Abstract
Maternal obesity during pregnancy increases the risk of obesity in the offspring. Obesity, arising from an imbalance of energy intake and expenditure, can be driven by the ingestion of palatable [high fat (HF), high sugar], energy-dense foods. Dopamine and opioid circuitry are neural substrates associated with reward that can affect animals’ preference for palatable foods. Using a mouse model, the long-term effect of maternal consumption of a HF diet on dopamine and opioid gene expression within the mesocorticolimbic reward circuitry and hypothalamus of the offspring was investigated. Mice from dams fed a HF diet during pregnancy and lactation showed an increased preference for sucrose and fat. Gene expression, measured using quantitative real-time PCR, revealed a significant approximately 3- to 10-fold up-regulation of dopamine reuptake transporter (DAT) in the ventral tegmental area, nucleus accumbens, and prefrontal cortex and a down-regulation of DAT in the hypothalamus. Additionally, expression of both μ-opioid receptor (MOR) and preproenkephalin (PENK) was increased in nucleus accumbens, prefrontal cortex, and hypothalamus of mice from dams that consumed the HF diet. Epigenetic mechanisms have been associated with long-term programming of gene expression after various in utero insults. We observed global and gene-specific (DAT, MOR, and PENK) promoter DNA hypomethylation in the brains of offspring from dams that consumed the HF diet. These data demonstrate that maternal consumption of a HF diet can change the offsprings’ epigenetic marks (DNA hypomethylation) in association with long-term alterations in gene expression (dopamine and opioids) and behavior (preference for palatable foods).
Maternal consumption of a high fat diet during pregnancy and lactation alters DNA methylation and gene expression of dopamine and opioid-related genes in the offspring.
Maternal obesity and consumption of a high-fat (HF) diet during pregnancy and lactation has been shown to increase the risk for development of obesity and related metabolic disorders in the offspring (1). Obesity develops from an imbalance of energy expenditure (resting metabolic rate and activity) and energy intake (the amount and type of food consumed). The central nervous system regulation of food intake is a complex process, involving homeostatic mechanisms seated largely in the hypothalamus and brainstem that interact extensively with circuitry that govern additional critical components, such as reward/motivation (mesocorticolimbic areas) and/or circadian rhythms (suprachiasmatic nucleus). Recent studies in the literature have focused on the effect of HF diet and/or maternal obesity on hypothalamic neuropeptides that affect food intake (2,3,4,5), noting (to varying extents) increased expression of NPY, AgRP, NPY Y1 receptor, and MC4R and, more consistently, an increase in POMC (2,6,7). It has also been reported that maternal HF diet can increase neurogenesis, specifically neurons that express galanin, enkephalin, dynorphin, orexin, and MCH (8). Aside from one report of differential expression of dopamine-related genes in the nucleus accumbens (NAc) (9), there has been little examination of circuitry outside the hypothalamus.
Animal studies have shown that maternal consumption of a palatable diet can increase the preference for fat and sugar in the offspring (8,9,10). This finding has recently been extended into a human clinical population in a report that prospectively followed a large number (∼4000) of mother, partner, and child groups, tracking food intake and body composition. They report that preference for protein, carbohydrates, and fat can be programmed by maternal but not paternal food intake during pregnancy, and prenatal rather than postnatal maternal fat intake was associated with fat preference in the child (11). Given these data, we hypothesized that molecules that participate in regulating consumption of palatable foods (dopamine and opioids) may be altered in offspring from mothers fed a HF diet. Dopamine projections from the ventral tegmental area (VTA) to frontal cortex (mesocortical) and NAc and amygdala (mesolimbic) projections have been implicated in reward and incentive motivation by natural and pharmacological reinforcers (12,13). The endogenous opioid system also plays a central role in driving reward-related behavior (for excellent review see Ref. 14), with broad expression of opioid precursor proteins and receptors throughout the brain. Additionally, there are extensive functional interactions between the dopamine and opioid systems within the reward circuitry (15).
Little is known about the mechanisms linking maternal consumption of HF diet with altered expression of brain neurotransmitters. Epigenetic alterations (i.e. DNA methylation and histone modifications) may be a potential mechanism linking environmental challenges with changes in gene expression. It has been suggested that because it is easier to shed a methyl group than change a base of DNA, epimutations may represent a way for an organism to more quickly adapt to environmental challenges, such as adverse in utero conditions, than through mutation and natural selection (16). Changes in early postnatal nutritional status have been shown to influence DNA metabolism and lead to altered patterns of both genome-wide and gene-specific DNA methylation (17). Therefore, in the current studies, experiments examined whether maternal consumption of HF diet would alter DNA methylation either globally or within the promoter regions of dopamine- and opioid-related genes.
Materials and Methods
Animals and experimental model
C57BL/6J females were maintained on a control or HF diet for approximately 3 months before breeding with DBA/2J males. Formulated diets (Test Diet, Richmond, IN) were as follows: control diet (no. 5755) with 18.5% protein, 12% fat, and 69.5% carbohydrate and HF diet (no. 58G9) with 18.5% protein, 60% fat, and 20.5% carbohydrate. Litter size was six to eight pups (one to two pups were culled when necessary), and there were no differences in sex balance between the groups. HF diet was maintained through lactation, and at weaning (d 21–22), all offspring were maintained on the control diet. One animal per litter from 10–15 different dams was used in individual experiments to control for any litter effect. Body weights were recorded weekly, and male mice (n = 5–8 per group) aged 18–24 wk of age were used in all experiments. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Glucose tolerance test
An ip glucose tolerance test was performed on control and HF-fed dams in late pregnancy (d 17) after an 18-h overnight fast. After determination of fasting blood glucose levels, each animal received an ip injection of 20% glucose (adjusted for body weight). Blood glucose levels were detected after 15, 30, 60, and 120 min. Glucose tolerance was calculated by adding the areas under the glucose-time curve for the intervals 0–15, 15–30, 30–60, and 60–120 min.
Sucrose and fat preference
Sucrose preference
For the duration of the test, offspring were caged individually and fed ad libitum. Mice were given simultaneous access to two bottles, one with water and one with 4% sucrose solution for 48 h. Bottle order was random and was switched after 24 h. Intake of consumed water and sucrose and animal weight were measured every 24 h. Water, sucrose, and the total fluid intake was normalized to body weight. Sucrose preference was calculated as a percentage of sucrose solution consumed in relation to total fluid intake.
Fat preference
Mice were presented with both control diet and HF diet (60%) for 24 h on 3 separate days. Data from d 1 were not analyzed, because animals acclimated to the novel diet, whereas data from d 2 and 3 were averaged for analysis. Intake of control diet and HF diet and animal weight were measured, and intake was normalized to body weight. Fat preference was calculated as a percentage of HF diet consumed in relation to total food intake.
Genomic DNA and total RNA isolation from brain
Animals were euthanized with an overdose of carbon dioxide, followed by cervical dislocation, a method consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. After the animals were killed, brains were rapidly removed and placed in RNAlater (Ambion, Austin, TX) for 4 h before dissections. Brain dissections were performed as previously described (18,19,20) and as detailed in Supplemental Methods (published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). Genomic DNA and total RNA were isolated simultaneously using AllPrep DNA/RNA Mini Kit (QIAGEN, Valencia, CA).
Gene expression analysis by quantitative real-time PCR (qRT-PCR)
For each individual sample, 500 ng total RNA was used in reverse transcription using the high-capacity reverse transcription kit (Applied Biosystems, Foster City, CA). Expression of target genes was determined by qRT-PCR using gene-specific TaqMan probes (Applied Biosystems) with TaqMan Gene Expression Master Mix (Applied Biosystems) on the ABI7900HT real-time PCR cycler. Probes used for RT-PCR are listed in Supplemental Table 1. The relative amount of each transcript was determined using the delta cycle threshold (ΔCt) values as previously described (21). Changes in gene expression were calculated using relative quantitation of a target gene against endogenous, unchanged glyceraldehyde 3-phosphate dehydrogenase (GAPDH) standard.
Global DNA methylation analysis
The amount of global, genome-wide DNA methylation was quantified using the Methylamp global DNA methylation quantification ultra kit (Epigentek, Brooklyn, NY) according to the manufacturer’s instructions. In this assay, 5-methylcytosine-modified genomic DNA is recognized by 5-methylcytosine antibody, and the bound DNA is quantified in a colorimetric reaction. Positive (methylated) and negative (unmethylated) control DNA was supplied with the kit. Absorbance was measured on an Emax Precision microplate reader (Molecular Devices, Sunnyvale, CA). The amount of DNA methylation (percent methylation) was calculated using the following formula: percent methylation = [OD (sample − negative control) × GC content]/[OD (positive control − negative control) × 10] × 100%. GC content for mouse genomic DNA is 42%.
Methylated DNA immunoprecipitation (MeDIP) assay
MeDIP assay was performed as described (22). Methylated DNA was immunoprecipitated using 10 μg mouse monoclonal 5-methylcytidine antibody (Eurogentec, Fremont, CA) or mouse preimmune serum. Enrichment in the MeDIP fraction was determined by qRT-PCR using ChIP-qPCR Assay Master Mix (SuperArray, Frederick, MD) on the ABI7900HT real-time PCR cycler. For all genes examined, primers were obtained from SuperArray [ChIP-qPCR assays (−01) kb tile] for the amplification of genomic regions spanning the CpG sites located approximately 300–500 bp upstream of the transcription start sites (see Supplemental Table 1 for primer sequences). MeDIP results are expressed as fold enrichment of immunoprecipitated DNA for each specific site. To calculate differential occupancy fold change (percent enrichment), the MeDIP DNA fractions’ Ct values were normalized to the input DNA fraction Ct value (see Supplemental Methods). Finally, the normalized level of DNA methylation at a particular site was expressed as relative to control group set to 1.
Statistical analyses
Data, presented as means ± sem, were analyzed using Prism 4 (GraphPad, San Diego, CA) and Excel tools for statistical analysis. Student’s t test was used to analyze differences in sucrose preference, fat preference, and gene expression between offspring from HF-fed and control dams, with Bonferroni correction for multiple t tests (multiple genes within a brain region) applied as warranted. A two-way ANOVA (group × region) was used to examine global methylation levels. A P value ≤ 0.05 was considered significant.
Results
Maternal and offspring characterization
To eliminate any potential adverse effect of handling on the development of offspring behavior and central nervous system (CNS) gene expression (23), a separate group of dams and offspring were used to determine maternal and offspring response to the HF diet (Table 1). On d 17 of the pregnancy, dams were weighed, and after an overnight fast, ip glucose tolerance tests were performed. HF-fed dams were significantly heavier; however, neither fasting glucose levels nor total area under the curve were different between the groups (although there was a nonsignificant trend for HF-fed dams to have an increased AUC). Offspring from obese dams weighed significantly more at d 3, 7, and 14 and at weaning than control pups (Table 1). By 5 wk of age and into adulthood, body weight did not differ between the groups.
Table 1.
Maternal and offspring characteristics
| Control-fed dams | HF-fed dams | |
|---|---|---|
| Maternal, d 17 of pregnancy | ||
| Weight (g) | 25.9 (2.05) | 41.9 (1.03)a |
| Fasting glucose (mg/dl) | 125.3 (19.7) | 128 (5.8) |
| GTT-AUC (mg/dl · h) | 920.8 (102.8) | 1352.3 (149.8)b |
| Offspring weight, preweaning (g) | ||
| PN3 | 2.63 (0.06) | 3.23 (0.07)c |
| PN7 | 4.78 (0.09) | 5.79 (.10)c |
| PN14 | 5.83 (.26) | 7.61 (.11)c |
| Weaning weight (g) | 9.07 (0.4) | 13.3 (0.5)c |
| Offspring weight, postweaning (g) | ||
| 5 wk | 20.1 (0.4) | 19.9 (0.7) |
| 10 wk | 27.3 (0.5) | 28.6 (0.9) |
| 20 wk | 36.5 (1.6) | 39.9 (1.2) |
Results are shown as mean (sem). For maternal weight and area under the curve (AUC) from glucose tolerance test (GTT), n = 4. Offspring were from two to three litters per group, four to six animals per litter, with separate groups at each time point. PN, Postnatal day.
P = 0.0004.
P = 0.06.
P < 0.0001.
Food preference
To evaluate whether maternal exposure to HF diet affected animals’ preference for palatable food, sucrose, or fat preference was examined in offspring from control or HF-fed dams. In separate experiments, animals (n = 6–8 per group, 24–26 wk of age) were allowed to freely choose between 1) 4% sucrose solution or water and 2) HF or control diet, and intake was recorded over 48 h. All animals preferred the palatable choice (HF or sucrose) over the control option (Fig. 1); however, the pups from HF-fed dams had a significantly increased preference for both sucrose (t test, P < 0.05) and HF diet (t test, P < 0.05).
Figure 1.
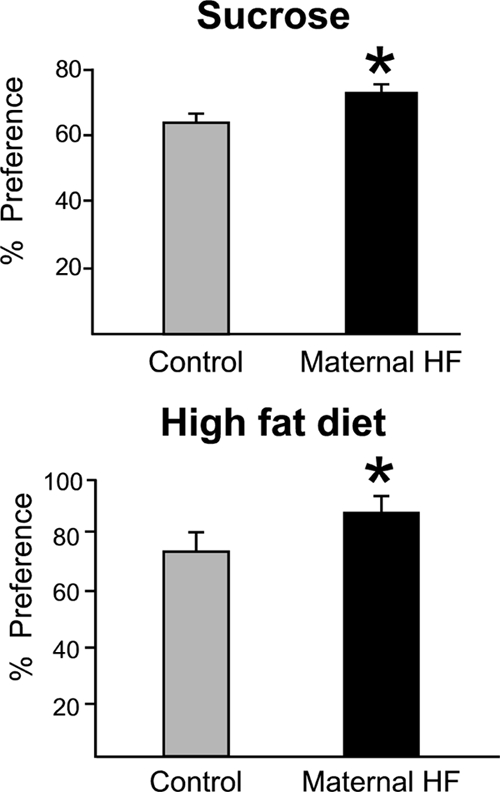
Preference for 4% sucrose solution (top) or 60% fat diet (bottom) was evaluated using preference tests in control offspring (gray bar) and offspring from HF-fed dams (black bar; n = 6–8 per group). Offspring from HF-fed dams have increased sucrose and fat preference compared with controls. Values represent mean ± sem. *, P < 0.05.
Dopamine-related gene expression is altered in offspring from HF-fed dams
We hypothesized that maternal exposure to a HF diet would alter the expression of CNS neurotransmitters/neuropeptides involved in the processing of reward, and we focused our studies on the dopamine and opioid systems. qRT-PCR was used to measure the relative expression of genes involved in the synthesis (TH, tyrosine hydroxylase), reuptake (DAT, dopamine reuptake transporter), and degradation of dopamine (COMT, catechol-O-methyltransferase) and modulation and transmission of dopamine signal (dopamine receptor D1, dopamine receptor D2, and dopamine- and cAMP-regulated phosphoprotein DARPP-32) in brain regions associated with reward processing [VTA, prefrontal cortex (PFC), and NAc] and homeostatic control of food intake (hypothalamus) of adult offspring (Fig. 2). The most notable differences were observed in DAT expression, with large, 3- to 10-fold increased expression in VTA, NAc, and PFC and a 50% reduction in expression in the hypothalamus of animals from HF-fed dams. The expression of TH was also up-regulated approximately 5-fold in the hypothalamus of these animals (n = 5; t test, P < 0.05), whereas expression of D1 and D2 receptors and DARPP-32 was reduced in both NAc and PFC, the primary target areas for mesocorticolimbic dopamine projections from the VTA. Expression levels of COMT, an enzyme involved in degradation of dopamine, did not differ (data not shown) regardless of the brain region. Taken together, these data demonstrate altered expression of key dopaminergic signaling molecules within the mesocorticolimbic circuitry and in the hypothalamus of offspring from HF-fed dams.
Figure 2.
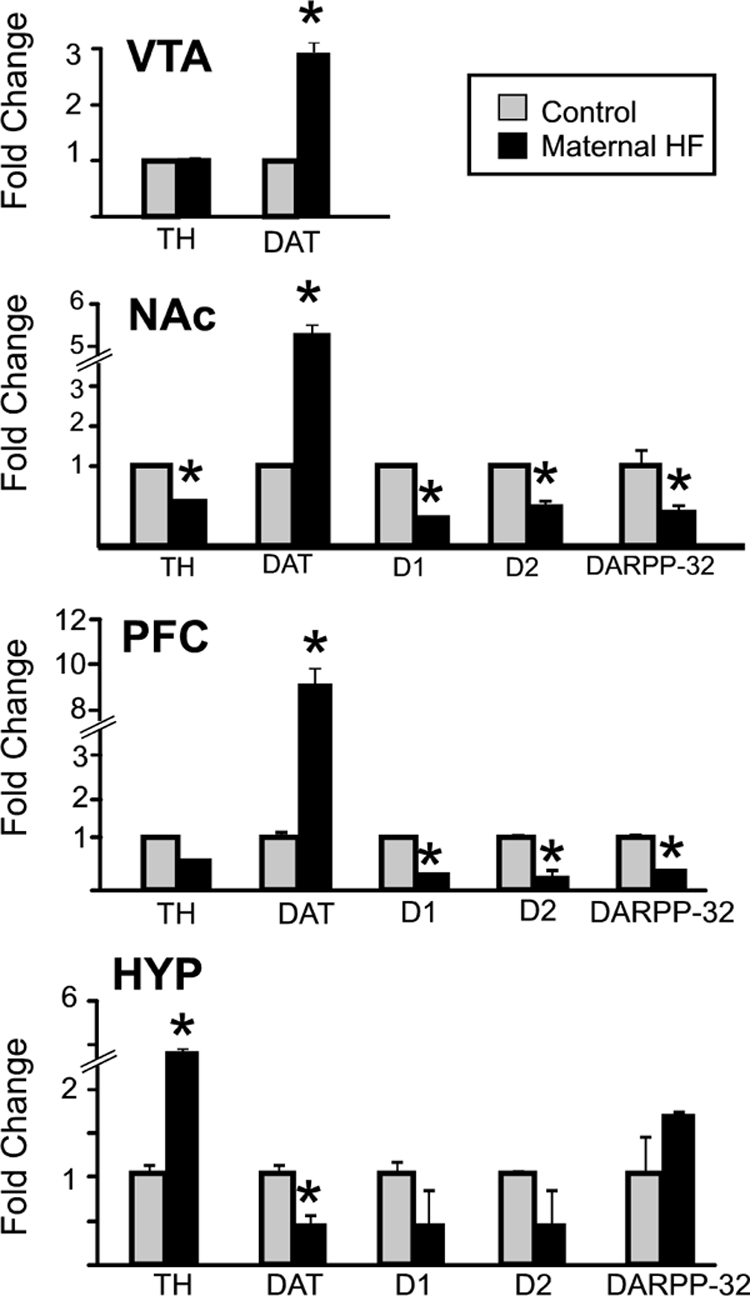
Dopamine-related gene expression. Quantitative RT-PCR was used to examine mRNA expression levels of dopamine pathway-related genes TH, DAT, D1, D2, and DARPP-32 in the CNS of adult male mice from dams fed either the control diet (control; gray bars) or HF diet (black bars). Mice from HF-fed dams demonstrate significant differences in multiple dopamine-related genes. Expression level of each gene examined was normalized to GAPDH levels and expressed relative to the control group set to 1. Values are mean ± sem. *, P values < 0.05 (n = 5 per group). HYP, Hypothalamus.
Expression of opioid ligands and receptors are altered in response to maternal HF diet
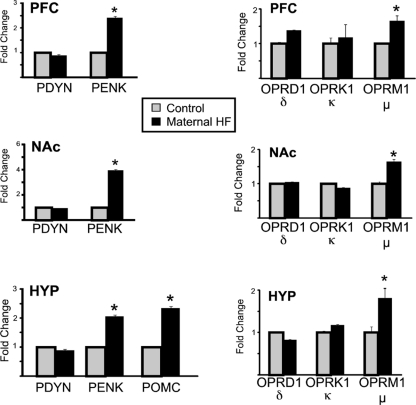
Significant differences in opioid ligands and receptors were observed in the offspring from dams fed the HF diet (Fig. 3; mean ± sem, P < 0.05; n = 6 per group). Preproenkephalin (PENK) was significantly up-regulated (∼2- to 4-fold) in the PFC, NAc, and hypothalamus of offspring from the maternal HF pregnancies, whereas (proopiomelanocortin) POMC was significantly increased in the hypothalamus. Of the three opioid receptor subtypes, only the μ-opioid receptor (MOR) was significantly altered (increased in all three regions: PFC, NAc, and hypothalamus). This up-regulation is notable, because both the μ-opioid receptor and PENK have been associated with an increase in the consumption of palatable foods.
Figure 3.
Opioid ligand and receptor gene expression. mRNA expression levels of the opioid prepropeptides (left) was examined in the PFC, NAc, and hypothalamus (HYP) of offspring from HF-fed dams (black bars) and control mice (gray bars) using qRT-PCR. PENK (NAc, PFC, and hypothalamus) and POMC (hypothalamus) were significantly up-regulated in mice from dams fed the HF diet. mRNA expression levels of opioid receptor genes OPRD1 (δ), OPRK1 (κ), and OPRM1 (μ) in the NAc, PFC, and hypothalamus of offspring from HF-fed dams (black bars) and control mice (gray bars) was determined by qRT-PCR (right). Mice from HF-fed dams demonstrate a significant up-regulation of μ-opioid receptor. Expression level of each gene examined was normalized to the GAPDH and β-actin levels and expressed relative to the control group set to 1. Values are mean ± sem. *, P values <0.05 (n = 6 per group).
Global methylation and methylation of the promoter regions of affected genes
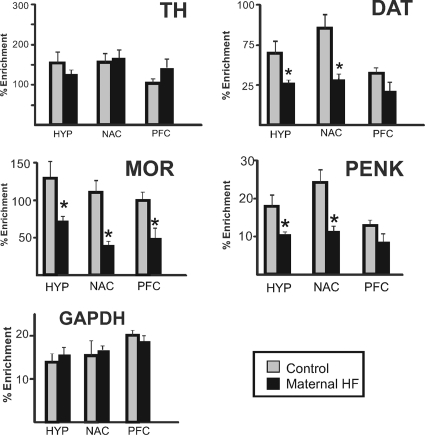
Given the altered gene expression observed in the brain, we examined differences in the total amount of genome-wide DNA methylation (Fig. 4). Two-way ANOVA revealed a significant decrease in global methylation in offspring from HF-fed dams [main effect for group: F(1,24) = 207.2; P < 0.0001] and a significant difference across the four brain regions tested [main effect for region: F(3,24) = 65.1; P < 0.0001]. Global methylation in the VTA was significantly lower than in other brain regions, and global methylation in the PFC was significantly lower than in the hypothalamus (groups compared with multiple t tests, Bonferroni correction for multiple comparisons α = 0.05/6 = 0.008). We then examined whether gene-specific promoter methylation of dopamine or opioid signaling components accompanied diet-induced gene expression changes. We used affinity purification of methylated DNA (MeDIP assay) followed by qRT-PCR to determine methylation levels of the proximal promoter regions of TH, DAT, MOR, and PENK (Fig. 5). In offspring from the HF pregnancies, there was significant hypomethylation of DAT, MOR, and PENK promoters in the HYP and NAc and significant hypomethylation of MOR in the PFC, which paralleled the increased expression of these genes in these regions. The amount of 5-methylcytosine-DNA-enriched fraction in the promoter region of TH of offspring from maternal HF diet did not differ significantly from the controls in any of the brain regions examined.
Figure 4.
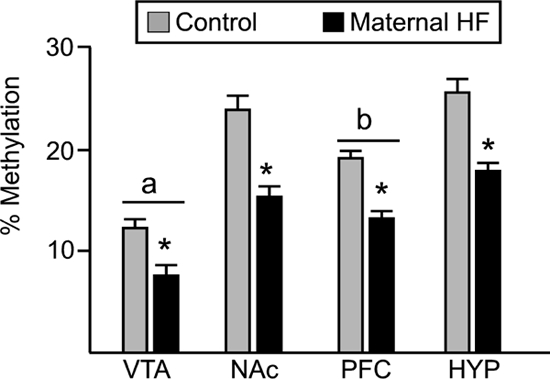
Global DNA methylation in brain regions of adult animals. Global methylation was analyzed using Methylamp kit (Epigentek) in animals from control (gray bars) or HF-fed (black bars) dams. Values are mean ± sem. Two-way ANOVA revealed a main effect for group (*, P < 0.0001), with offspring from HF-fed dams demonstrating a significant reduction in global DNA methylation. Furthermore, global methylation levels in the VTA were significantly lower than in all other brain regions (a, P < 0.008), and global methylation in PFC was significantly lower than in the hypothalamus (b, P < 0.008) (n = 5 per group).
Figure 5.
DNA methylation changes at regulatory regions of reward-associated genes in offspring from HF-fed dams. Genomic DNA was isolated, sheared, and immunoprecipitated with 5-methylcytosine-specific antibody from brain regions from offspring of control (gray bars) or HF-fed (black bars) dams. The enrichment of DNA methylation relative to input genomic DNA at proximal promoter regions of TH, DAT, PENK, μ-opioid receptor (MOR), and GAPDH was quantified by qRT-PCR. Significant hypomethylation was observed in DAT, MOR, and PENK in offspring from HF-fed dams, which paralleled the increased expression of these genes. Values are mean ± sem. *, P values < 0.05 (n = 6 per group, two-tailed t test).
Discussion
Increasing evidence supports the idea that maternal obesity and/or consumption of a HF diet during early fetal life (pregnancy and/or lactation) can increase the risk for development of obesity and related negative metabolic outcomes in the offspring. The present studies examine potential underlying mechanisms linking maternal consumption of HF diet to adverse offspring development, with a number of novel findings, including identifying alterations in the dopamine and opioid systems and both global and gene-specific differential DNA methylation.
It has been reported that maternal consumption of palatable foods (e.g. sugar, fat, and salt) can program taste preference in the offspring, such that there is an increased preference for palatable foods in the offspring (9,10,11), but to date, the mechanism(s) underlying the programming of preference for palatable foods remain unclear. We hypothesized that dopamine- and opioid-related gene expression within mesocorticolimbic circuitry, which underlies the processing of the rewarding properties of food, may be altered in offspring from HF-fed dams. We report significant increases in DAT expression in the VTA, NAc, and PFC as well as decreased expression of D1, D2, and DARPP-32 within NAc and PFC. Collectively, these changes can result in a hypodopaminergic state, with increased reuptake of dopamine [via increased DAT expression (24) and decreased signaling through the receptors]. A hypodopaminergic state has been linked to increased consumption of rewarding stimuli in an effort to restore homeostasis within this circuitry (25). Dopamine-related gene expression was also altered in the hypothalamus, but in an opposing pattern to that seen within the mesocorticolimbic structures, with increased expression of TH and decreased expression of DAT. In line with these findings, dopamine acting within the hypothalamus is known to promote food intake (26,27).
In addition to altered expression of dopamine-related genes, this is the first report showing that HF diet consumption during pregnancy and lactation is associated with altered expression of μ-opioid receptor and the opiate ligand preproenkephalin, genes specifically linked to the intake of palatable foods. These findings extend a recent report that showed maternal consumption of HF diet in pregnant rats increased PENK expression in hypothalamus (8). Studies have shown that opioid antagonists, such as naloxone or naltrexone, reduce the consumption of a palatable food, whereas agonists, such as morphine or the μ-opioid receptor agonist [D-Ala2, N-MePhe4, Gly-ol]-enkephalin, increase food consumption (28). Injection of [D-Ala2, N-MePhe4, Gly-ol]-enkephalin into the NAc was found to selectively increase the intake of HF diet (29). Injections of the enkephalin and dynorphin peptides have been shown to stimulate feeding, particularly that of a HF diet more than a low-fat diet and a fat-rich diet more than a carbohydrate-rich diet (30). Furthermore, it is possible that our findings of increased opioid ligand and receptor expression may participate in our observations of altered dopamine-related molecules, as opioid-receptor activation or blockade in the hypothalamus has been shown to stimulate or inhibit, respectively, release of dopamine in the NAc (31). The use of qRT-PCR using TaqMan-labeled probes allowed for the highly sensitive and specific quantification of numerous genes per sample in macrodissected tissue. To improve the anatomical specificity in future experiments, in situ hybridization experiments could be used to identify subregions that account for the observed responses.
Global DNA hypomethylation was observed in all brain regions examined in the animals from HF-fed dams. The effect of maternal diet on global DNA methylation has not been previously reported; however, global hypomethylation has been observed in other models of adverse in utero environment, including in whole brain from pups that experienced bilateral uterine artery ligation (32) or hippocampal samples from pups with prenatal cocaine exposure (33). The amount of global DNA methylation represents methylation status of repetitive DNA elements, transposons and intergenic sequences, which are typically heavily methylated. Loss of genomic DNA methylation not only contributes to locus- and gene-specific changes in DNA methylation but also can have multiple genome-wide consequences including chromosomal instability, aberrant activation of endogenous retroviral elements, and loss of imprinting (34). A large literature supports the association between DNA hypomethylation and increased cancer risk (35); however, little is known about the effects of global DNA hypomethylation in the CNS. Forebrain-specific DNA methyltransferase 1 (DNMT1) knockout animals demonstrate global hypomethylation and provide some information on potential effects of global hypomethylation in the CNS. These animals have progressive neurodegeneration, altered neuronal gene expression, and severe degeneration of cortical neurons (36,37). Therefore, global hypomethylation has been associated with altered neuronal viability, which may have broad effects on CNS gene expression and behavioral endpoints.
Given the observed global DNA hypomethylation and in an effort to determine whether differential methylation patterns were related to increased gene expression, we examined the methylation status of the promoter region of the genes that demonstrated altered expression. DAT, MOR, and PENK all showed hypomethylation within their promoter regions, in accordance with increased expression of each transcript, suggesting that maternal HF diet during pregnancy and lactation can alter the methylation status of specific gene promoters leading to persistent changes in gene expression. Expression of MOR in cultured cell lines and in the mouse brain has been shown to be under epigenetic control occurring through histone and DNA methylation changes (38,39,40). Epigenetic regulation of DAT and PENK promoters is not well understood; however, studies that identified altered DAT promoter methylation in patients with eating disorders (41) and in alcohol-dependent patients (42) suggest that epigenetic mechanisms may underlie dysregulation of dopamine signaling in these diseases. This is the first report of altered methylation status of MOR, DAT, or PENK in response to an in vivo challenge in the mouse and suggests these genes may be particularly vulnerable to differential methylation due to suboptimal early life conditions. In our study, as would be predicted from similar experimental strategies (43), the epigenetic effect did not apply to all genes, because methylation of the promoter region of TH was not different in any region, suggesting that mechanisms other than differential DNA methylation were responsible for alterations in TH gene expression seen in NAc and hypothalamus, such as histone modifications or differential recruitment of specific transcription factors on the TH promoter protecting it from DNA methylation changes. It is not well understood why just a subset of gene-specific regions is targeted and corresponds to global changes in DNA methylation. A potential mechanism could be that certain genes are preferentially susceptible to acquiring DNA methylation changes based on the surrounding chromatin structure (e.g. enriched in certain histone marks) or are more easily targeted for DNA methylation changes because of association with specific transcription factors (44,45,46). Recently, it has been shown that maternal intake of a HF diet decreased methylation of the promoter region of GH secretagogue receptor, an effect persisting into at least the second generation (47), suggesting the possibility that our observed epigenetic effects on dopamine and opioid gene expression may extend beyond the F1 generation, although this remains to be empirically tested. And although the current studies focused on DNA methylation, additional epigenetic modifications that can alter transcriptional activity (e.g. histone methylation or acetylation) may well play a role in the observed effects.
In our studies, dams were fed the HF diet approximately 3 months before breeding so that they would be significantly heavier at the time of mating. The dams weighed more but were not diabetic, because they had normal fasting glucose levels and the response in the glucose tolerance test was not statistically different between the groups (HF-fed dams had an elevated glucose response, but the difference was not statistically reliable). One question that remains unresolved by the current experiments is the respective contributions of the HF diet vs. the maternal response to the diet (i.e. increased adiposity and increased adipokine secretion). In a recent study that included a pair-fed group of rats that were given the HF diet restricted in amount to match the caloric intake of the control group, it was found that maternal adiposity, and not dietary fat per se, was found to induce hyperleptinemia and insulin resistance in offspring as well as an increased body weight that persisted into adulthood (48). An important caveat, however, is that pair feeding has been shown to induce a pronounced stress response (49), which can independently impact fetal development. Conversely, in rats, HF diet consumption during pregnancy in the absence of significant weight gain altered expression of orexigenic neuropeptides within the hypothalamus (8). Parsing out the respective contributions of the maternal HF diet vs. the maternal response to the diet with regard to specific outcomes in the offspring remains an important issue of study. Additionally, the HF-fed dams were maintained on the HF diet through the end of lactation in an effort to more fully cover the period of offspring brain development. Although the majority of neurogenesis of dopaminergic and opioidergic neurons occurs in utero, significant additional brain development does occur postnatally (e.g. postnatal d 3–11 are thought to mirror the third trimester of a human pregnancy) (50). And in fact, a study that restricted HF diet exposure to the last week of gestation and through lactation, a time at which neurogenesis for dopaminergic neurons is complete, found significant effects of maternal HF diet on the development and function of the dopamine system in the offspring (51), highlighting the vulnerability of this later period of brain development to changes in maternal diet and, specifically, exposure to HF diet. Because estrogen is known to affect dopamine (52), MOR (53), response to sucrose (54), and food intake (55), only male offspring were studied in the current experiments. Future studies with female offspring, controlling for cycling status, will be an important future direction.
Collectively, our data show that maternal consumption of HF diet during pregnancy and lactation can program an increased drive for the consumption of palatable foods in parallel with alterations in dopamine and opioid gene expression within mesocorticolimbic and hypothalamic circuitry. An overall increase in food intake will contribute to the risk for obesity, but it is the overconsumption of highly palatable and energy-dense foods (e.g. HF, high-sugar foods) that more rapidly drives the development of obesity. Furthermore, these data identify an epigenetic modification (promoter region hypomethylation) as a potential mechanism for increased long-term expression of dopamine and opioid-related genes (DAT, MOR, and PENK). These findings add to a growing literature that detail adverse neurodevelopmental consequences of maternal obesity and/or ingestion of a HF diet during pregnancy.
Supplementary Material
Footnotes
The current work was supported by NIH DK064086 (to T.M.R.) and MH087978 (to T.M.R.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online August 4, 2010
Abbreviations: CNS, Central nervous system; ΔCt, delta cycle threshold; HF, high fat; MeDIP, methylated DNA immunoprecipitation; NAc, nucleus accumbens; PFC, prefrontal cortex; qRT-PCR, quantitative real-time PCR; VTA, ventral tegmental area.
References
- Howie GJ, Sloboda DM, Kamal T, Vickers MH 2009 Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol 587:905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Morris MJ 2009 Differential responses of orexigenic neuropeptides to fasting in offspring of obese mothers. Obesity (Silver Spring) 17:1356–1362 [DOI] [PubMed] [Google Scholar]
- Gupta A, Srinivasan M, Thamadilok S, Patel MS 2009 Hypothalamic alterations in fetuses of high fat diet-fed obese female rats. J Endocrinol 200:293–300 [DOI] [PubMed] [Google Scholar]
- Morris MJ, Chen H 2009 Established maternal obesity in the rat reprograms hypothalamic appetite regulators and leptin signaling at birth. Int J Obes (Lond) 33:115–122 [DOI] [PubMed] [Google Scholar]
- Chen H, Simar D, Morris MJ 2009 Hypothalamic neuroendocrine circuitry is programmed by maternal obesity: interaction with postnatal nutritional environment. PLoS One 4:e6259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlhausler BS, Adam CL, Findlay PA, Duffield JA, McMillen IC 2006 Increased maternal nutrition alters development of the appetite-regulating network in the brain. FASEB J 20:1257–1259 [DOI] [PubMed] [Google Scholar]
- Chen H, Simar D, Lambert K, Mercier J, Morris MJ 2008 Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology 149:5348–5356 [DOI] [PubMed] [Google Scholar]
- Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF 2008 Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci 28:12107–12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teegarden SL, Scott AN, Bale TL 2009 Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience 162:924–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayol SA, Farrington SJ, Stickland NC 2007 A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br J Nutr 98:843–851 [DOI] [PubMed] [Google Scholar]
- Brion MJ, Ness AR, Rogers I, Emmett P, Cribb V, Smith GD, Lawlor DA 2010 Maternal macronutrient and energy intakes in pregnancy and offspring intake at 10 y: exploring parental comparisons and prenatal effects. Am J Clin Nutr 91:748–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G 1999 Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience 89:637–641 [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M 1997 Drug abuse: hedonic homeostatic dysregulation. Science 278:52–58 [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL 2009 Reward processing by the opioid system in the brain. Physiol Rev 89:1379–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinus L, Cador M, Le Moal M 1992 Interaction between endogenous opioids and dopamine within the nucleus accumbens. Ann NY Acad Sci 654:254–273 [DOI] [PubMed] [Google Scholar]
- Murphy SK, Jirtle RL 2003 Imprinting evolution and the price of silence. Bioessays 25:577–588 [DOI] [PubMed] [Google Scholar]
- Burdge GC, Hanson MA, Slater-Jefferies JL, Lillycrop KA 2007 Epigenetic regulation of transcription: a mechanism for inducing variations in phenotype (fetal programming) by differences in nutrition during early life? Br J Nutr 97:1036–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z, Totoki K, Schoch H, Whitaker KW, Hill-Smith T, Lucki I, Reyes TM 2010 Early life protein restriction alters dopamine circuitry. Neuroscience 168:359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes TM, Walker JR, DeCino C, Hogenesch JB, Sawchenko PE 2003 Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci 23:5607–5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleck JN, Ecke LE, Blendy JA 2008 Endocrine and gene expression changes following forced swim stress exposure during cocaine abstinence in mice. Psychopharmacology (Berl) 201:15–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW 2001 A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Davies JJ, Wittig D, Oakeley EJ, Haase M, Lam WL, Schübeler D 2005 Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nat Genet 37:853–862 [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmühl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D 2009 Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci 12:1559–1566 [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Jones SR, Caron MG 1999 Functional hyperdopaminergia in dopamine transporter knock-out mice. Biol Psychiatry 46:303–311 [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M 2008 Addiction and the brain antireward system. Annu Rev Psychol 59:29–53 [DOI] [PubMed] [Google Scholar]
- Meguid MM, Fetissov SO, Varma M, Sato T, Zhang L, Laviano A, Rossi-Fanelli F 2000 Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition 16:843–857 [DOI] [PubMed] [Google Scholar]
- Meguid MM, Yang ZJ, Laviano A 1997 Meal size and number: relationship to dopamine levels in the ventromedial hypothalamic nucleus. Am J Physiol 272(6 Pt 2):R1295–R1230 [DOI] [PubMed] [Google Scholar]
- Barbano MF, Cador M 2007 Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology (Berl) 191:497–506 [DOI] [PubMed] [Google Scholar]
- Zhang M, Gosnell BA, Kelley AE 1998 Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther 285:908–914 [PubMed] [Google Scholar]
- Leibowitz SF 2007 Overconsumption of dietary fat and alcohol: mechanisms involving lipids and hypothalamic peptides. Physiol Behav 91:513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Barson JR, Leibowitz SF, Hoebel BG 2010 Opioids in the hypothalamus control dopamine and acetylcholine levels in the nucleus accumbens. Brain Res 1312:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke X, Lei Q, James SJ, Kelleher SL, Melnyk S, Jernigan S, Yu X, Wang L, Callaway CW, Gill G, Chan GM, Albertine KH, McKnight RA, Lane RH 2006 Uteroplacental insufficiency affects epigenetic determinants of chromatin structure in brains of neonatal and juvenile IUGR rats. Physiol Genomics 25:16–28 [DOI] [PubMed] [Google Scholar]
- Novikova SI, He F, Bai J, Cutrufello NJ, Lidow MS, Undieh AS 2008 Maternal cocaine administration in mice alters DNA methylation and gene expression in hippocampal neurons of neonatal and prepubertal offspring. PLoS One 3:e1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AS, Power BE, Molloy PL 2007 DNA hypomethylation and human diseases. Biochim Biophys Acta 1775:138–162 [DOI] [PubMed] [Google Scholar]
- Dunn BK 2003 Hypomethylation: one side of a larger picture. Ann NY Acad Sci 983:28–42 [DOI] [PubMed] [Google Scholar]
- Golshani P, Hutnick L, Schweizer F, Fan G 2005 Conditional Dnmt1 deletion in dorsal forebrain disrupts development of somatosensory barrel cortex and thalamocortical long-term potentiation. Thalamus Relat Syst 3:227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutnick LK, Golshani P, Namihira M, Xue Z, Matynia A, Yang XW, Silva AJ, Schweizer FE, Fan G 2009 DNA hypomethylation restricted to the murine forebrain induces cortical degeneration and impairs postnatal neuronal maturation. Hum Mol Genet 18:2875–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CK, Song KY, Kim CS, Choi HS, Guo XH, Law PY, Wei LN, Loh HH 2009 Epigenetic programming of μ-opioid receptor gene in mouse brain is regulated by MeCP2 and brg1 chromatin remodelling factor. J Cell Mol Med 13:3591–3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CK, Song KY, Kim CS, Choi HS, Guo XH, Law PY, Wei LN, Loh HH 2007 Evidence of endogenous μ-opioid receptor regulation by epigenetic control of the promoters. Mol Cell Biol 27:4720–4736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LN 2008 Epigenetic control of the expression of opioid receptor genes. Epigenetics 3:119–121 [DOI] [PubMed] [Google Scholar]
- Frieling H, Römer KD, Scholz S, Mittelbach F, Wilhelm J, De Zwaan M, Jacoby GE, Kornhuber J, Hillemacher T, Bleich S 2 September 2009 Epigenetic dysregulation of dopaminergic genes in eating disorders. Int J Eat Disord 10.1002/eat.20745 [DOI] [PubMed] [Google Scholar]
- Hillemacher T, Frieling H, Hartl T, Wilhelm J, Kornhuber J, Bleich S 2009 Promoter specific methylation of the dopamine transporter gene is altered in alcohol dependence and associated with craving. J Psychiatr Res 43:388–392 [DOI] [PubMed] [Google Scholar]
- van Straten EM, Bloks VW, Huijkman NC, Baller JF, Meer H, Lütjohann D, Kuipers F, Plösch T 2010 The liver X-receptor gene promoter is hypermethylated in a mouse model of prenatal protein restriction. Am J Physiol Regul Integr Comp Physiol 298:R275–R282 [DOI] [PubMed] [Google Scholar]
- Schlesinger Y, Straussman R, Keshet I, Farkash S, Hecht M, Zimmerman J, Eden E, Yakhini Z, Ben-Shushan E, Reubinoff BE, Bergman Y, Simon I, Cedar H 2007 Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nat Genet 39:232–236 [DOI] [PubMed] [Google Scholar]
- Ohm JE, McGarvey KM, Yu X, Cheng L, Schuebel KE, Cope L, Mohammad HP, Chen W, Daniel VC, Yu W, Berman DM, Jenuwein T, Pruitt K, Sharkis SJ, Watkins DN, Herman JG, Baylin SB 2007 A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet 39:237–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan S, Van Emburgh BO, Robertson KD 2008 DNA methylation in development and human disease. Mutat Res 647:30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GA, Bale TL 2009 Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology 150:4999–5009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CL, Purpera MN, Morrison CD 2009 Maternal obesity is necessary for programming effect of high-fat diet on offspring. Am J Physiol Regul Integr Comp Physiol 296:R1464–R1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Luna C, Amaya MI, Alvarez-Salas E, de Gortari P 2010 Prepro-orexin and feeding-related peptide receptor expression in dehydration-induced anorexia. Regul Pept 159:54–60 [DOI] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR 2003 Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol 25:447–458 [DOI] [PubMed] [Google Scholar]
- Naef L, Srivastava L, Gratton A, Hendrickson H, Owens SM, Walker CD 2008 Maternal high fat diet during the perinatal period alters mesocorticolimbic dopamine in the adult rat offspring: reduction in the behavioral responses to repeated amphetamine administration. Psychopharmacology (Berl) 197:83–94 [DOI] [PubMed] [Google Scholar]
- Afonso VM, King S, Chatterjee D, Fleming AS 2009 Hormones that increase maternal responsiveness affect accumbal dopaminergic responses to pup- and food-stimuli in the female rat. Horm Behav 56:11–23 [DOI] [PubMed] [Google Scholar]
- Torres-Reveron A, Williams TJ, Chapleau JD, Waters EM, McEwen BS, Drake CT, Milner TA 2009 Ovarian steroids alter mu opioid receptor trafficking in hippocampal parvalbumin GABAergic interneurons. Exp Neurol 219:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis KS, Stratford JM, Contreras RJ 2005 Estrogen increases the taste threshold for sucrose in rats. Physiol Behav 86:281–286 [DOI] [PubMed] [Google Scholar]
- Butera PC, Wojcik DM, Clough SJ 2010 Effects of estradiol on food intake and meal patterns for diets that differ in flavor and fat content. Physiol Behav 99:142–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.