | 49.44 ± 18.82 | 51.60 ± 18.29 | 53.17 ± 17.47 | 54.10 ± 18.53 | 52.04 ± 18.54 |
| Comparison | 0–100 | 46.02 ± 16.28 | 45.35 ± 16.88 | 47.51 ± 17.09 | 47.60 ± 19.69 | 47.38 ± 15.65 |
| External Strategies | ||||||
| Intervention | 1–5 | 3.90 ± 0.56 | 4.01 ± 0.57 | 4.08 ± 0.59 | 4.02 ± 0.60 | 4.06 ± 0.54 |
| Comparison | 1–5 | 4.01 ± 0.59 | 3.97 ± 0.60 | 4.05 ± 0.57 | 4.05 ± 0.59 | 4.07 ± 0.61 |
| Internal Strategies | ||||||
| Intervention | 1–5 | 3.56 ± 0.51 | 3.67 ± 0.59 | 3.74 ± 0.51 | 3.73 ± 0.58 | 3.72 ± 0.57 |
| Comparison | 1–5 | 3.54 ± 0.58 | 3.53 ± 0.59 | 3.66 ± 0.53 | 3.53 ± 0.62 | 3.58 ± 0.53 |
Note. Visual Memory = Delayed recall T score from the Brief Visuospatial Memory Test-Revised; Instrumental Activities = Direct Assessment of Functional Status; Verbal Memory = Delayed recall T score from the Hopkins Verbal Learning Test-Revised; Cognition = Mini Mental State Examination; Everyday Memory = Standard profile score from the Rivermead Behavioral Memory Test; Memory Complaints = Memory Complaints Inventory from the Metamemory in Adulthood Questionnaire; Memory Self Efficacy = Memory Self Efficacy Questionnaire; External Strategies = External strategies scale from the Metamemory in Adulthood Questionnaire; Internal Strategies = Internal strategies scale from the Metamemory in Adulthood Questionnaire.
The HLM results for measures of cognitive abilities and instrumental functional abilities are shown in Table 3. At baseline, older, less educated, Black, and Hispanic participants had significantly lower scores on visual memory. Less educated, Black, and Hispanic participants also had lower baseline scores on verbal memory. At baseline, scores on the MMSE were positively associated with years of education and negatively associated with being Hispanic or Black.
Table 3.
HLM Results for Memory Performance Measures
| Baseline | Baseline to Post-Classes | Baseline to End of Study | ||||
|---|---|---|---|---|---|---|
| Coefficient | SE | Coefficient | SE | Coefficient | SE | |
| Brief Visuospatial Memory Test-Revised | ||||||
| Treatment | 0.758 | 1.437 | 0.122 | 0.483 | −0.062 | 0.080 |
| Age | −0.495** | 0.123 | 0.121* | 0.051 | 0.022** | 0.007 |
| Education | 0.811** | 0.236 | −0.071 | 0.093 | −0.016 | 0.013 |
| Hispanic | −8.901** | 2.399 | −0.612 | 1.002 | 0.336* | 0.132 |
| Black | −12.818** | 2.329 | 0.307 | 0.984 | 0.538** | 0.134 |
| Class Attendance | 0.105 | 0.279 | 0.040 | 0.038 | ||
| Pretest | 0.007 | 0.023 | 0.027** | 0.003 | ||
| Intercept | 71.030** | 10.295 | −9.294 | 4.744 | −2.992** | 0.672 |
| Direct Assessment Functional Status | ||||||
| Treatment | 0.952 | 0.523 | −0.028 | 0.149 | −0.031 | 0.023 |
| Age | −0.119** | 0.045 | −0.008 | 0.015 | −0.002 | 0.002 |
| Education | 0.490** | 0.085 | −0.020 | 0.029 | −0.008* | 0.004 |
| Hispanic | −3.305** | 0.862 | 0.189 | 0.302 | 0.016 | 0.039 |
| Black | −4.842** | 0.844 | 0.072 | 0.275 | 0.104** | 0.040 |
| Class Attendance | 0.105 | 0.087 | 0.026* | 0.013 | ||
| Pretest | −0.001 | 0.020 | 0.011** | 0.002 | ||
| Intercept | 85.012** | 3.741 | 0.375 | 2.161 | −0.890* | 0.293 |
| Hopkins Verbal Learning Test-Revised | ||||||
| Treatment | 1.778 | 1.174 | 0.173 | 0.372 | −0.008 | 0.051 |
| Age | −0.081 | 0.101 | −0.039 | 0.037 | −0.006 | 0.004 |
| Education | 0.594** | 0.195 | 0.037 | 0.071 | −0.006 | 0.008 |
| Hispanic | −5.997** | 2.013 | −0.380 | 0.726 | 0.041 | 0.086 |
| Black | −12.157** | 1.967 | 0.156 | 0.700 | 0.168 | 0.090 |
| Class Attendance | 0.098 | 0.216 | 0.031 | 0.029 | ||
| Pretest | - | - | −0.031 | 0.018 | 0.009** | 0.002 |
| Intercept | 47.193** | 8.455 | 3.406 | 3.473 | −0.062 | 0.439 |
| Mini Mental State Exam | ||||||
| Treatment | 0.270 | 0.197 | 0.208** | 0.079 | −0.006 | 0.010 |
| Age | −0.010 | 0.017 | −0.014 | 0.008 | −0.002 | 0.001 |
| Education | 0.210** | 0.034 | −0.033* | 0.016 | −0.005** | 0.002 |
| Hispanic | −0.878* | 0.364 | −0.327* | 0.161 | 0.003 | 0.017 |
| Black | −2.095** | 0.350 | 0.106 | 0.145 | 0.028 | 0.017 |
| Class Attendance | - | - | 0.010 | 0.044 | 0.001 | 0.005 |
| Pretest | - | - | 0.004 | 0.022 | 0.009** | 0.003 |
| Intercept | 26.086** | 1.440 | 1.257 | 0.934 | −0.086 | 0.109 |
| Rivermead Behavioral Memory Test | ||||||
| Treatment | 0.847* | 0.412 | 0.136 | 0.139 | −0.011 | 0.018 |
| Age | −0.154** | 0.036 | −0.012 | 0.015 | 0.000 | 0.002 |
| Education | 0.224** | 0.069 | −0.017 | 0.026 | −0.005 | 0.003 |
| Hispanic | −2.713** | 0.720 | 0.482 | 0.282 | −0.003 | 0.032 |
| Black | −3.578** | 0.696 | 0.089 | 0.268 | 0.037 | 0.033 |
| Class Attendance | - | - | 0.121 | 0.081 | −0.002 | 0.010 |
| Pretest | - | - | −0.026 | 0.022 | 0.008** | 0.002 |
| Intercept | 27.517** | 2.969 | 0.671 | 1.422 | −0.076 | 0.163 |
Note. p≤.05,
p≤.01 Visual Memory = Delayed recall T score from the Brief Visuospatial Memory Test-Revised; Instrumental Activities = Direct Assessment of Functional Status; Verbal Memory = Delayed recall T score from the Hopkins Verbal Learning Test-Revised; Cognition = Mini Mental State Examination; Everyday Memory = Standard profile score from the Rivermead Behavioral Memory Test. Treatment was coded as 1 = received Memory Intervention, 0 = received Health Intervention; Age and Education are in years; Hispanic was coded as 1 = Hispanic, 0 = not Hispanic (White is the reference group); Black was coded as 1 = Black, 0 = not Black (White is the reference group); Class Attendance refers to the number of intervention classes attended by the participant not including booster sessions; Pretest = the baseline score on the dependent variable of interest.
Lower DAFS scores at baseline were related to being older, less educated, Hispanic, and Black. Although in preliminary univariate tests the memory and health promotion groups did not differ at baseline on outcome measures, the HLM results suggested that the memory group scored significantly higher at baseline on everyday memory than did the health promotion group.
Younger participants reported fewer memory complaints at baseline than older participants (Table 4). In addition, younger participants rated their memory self-efficacy higher than older participants did. Also, the memory training group reported significantly higher memory self-efficacy at baseline than the health promotion group. Hispanics and Blacks reported using fewer external memory strategies than Whites did at baseline. In addition, Hispanics used fewer internal strategies than Whites did.
Table 4.
HLM Results for Memory Self-Report Measures
| Baseline | Baseline to Post-Classes | Baseline to End of Study | ||||
|---|---|---|---|---|---|---|
| Coefficient | SE | Coefficient | SE | Coefficient | SE | |
| Memory Complaints | ||||||
| Treatment | −0.014 | 0.068 | 0.050** | 0.019 | 0.006** | 0.002 |
| Age | −0.017** | 0.006 | −0.003 | 0.002 | −0.001** | 0.000 |
| Education | 0.011 | 0.011 | 0.002 | 0.004 | 0.000 | 0.000 |
| Hispanic | 0.123 | 0.111 | 0.007 | 0.037 | 0.000 | 0.003 |
| Black | −0.171 | 0.110 | −0.010 | 0.033 | −0.004 | 0.003 |
| Class Attendance | 0.010 | 0.011 | 0.000 | 0.001 | ||
| Pretest | 0.010 | 0.017 | 0.002 | 0.002 | ||
| Intercept | 3.836** | 0.483 | 0.072 | 0.187 | 0.039* | 0.018 |
| Memory Self Efficacy | ||||||
| Treatment | 4.033* | 2.082 | 0.308 | 0.651 | 0.035 | 0.077 |
| Age | −0.611** | 0.179 | −0.090 | 0.063 | −0.008 | 0.007 |
| Education | 0.571 | 0.349 | −0.028 | 0.118 | 0.015 | 0.012 |
| Hispanic | −0.669 | 3.533 | −1.083 | 1.253 | −0.209 | 0.124 |
| Black | −2.209 | 3.475 | −0.772 | 1.055 | −0.307* | 0.126 |
| Class Attendance | −0.114 | 0.376 | 0.030 | 0.045 | ||
| Pretest | −0.001 | 0.019 | −0.006** | 0.002 | ||
| Intercept | 83.913** | 14.918 | 8.419 | 5.926 | 0.567 | 0.657 |
| External Strategies | ||||||
| Treatment | −0.068 | 0.067 | 0.026 | 0.020 | 0.005 | 0.003 |
| Age | −0.001 | 0.006 | 0.001 | 0.002 | 0.000 | 0.000 |
| Education | −0.006 | 0.011 | −0.003 | 0.004 | 0.000 | 0.000 |
| Hispanic | −0.487** | 0.114 | −0.021 | 0.042 | 0.005 | 0.004 |
| Black | −0.259* | 0.111 | 0.019 | 0.036 | 0.004 | 0.004 |
| Class Attendance | 0.019 | 0.012 | −0.001 | 0.001 | ||
| Pretest | −0.029 | 0.019 | 0.001 | 0.002 | ||
| Intercept | 4.262** | 0.479 | −0.051 | 0.190 | −0.025 | 0.019 |
| Internal Strategies | ||||||
| Treatment | 0.109 | 0.062 | 0.027 | 0.019 | 0.004 | 0.002 |
| Age | −0.003 | 0.005 | 0.002 | 0.002 | 0 | 0 |
| Education | 0.015 | 0.011 | 0.004 | 0.004 | 0.001 | 0 |
| Hispanic | −0.321** | 0.109 | 0.022 | 0.038 | 0.004 | 0.004 |
| Black | −0.13 | 0.107 | 0.036 | 0.032 | 0.001 | 0.004 |
| Class Attendance | 0.022* | 0.011 | 0 | 0.001 | ||
| Pretest | 0.02 | 0.02 | 0 | 0.002 | ||
| Intercept | 3.572** | 0.451 | −0.413* | 0.18 | −0.012 | 0.02 |
Note. p≤.05,
p≤.01 Memory Complaints = Memory Complaints Inventory from the Metamemory in Adulthood Questionnaire; Memory Self Efficacy = Memory Self Efficacy Questionnaire; External Strategies = External strategies scale from the Metamemory in Adulthood Questionnaire; Internal Strategies = Internal strategies scale from the Metamemory in Adulthood Questionnaire. Treatment was coded as 1 = received Memory Intervention, 0 = received Health Intervention; Age and Education are in years; Hispanic was coded as 1 = Hispanic, 0 = not Hispanic (White is the reference group); Black was coded as 1 = Black, 0 = not Black (White is the reference group); Class Attendance refers to the number of intervention classes attended by the participant not including booster sessions; Pretest = the baseline score on the dependent variable of interest.
Training Effects from Baseline to Post-Class: Performance Measures
The memory and health promotion groups did not differ significantly in gains on visual memory from baseline to post-classes (Table 3). Performance for the entire group also did not change significantly (b=−0.20, p=.50). The memory and health groups did differ significantly in gains from baseline to post-classes on the DAFS performance measure, thoughboth groups, improved on the DAFS (b = 0.23, p = .04). On verbal memory, overall performance did not significantly change from baseline to end of classes (b = 0.36, p = .10). However, the memory group showed greater gains from baseline to post-classes on MMSE scores, a measure of global cognition. On everyday memory performance, there were no significant gains for the memory group from baseline to end of classes (b = 0.14, p = .07).
Training Effects from Baseline to End of Study: Performance Measures
Overall performance on visual memory did not significantly increase or decline for the sample as a whole (b = −0.08, p = .11). Hispanic and Black participants gained more than Whites, did and individuals with less education also gained relatively more.
Participants showed no significant changes in DAFS scores from baseline to end of study (b = −0.01, p = .68). However, those with initially higher DAFS scores, and those who attended more classes gained more from baseline to end of study. Those with fewer years of education gained more on the DAFS from baseline to end of study, and Blacks gained more than Whites.
Although the memory group did not outperform the health promotion group, those with higher verbal memory scores at baseline performed relatively better from baseline to end of study. However, the overall performance of both groups, on verbal memory did not significantly increase or decline from baseline to end of study (b = 0.04, p = .19). The MMSE scores for the sample as a whole also did not significantly change from baseline to end of study (b = 0.00, p = .94).
The participants who scored higher on everyday memory in the pretest at the beginning of the study also gained more from baseline to end of study. Older participants gained relatively more than younger participants. Everyday memory performance gains did not differ significantly from baseline to end of study by treatment condition. Overall performance on everyday memory for the entire sample did not significantly change (b = 0.01, p = .69).
Training Effects from Baseline to Post-Test Classes: Self-Report Measures
Table 4 presents the HLM results for the self-report measures of memory complaints, self-efficacy, and strategies. Memory group participants reported a greater reduction in memory complaints from baseline to post-classes. Memory self-efficacy gains, however, did not significantly differ by training group (b = 0.53, p = .17). For internal strategies, scores for the sample did not significantly increase or decline (b = 0.01, p = .33). However, those who attended more classes reported greater use of internal strategies over time. Similarly, overall scores for external strategies did not change significantly (b = 0.00, p = .79). On the psychological measures of anxiety (state/trait) and depression, controlling for education, there were no significant effects of time, ethnic group, or intervention.
Training Effects from Baseline to End of Study: Self-Report Measures
Memory training participants who were younger reported a greater reduction in memory complaints than older participants. On the memory self-efficacy measure, Whites made relatively greater gains than Blacks did, but neither group changed significantly (b = 0.07, p = .33). Both groups improved their use of external and internal memory strategies over time. Internal strategies increased significantly (b = 0.004, p < .01), as did external strategies (b = 0.004, p = .03). The memory group did not make significantly greater gains than the health promotion group in strategy use.
Discussion
This study had many strengths. First, the tri-ethnic sample was recruited in the community, and volunteer participants were highly motivated and maintained their interest in the study, as evidenced by a 79% retention rate over the 26-month study. The ACTIVE trials reported 80% retention at 24-month follow-up (Ball et al., 2002). Further, the range of outcomes was comprehensive and administered at each measurement occasion. Finally, we explored differences in racial and ethnic group responses to the memory training. Study limitations included the lack of a no-treatment control group. Further, the generalizability of the findings from the minority sample is not clear.
The participants in both the memory and health promotion groups improved their use of external and internal memory strategies. This is a unique finding for an intervention that did not place any emphasis on memory strategies in the health training (Lachman et al., 1992; Lachman, Andreoletti, & Pearman, 2006). However, both groups received mental stimulation and social engagement, in part that may explain the benefits to memory. Participants may have engaged in self-study to improve memory strategies as a result of their participation in the study. Health promotion training is not a normative intervention in people’s daily lives. When this study was launched in 2001, evidence of the effectiveness of memory training with older adults was known (Floyd & Scogin, 1997; Verhaeghen et al., 1992), but, the benefits of health promotion as mental stimulation were less well known. Nevertheless, the health promotion group’s maintenance of cognitive functioning is consistent with growing scientific evidence that health promotion interventions, including physical activity, may prevent decline (Abbott, White, Ross, Masaki, Curb, & Petrovitch, 2004; Boron, Willis, & Schaie, 2007; Salthouse, 2006; Salthouse, Berish, & Miles, 2002; Verghese et al., 2003; Weuve et al., 2004).
In the analyses, race and ethnicity were covariates, which suggests that memory performance may change differently over time based on demographic characteristics. Both Hispanics and Blacks tended to make greater gains than Whites on visual memory; Blacks also performed better over time on instrumental functional abilities, though Hispanics performed worse on the memory measure in the short run. These results raise the question: Are minorities more likely to gain from health promotion or memory training interventions? Because pretest scores were included in the model as covariates, the differential changes over time for Black and Hispanic participants as compared to Whites were not due to lower pretest scores.
The participants improved on the DAFS performance measure from baseline to end of classes, but there was no significant change in scores at the end of the study. Transfer of learning or practice effects may have occurred as a result of taking the DAFS repeatedly. Even though our participants were screened out for dementia, we found that the DAFS (even excluding the eating and dressing/grooming skills subscales) had a non-normal distribution and a ceiling effect despite the fact that two-thirds of these same respondents scored in the poor or impaired ranges of a test of everyday memory performance. This pattern of results suggested that the DAFS might not be sensitive to the early deficits in functioning exhibited by older adults who are experiencing poor memory performance but are still living independently in the community.
Also, our study included four required 90-minute booster sessions at 3 months post-training for all participants. These additional sessions may have increased learning and retention through practice effects with everyday activities. The four booster sessions for the health promotion group may have increased the mental stimulation for this group (Austin-Wells, McDougall, & Becker, 2006).
Less educated, Black, and Hispanic participants had lower scores than Whites on verbal memory at baseline. In our HLM analyses, we examined the verbal memory delayed recall T-score as an outcome at Times 2 and 5. Controlling for ethnicity, age, education, and verbal memory scores at Time 1, there were no treatment effects. However, testing with Hispanic elders was often done over many days to accommodate their low literacy skills and level of frustration with the complex battery of measures. Practice effects may have been greater for Blacks and Hispanics, or the interventions may have been more effective for minority participants (McDougall et al., 2008). Since different patterns were observed for Blacks and Hispanics, future research should examine ethnic/racial groups separately and consider the moderator effects of race and ethnicity on memory training.
Following the training, we observed an improvement in global cognition; however, there was no change from baseline to end of study. The initial improvement may reflect a practice effect induced by repetition because the period between baseline testing and post-class was approximately 8 weeks. The MMSE has a documented selective bias toward episodic memory and visuospatial skills in normal elderly adults without dementia (Hill & Backman, 1995). Also, in a longitudinal analysis, Rabbitt, Diggle, Holland, and McInnes (2004) found that practice improvements were greatest between the first and second experiences with a cognitive task.
The memory group showed no greater gains in everyday memory performance, than the health promotion group. These findings contrast with the ACTIVE trial findings, in which the participants in each intervention group improved targeted cognitive abilities by an amount approximately equal to the cognitive decline that would naturally occur in older adults without dementia, and maintained these gains for 5 years (Ball et al., 2002; Willis et al., 2006).
The participants in our study did not have dementia. However, 169 (64%) individuals had measurable problems in one or more domains of everyday function, and 46 (17%) had problems that would be of serious enough concern for further neuropsychological referral and would potentially meet the criteria for mild cognitive impairment (MCI). The MCI prevalence rate in a nationally representative sample was 22% (Plassman et al., 2008). In recent studies, individuals with MCI might have received differential benefits from cognitive training (Belleville, 2008; Unverzagt et al., 2007). The issue of memory complaints deserves further discussion because these subjective concerns are often predictive of a future diagnosis of MCI (Pearman & Storandt, 2004; Purser, Fillenbaum, & Wallace, 2006; Winblad et al., 2004; Zandi, 2004; Zeintl et al., 2006). We found a reduction in memory complaints for participants in the memory group. At 26 months, they continued to report fewer complaints.
Whites made relatively greater gains than Blacks did in memory self-efficacy. We expected memory self-efficacy to improve based on evidence from previous studies with diverse older adults (Dittmann-Kohli et al., 1990; Lachman et al., 1992; McDougall, 1998, 1999, 2000, 2002; Rebok, & Balcerak, 1989; West et al., 2008). However, all previous intervention studies used different memory efficacy questionnaires. Difficulty in comprehending the MSEQ was reflected in our findings. Future studies will require further adjustment of this measure, and possibly other measures, to accommodate the literacy level of participants (Institute of Medicine, 2004; Scott et al., 2002).
In a recent study, Algase, Souder, Roberts, & Beattie (2006) elevated the urgency to prepare advanced practice geropsychiatric nurses to care for the projected 15 million older adults by 2030 with major psychiatric illness. The Cognitive Behavioral Model of Everyday Memory provides a framework for intervening with the everyday memory concerns of older adults striving for independence. Nevertheless, the findings that Black and Hispanic elderly made greater gains than Whites on some memory performance measures merits further study.
Figure 2.
Flow chart
Table 1.
Demographics and Screening Measures at Baseline
| Comparison Group N=130 | Intervention Group N=135 | ||
|---|---|---|---|
| Variables | Score Range | Mean ± SD N (%) | Mean ± SD N (%) |
| Age | 65–94 | 74.83 ± 6.22 | 74.69 ± 5.74 |
| Education | 0–22 | 13.81 ± 3.71 | 13.39 ± 3.9 |
| Number of people in house | 1–6 | 1.65 ± 1.42 | 1.53 ± .81 |
| Attention | 0–324 | 45.53 ± 36.73 | 44.95 ± 34.16 |
| Cognitive Flexibility | 0–630 | 134.91 ± 98.62 | 125.3 ± 74.27 |
| Organizational Strategy | 0–79 | 40.58 ± 11.16 | 41.27 ± 11.46 |
| Depression | 0–60 | 9.47 ± 6.87 | 9.13 ± 6.71 |
| State Anxiety | 20–80 | 29.32 ± 8.12 | 29.72 ± 8.18 |
| Trait Anxiety | 20–80 | 32.15 ± 8.55 | 31.90 ± 9.24 |
| Gender | |||
| Male | 30 (23.1) | 30 (22.2) | |
| Female | 100 (76.9) | 105 (77.8) | |
| Race | |||
| White | 93 (71.5) | 96 (71.1) | |
| Black | 15 (11.5) | 15 (11.1) | |
| Hispanic | 22 (16.9) | 24 (17.8) | |
| Marital Status | |||
| Married | 37 (28.5) | 45 (33.8) | |
| Never Married | 3 (2.3) | 5 (3.8) | |
| Divorced | 26 (20) | 25 (18.8) | |
| Widowed | 64 (49.2) | 58 (43.6) | |
Note. Attention = Trail-Making Test A; Cognitive Flexibility = Trail-Making Test B; Organizational Strategy = Controlled Oral Word Association Test; Depression = Centers for Epidemiologic Studies Scale (CES-D); State and Trait Anxiety = Spielberger State-Trait Anxiety Inventory.
Acknowledgments
Support for this research was provided by NIA Grant R01 AG 15384
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA. 2004;292(12):1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- Algase DL, Souder E, Roberts B, Beattie E. Enriching geropsychiatric nursing in advanced practice nursing programs. Journal of Professional Nursing. 2006;22(2):129–136. doi: 10.1016/j.profnurs.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Artistico D, Cervone D, Pezzuti L. Perceived self-efficacy and everyday problem solving among you and other adults. Psychology and Aging. 2003;18:68–79. doi: 10.1037/0882-7974.18.1.68. [DOI] [PubMed] [Google Scholar]
- Austin-Wells V, Zimmerman T, McDougall GJ. An optimal delivery format for lectures targeting mature adults. Educational Gerontology. 2003;29(6):493–501. doi: 10.1080/713844396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin-Wells V, McDougall GJ, Becker H. Recruiting and retaining an ethnically diverse sample of older adults in a longitudinal intervention study. Educational Gerontology. 2006;32:159–170. doi: 10.1080/03601270500388190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA. 2002;288(18):2271–2278. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Regulation of cognitive processes through perceived self-efficacy. Developmental Psychology. 1989;25(5):729–735. [Google Scholar]
- Bandura A, Locke EA. Negative self-efficacy and goal effects revisited. Journal of Applied Psychology. 2003;88(1):87–99. doi: 10.1037/0021-9010.88.1.87. [DOI] [PubMed] [Google Scholar]
- Becker H, McDougall GJ, Douglas N, Arheart KL. Comparison of the impact of an eight-session and four-session memory intervention. Archives of Psychiatric Nursing. 2008;22(2):87–94. doi: 10.1016/j.apnu.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleville S. Cognitive training for persons with mild cognitive impairment. International Psychogeriatrics. 2008;20(1):57–66. doi: 10.1017/S104161020700631X. [DOI] [PubMed] [Google Scholar]
- Benedict RHB. Brief visuospatial memory test-revised: Professional manual. Odessa, FL: Psychological Assessment Resources, Inc; 1997. [Google Scholar]
- Berry JM. Cognitive efficacy across the life span: Introduction to the special series. Developmental Psychology. 1989;25:683–686. [Google Scholar]
- Berry JM, West RL, Dennehey DM. Reliability and validity of the memory self-efficacy questionnaire. Developmental Psychologist. 1989;25:701–713. [Google Scholar]
- Berry JM, West RL. Cognitive self-efficacy in relation to personal mastery and goal setting across the life span. International Journal of Behavioral Development. 1993;16:351–379. [Google Scholar]
- Boron JB, Willis SL, Schaie KW. Cognitive training gain as a predictor of mental status. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2007;62(1):P45–P52. doi: 10.1093/geronb/62.1.p45. [DOI] [PubMed] [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. The Clinical Neuropsychologist. 1991;5:125–142. [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical linear models. Newbury Park, CA: Sage Publications; 1992. [Google Scholar]
- Butters MA, Young JB, Lopez O, Aizenstein HJ, Mulsant BH, Reynolds CF, 3rd, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues in Clinical Neursoscience. 2008;10(3):345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey B. A ‘senior moment’ or a self-fulfilling prophecy? The New York Times. 2006:D6. [Google Scholar]
- Carlson MC, Xue QL, Zhou J, Fried LP. Executive decline and dysfunction precedes decline in memory: The women’s health and aging study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2009;64(1):12–18. doi: 10.1093/gerona/gln008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattan M, White M, Bond J, Learmouth A. Preventing social isolation and loneliness among older people: A systematic review of health promotion interventions. Ageing & Society. 2005;25:41–67. doi: 10.7748/nop.17.1.40.s11. [DOI] [PubMed] [Google Scholar]
- Cockburn J, Smith PT. The Rivermead Behavioural Memory Test. Supplement 3: Elderly people. Bury St. Edmonds, Suffolk: Thames Valley Test Company; 1989. [Google Scholar]
- Cockburn J, Smith PT. Anxiety and errors of prospective memory among elderly people. British Journal of Psychology. 1994;85:273–282. doi: 10.1111/j.2044-8295.1994.tb02523.x. [DOI] [PubMed] [Google Scholar]
- Comijs HC, Deeg DJ, Dik MG, Twisk JW, Jonker C. Memory complaints: The association with psycho-affective and health problems and the role of personality characteristics. A 6-year follow-up study. Journal of Affective Disorders. 2002;72:157–165. doi: 10.1016/s0165-0327(01)00453-0. [DOI] [PubMed] [Google Scholar]
- Dellefield KS, McDougall GJ. Increasing metamemory in older adults. Nursing Research. 1996;45(5):284–290. doi: 10.1097/00006199-199609000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Hultsch DF, Hertzog C. The metamemory in adulthood (MIA) questionnaire. Psychopharmacology Bulletin. 1988;24:671–688. [PubMed] [Google Scholar]
- Dodge HH, Du Y, Saxton JA, Ganguli M. Cognitive domains and trajectories of functional independence in nondemented elderly persons. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2006;61(12):1330–1337. doi: 10.1093/gerona/61.12.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dux MC, Woodard JL, Calamari JE, Messina M, Arora S, Chik H, et al. The moderating role of negative affect on objective verbal memory performance and subjective memory complaints in healthy older adults. Journal of the International Neuropsychological Society. 2008;14(2):327–336. doi: 10.1017/S1355617708080363. [DOI] [PubMed] [Google Scholar]
- Federal Interagency Forum on Aging Related Statistics. Older Americans 2008: Key indicators of well being. Washington, DC: U.S. Government Printing Office; 2008. [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Cohort differences in trajectories of cognitive aging. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2007;62(5):P286–P294. doi: 10.1093/geronb/62.5.p286. [DOI] [PubMed] [Google Scholar]
- Fiocco AJ, Wan N, Weekes N, Pim H, Lupien SJ. Diurnal cycle of salivary cortisol in older adult men and women with subjective complaints of memory deficits and/or depressive symptoms: Relation to cognitive functioning. Stress. 2006;9(3):143–152. doi: 10.1080/10253890600965674. [DOI] [PubMed] [Google Scholar]
- Floyd M, Scogin F. Effects of memory training on the subjective memory functioning and mental health of older adults: A meta-analysis. Psychology and Aging. 1997;12(1):150–161. doi: 10.1037//0882-7974.12.1.150. [DOI] [PubMed] [Google Scholar]
- Flynn T, Storandt M. Supplemental group discussions in memory training for older adults. Psychology and Aging. 1990;5(2):178–181. doi: 10.1037//0882-7974.5.2.178. [DOI] [PubMed] [Google Scholar]
- Fogler J, Stern L. Improving your memory: How to remember what you are starting to forget. Baltimore: John Hopkins University Press; 1994. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-Mental State’: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hayslip B, Maloy RM, Kohl R. Long-term efficacy of fluid ability interventions with older adults. Journal of Gerontology: Psychological Sciences. 1995;50B(3):P141–P149. doi: 10.1093/geronb/50b.3.p141. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the U.S. population: Prevalence estimates using the 2000 census. Archives of Neurology. 2003;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Herrmann LL, Goodwin GM, Ebmeler KP. The cognitive neuropsychology of depression in the elderly. Psychological Medicine. 2007;37(12):1693–1702. doi: 10.1017/S0033291707001134. [DOI] [PubMed] [Google Scholar]
- Hill RD, Backman L. The relationship between the Mini-mental State Examination and cognitive functioning in normal elderly adults: A componential analysis. Age and Ageing. 1995;24:440–446. doi: 10.1093/ageing/24.5.440. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Health literacy: A prescription to end confusion. Washington, DC: The National Academies Press; 2004. [PubMed] [Google Scholar]
- Jennings JM, Darwin AL. Efficacy beliefs, everyday behavior, and memory performance among older elderly adults. Educational Gerontology. 2003;29:71–91. [Google Scholar]
- Jobe JB, Smith DM, Ball K, Tennstedt SL, Marsiske M, Willis, et al. ACTIVE: A cognitive intervention trial to promote independence in older adults. Controlled Clinical Trials. 2001;22:453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JK, Lui LY, Yaffe K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. The Journals of Gerontology. Series B, Biological Sciences and Medical Sciences. 2007;62(10):M1134–M1141. doi: 10.1093/gerona/62.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman ME, Steinberg ES, Trotter SD. Effects of control beliefs and attributions on memory self-assessments and performance. Psychology and Aging. 1987;2(3):266–271. doi: 10.1037//0882-7974.2.3.266. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Weaver SL, Bandura M, Elliott E, Lewkowicz CJ. Improving memory and control beliefs through cognitive restructuring and self-generated strategies. Journal of Gerontology. 1992;47(50):P293–P299. doi: 10.1093/geronj/47.5.p293. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Andreoletti C, Pearman A. Memory control beliefs: How are they related to age, strategy use and memory improvement? Social Cognition. 2006;24(3):359–385. [Google Scholar]
- Light LL. Memory and aging: Four hypotheses in search of data. Annual Review of Psychology. 1991;42:333–376. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- Loewenstein DA, Amigo E, Duara R, Guterman A, Hurwitz D, Berkowitz N, et al. A new scale for the assessment of functional status in Alzheimer’s disease and related disorders. Journal of Gerontology. 1989;44:P114–P121. doi: 10.1093/geronj/44.4.p114. [DOI] [PubMed] [Google Scholar]
- Loewenstein DA, Ardila A, Rosselli M, Hayden S, Duara R, Berkowitz N, et al. A comparative analysis of functional status among Spanish- and English-speaking patients with dementia. Journal of Gerontology. 1992;47:P389–P394. doi: 10.1093/geronj/47.6.p389. [DOI] [PubMed] [Google Scholar]
- McDougall GJ. Increasing memory strategy use and self-efficacy in Hispanic elders. Clinical Gerontologist. 1998;19(2):57–76. doi: 10.1300/j018v19n02_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall GJ, Strauss ME, Holston E, Martin M. Memory self-efficacy and memory anxiety as predictors of memory performance in at-risk elderly. In: Kwon S, editor. Memory and cognition: Theoretical and methodological approaches to self-ratings, control beliefs, and self-efficacy; Symposium conducted at the annual meeting of the Gerontological Society of America; San Francisco, CA. 1999. Nov, [Google Scholar]
- McDougall GJ. Cognitive interventions among older adults. In: Fitzpatrick JJ, editor. Annual Review of Nursing Research. 17. New York: Springer; 1999. pp. 219–240. [PMC free article] [PubMed] [Google Scholar]
- McDougall GJ. Memory improvement in assisted living elders. Issues in Mental Health Nursing. 2000;21(2):217–233. doi: 10.1080/016128400248202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall GJ, Holston EC, Wilke P. Recruiting African-Americans into cognitive aging research. Ethnicity & Disease. 2001;11:124–133. [PMC free article] [PubMed] [Google Scholar]
- McDougall GJ. Memory improvement in octogenarians. Applied Nursing Research. 2002;15(1):2–10. doi: 10.1053/apnr.2002.29518. [DOI] [PubMed] [Google Scholar]
- McDougall GJ. Memory self-efficacy and memory performance among black and white elders. Nursing Research. 2004;53(5):323–331. doi: 10.1097/00006199-200409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall GJ, Becker H, Arheart KL. Older adults in the SeniorWISE study at-risk for mild cognitive impairment. Archives of Psychiatric Nursing. 2006;20(3):126–134. doi: 10.1016/j.apnu.2005.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall GJ, Becker H, Pituch K, Acee TW, Vaughan PW, Delville CL. Emerging evidence for memory training with community-residing minority older adults. Research poster presented at the annual meeting of Academy Health; Washington, DC. 2008. Jun, [Google Scholar]
- McDougall GJ. A framework for cognitive interventions targeting everyday memory performance and memory self-efficacy. Family and Community Health, Supplement 1. 2009;32(18):S15–S26. doi: 10.1097/01.FCH.0000342836.20854.fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes de Leon CF, Gold DT, Glass TA, Kaplan L, George LK. Disability as a function of social networks and support in elderly African Americans and whites: The Duke EPESE 1986–1992. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2001;56(3):S179–S190. doi: 10.1093/geronb/56.3.s179. [DOI] [PubMed] [Google Scholar]
- Michael YL, Berkman LF, Colditz GA, Holmes MD, Kawachi I. Social networks and health-related quality of life in breast cancer survivors: A prospective study. Journal of Psychosomatic Research. 2002;52(5):285–293. doi: 10.1016/s0022-3999(01)00270-7. [DOI] [PubMed] [Google Scholar]
- Morgan MD, Mielke MM, O’Brien R, Troncoso JC, Zonderman AB, Lyketsos CG. Rates of depression in individuals with pathologic but not clinical Alzheimer disease are lower than those in individuals without the disease: Findings from the Baltimore Longitudinal Study on Aging (BLSA) Alzheimer Disease & Associated Disorders. 2007;21(3):199–204. doi: 10.1097/WAD.0b013e3181461932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald WD, Rupprecht R, Gunzelmann T, Tritt K. The SIMA-project: Effects of 1 year cognitive and psychomotor training on cognitive abilities of elderly. Behavioural Brain Research. 1996;78:67–72. doi: 10.1016/0166-4328(95)00219-7. [DOI] [PubMed] [Google Scholar]
- Papp KV, Walsh SJ, Snyder PJ. Immediate and delayed effects of cognitive interventions in healthy elderly: a review of current literature and future directions. Alzheimers and Dementia. 2009;5(1):50–60. doi: 10.1016/j.jalz.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Pearman A, Storandt M. Predictors of subjective memory in older adults. Journals Gerontology B Psychological Sciences Social Sciences. 2004;59(1):P4–P6. doi: 10.1093/geronb/59.1.p4. [DOI] [PubMed] [Google Scholar]
- Phelan EA, Williams B, Penninx BWJH, LoGerfo JP, Leveille SG. Activities of daily living function and disability in older adults in a randomized trial of the health enhancement program. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2004;59(8):M838–M843. doi: 10.1093/gerona/59.8.m838. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of cognitive impairment without dementia in the United States. Annals of Internal Medicine. 2008;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purser JL, Fillenbaum GG, Wallace RB. Memory complaint is not necessary for diagnosis of mild cognitive impairment and does not predict 10-year trajectories of functional disability, word recall, or short portable mental status questionnaire limitations. Journal of the American Geriatrics Society. 2006;54(2):335–338. doi: 10.1111/j.1532-5415.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Diggle P, Holland F, McInnes L. Practice and drop-out effects during a 17-year longitudinal study of cognitive aging. Journal of Gerontology: Psychological Sciences. 2004;59B:84–97. doi: 10.1093/geronb/59.2.p84. [DOI] [PubMed] [Google Scholar]
- Radloff LS, Teri L. Use of the Center for Epidemiological Studies-Depression Scale with older adults. Clinical Gerontologist. 1986;5:119–136. [Google Scholar]
- Rebok GW, Balcerak LJ. Memory self-efficacy and performance differences in young and old adults: The effect of mnemonic training. Developmental Psychology. 1989;25(5):714–721. [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Royall DR, Palmer R, Chiodo LK, Polk MJ. Declining executive control in normal aging predicts change in functional status: The Freedom House study. Journal of the American Geriatrics Society. 2005;52:346–352. doi: 10.1111/j.1532-5415.2004.52104.x. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Theoretical perspectives on cognitive aging. Hillsdale, NJ: Lawrence Erlbaum Associates; 1991. [Google Scholar]
- Salthouse TA. Memory aging from 18 to 80. Alzheimer Disease Associated Disorders. 2003;17(3):162–167. doi: 10.1097/00002093-200307000-00008. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Mental exercise and mental aging: Evaluating the validity of the “use it or lose it” hypothesis. Perspectives on Psychological Science. 2006;1(1):68–87. doi: 10.1111/j.1745-6916.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Berish DE, Miles JD. The role of cognitive stimulation on the relations between age and cognitive functioning. Psychology and Aging. 2002;17(4):548–557. doi: 10.1037//0882-7974.17.4.548. [DOI] [PubMed] [Google Scholar]
- Scogin FM. Effects of memory training on the subjective memory functioning and mental health of older adults: A meta-analysis. Psychology and Aging. 1997;12(1):150–161. doi: 10.1037//0882-7974.12.1.150. [DOI] [PubMed] [Google Scholar]
- Slegers K, van Boxtel M, Jolles J. Effects of computer training and internet usage on cognitive abilities in older adults: a randomized controlled study. Aging Clinical and Expirmental Research. 2009;21(1):43–54. doi: 10.1007/BF03324898. [DOI] [PubMed] [Google Scholar]
- Smith GE, Housen P, Yaffe K, Ruff R, Kennison RF, Mahncke HW, Zelinski EM. A cognitive training program based on principles of brain plasticity: results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) study. Journal American Geriatrics Society. 2009;57(4):594–603. doi: 10.1111/j.1532-5415.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory (Self-Evaluation Questionnaire) Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Sumerall SW, Timmons PL, James AL, Ewing MJM, Oehlert ME. Expanded norms for the Controlled Oral Word Association Test. Journal of Clinical Psychology. 1997;53:517–521. doi: 10.1002/(sici)1097-4679(199708)53:5<517::aid-jclp14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Unverzagt FW, Kasten L, Johnson KE, Rebok GW, Marsiske M, Koepke KM, et al. Effect of memory impairment on training outcomes in ACTIVE. Journal of the International Neuropsychological Society. 2007;13:953–960. doi: 10.1017/S1355617707071512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentijn SA, Van Hooren SA, Bosma H, Touw MS, Jolles J, Van Boxtel MP, et al. The effect of two types of memory training on subjective and objective memory performance in healthy individuals aged 55 years and older: A randomized controlled trial. Patient Education and Counseling. 2005;57:106–114. doi: 10.1016/j.pec.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, et al. Leisure activities and the risk of dementia in the elderly. New England Journal of Medicine. 2003;348(25):2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A, Goossens L. Improving memory performance in the aged through mnemonic training: A meta-analytic study. Psychology and Aging. 1992;7(2):242–251. doi: 10.1037//0882-7974.7.2.242. [DOI] [PubMed] [Google Scholar]
- West RL, Sinnott JD. Everyday memory and aging. New York: Springer-Verlag; 1991. [Google Scholar]
- West RL, Crook TH, Barron KL. Everyday memory performance across the life span: Effects of age and noncognitive individual differences. Psychology and Aging. 1992;7(1):72–82. doi: 10.1037//0882-7974.7.1.72. [DOI] [PubMed] [Google Scholar]
- West RL, Bagwell DK, Dark-Freudeman A. Self-efficacy and memory aging: The impact of a memory intervention based on self-efficacy. Neuropsychology Development and Cognition B Aging Neuropsychology and Cognition. 2008;15(3):302–329. doi: 10.1080/13825580701440510. [DOI] [PubMed] [Google Scholar]
- Weuve J, Kang JH, Manson JE, Breteler MMB, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292(12):1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296(23):2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging. 2002;17(2):179–193. [PubMed] [Google Scholar]
- Wilson B, Cockburn J, Baddeley A, Hiorns R. The development and validation of a test battery for detecting and monitoring everyday memory problems. Journal of Clinical and Experimental Neuropsychology. 1989;11:855–870. doi: 10.1080/01688638908400940. [DOI] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment—beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Zandi T. Relationship between subjective memory complaints, objective memory performance, and depression among older adults. American Journal of Alzheimer’s Disease and Related Disorders. 2004;19(6):353–360. doi: 10.1177/153331750401900610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeintl M, Kliegel M, Rast P, Zimprich D. Prospective memory complaints can be predicted by prospective memory performance in older adults. Dementia and Geriatric Cognitive Disorders. 2006;22(3):209–215. doi: 10.1159/000094915. [DOI] [PubMed] [Google Scholar]