Abstract
Regulation of cytosolic Ca2+ and cytosolic Na+ is critical for lymphocyte cation homeostasis and function. To examine the influence of cytosolic Na+ on Ca2+ regulation in human peripheral blood lymphocytes, Ca2+ entry and cytosolic Ca2+ (measured with fura-2) were monitored in cells in which cytosolic Na+ was increased and/or the Na+ gradient was decreased by reduction of external Na+ concentration. Ouabain-treated cells (0.1 mM for 30 min at 37 degrees C), suspended in Na(+)-free medium, showed a 30-65% increase in Ca2+ uptake compared to cells in 140 mM Na+ medium. Enhanced Ca2+ influx was entirely dependent on ouabain pretreatment and reversal of the Na+ gradient. Na pump inhibition or Na ionophore addition and subsequent exposure to Na(+)-free medium resulted in a sustained elevation of cytosolic Ca2+. As preincubation of cells in Ca(2+)-free medium further enhanced the ouabain-dependent increase in cytosolic Ca2+, the effects of the microsomal Ca(2+)-ATPase inhibitor thapsigargin on Ca2+ influx and cytosolic Ca2+ were studied. Thapsigargin stimulated Ca2+ entry following ouabain pretreatment and reversal of the Na+ gradient; the effects of thapsigargin were retained in the presence of LaCl3, a potent inhibitor of store-dependent calcium influx pathways. These results show lymphocytes demonstrate Na+/Ca2+ exchange activity and suggest the Na+/Ca2+ exchanger modulates cytosolic Ca2+ following intracellular Ca2+ store depletion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexiewicz J. M., Gaciong Z., Parise M., Karubian F., Massry S. G., Campese V. M. Effect of dietary sodium intake on intracellular calcium in lymphocytes of salt-sensitive hypertensive patients. Am J Hypertens. 1992 Aug;5(8):536–541. doi: 10.1093/ajh/5.8.536. [DOI] [PubMed] [Google Scholar]
- Ambrosioni E., Costa F. V., Montebugnoli L., Tartagni F., Magnani B. Increased intralymphocytic sodium content in essential hypertension: an index of impaired Na+ cellular metabolism. Clin Sci (Lond) 1981 Aug;61(2):181–186. doi: 10.1042/cs0610181. [DOI] [PubMed] [Google Scholar]
- Aviv A. Cytosolic Ca2+, Na+/H+ antiport, protein kinase C trio in essential hypertension. Am J Hypertens. 1994 Feb;7(2):205–212. doi: 10.1093/ajh/7.2.205. [DOI] [PubMed] [Google Scholar]
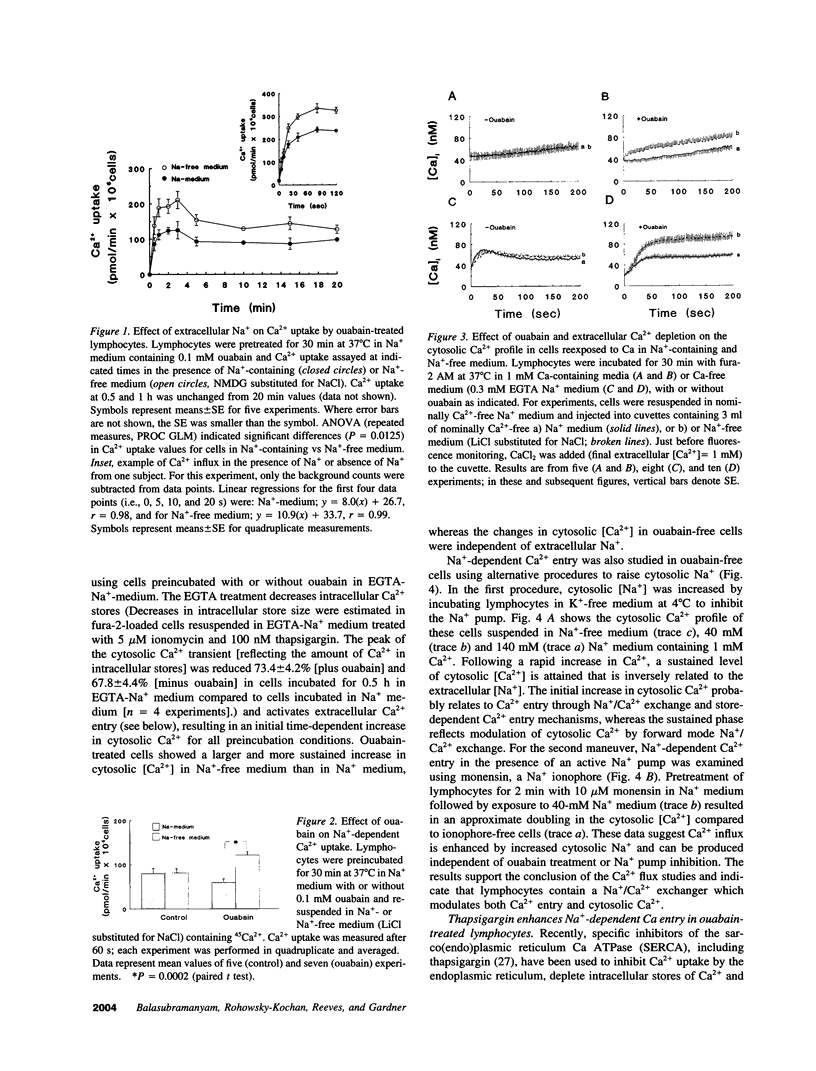
- Balasubramanyam M., Kimura M., Aviv A., Gardner J. P. Kinetics of calcium transport across the lymphocyte plasma membrane. Am J Physiol. 1993 Aug;265(2 Pt 1):C321–C327. doi: 10.1152/ajpcell.1993.265.2.C321. [DOI] [PubMed] [Google Scholar]
- Breittmayer J. P., Ticchioni M., Ferrua B., Bernard A., Aussel C. Ca(2+)-ATPase inhibitors induce interleukin-2 synthesis and T cell proliferation. Cell Immunol. 1993 Jul;149(2):248–257. doi: 10.1006/cimm.1993.1152. [DOI] [PubMed] [Google Scholar]
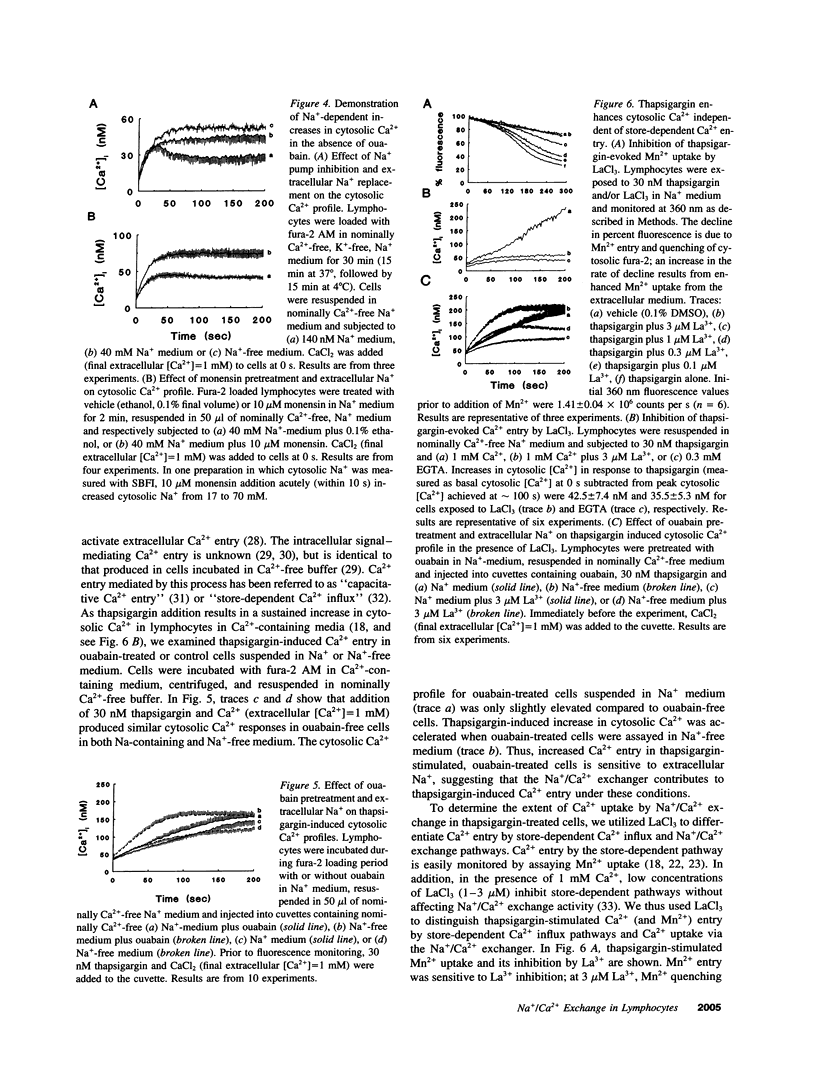
- Clementi E., Scheer H., Zacchetti D., Fasolato C., Pozzan T., Meldolesi J. Receptor-activated Ca2+ influx. Two independently regulated mechanisms of influx stimulation coexist in neurosecretory PC12 cells. J Biol Chem. 1992 Feb 5;267(4):2164–2172. [PubMed] [Google Scholar]
- Dipolo R., Beaugé L. The effect of pH on Ca2+ extrusion mechanisms in dialyzed squid axons. Biochim Biophys Acta. 1982 May 21;688(1):237–245. doi: 10.1016/0005-2736(82)90599-5. [DOI] [PubMed] [Google Scholar]
- Dolmetsch R. E., Lewis R. S. Signaling between intracellular Ca2+ stores and depletion-activated Ca2+ channels generates [Ca2+]i oscillations in T lymphocytes. J Gen Physiol. 1994 Mar;103(3):365–388. doi: 10.1085/jgp.103.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnadieu E., Bismuth G., Trautmann A. Calcium fluxes in T lymphocytes. J Biol Chem. 1992 Dec 25;267(36):25864–25872. [PubMed] [Google Scholar]
- Donnadieu E., Cefai D., Tan Y. P., Paresys G., Bismuth G., Trautmann A. Imaging early steps of human T cell activation by antigen-presenting cells. J Immunol. 1992 May 1;148(9):2643–2653. [PubMed] [Google Scholar]
- Donnadieu E., Trautmann A. Is there a Na+/Ca2+ exchanger in macrophages and in lymphocytes? Pflugers Arch. 1993 Sep;424(5-6):448–455. doi: 10.1007/BF00374907. [DOI] [PubMed] [Google Scholar]
- Edmondson R. P., Thomas R. D., Hilton P. J., Patrick J., Jones N. F. Abnormal leucocyte composition and sodium transport in essential hypertension. Lancet. 1975 May 3;1(7914):1003–1005. doi: 10.1016/s0140-6736(75)91947-9. [DOI] [PubMed] [Google Scholar]
- Gouy H., Cefai D., Christensen S. B., Debré P., Bismuth G. Ca2+ influx in human T lymphocytes is induced independently of inositol phosphate production by mobilization of intracellular Ca2+ stores. A study with the Ca2+ endoplasmic reticulum-ATPase inhibitor thapsigargin. Eur J Immunol. 1990 Oct;20(10):2269–2275. doi: 10.1002/eji.1830201016. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hallam T. J., Rink T. J. Agonists stimulate divalent cation channels in the plasma membrane of human platelets. FEBS Lett. 1985 Jul 8;186(2):175–179. doi: 10.1016/0014-5793(85)80703-1. [DOI] [PubMed] [Google Scholar]
- Huot S. J., Aronson P. S. Na(+)-H+ exchanger and its role in essential hypertension and diabetes mellitus. Diabetes Care. 1991 Jun;14(6):521–535. doi: 10.2337/diacare.14.6.521. [DOI] [PubMed] [Google Scholar]
- Imboden J. B., Weiss A. The T-cell antigen receptor regulates sustained increases in cytoplasmic free Ca2+ through extracellular Ca2+ influx and ongoing intracellular Ca2+ mobilization. Biochem J. 1987 Nov 1;247(3):695–700. doi: 10.1042/bj2470695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechene C., Crabos M., Bianchi G., Cantiello H. Rôle physiologique de la pompe à sodium. Implications pour l'étude de l'hypertension artérielle. Nephrologie. 1989;10(2):59–64. [PubMed] [Google Scholar]
- Lichtman A. H., Segel G. B., Lichtman M. A. Calcium transport and calcium-ATPase activity in human lymphocyte plasma membrane vesicles. J Biol Chem. 1981 Jun 25;256(12):6148–6154. [PubMed] [Google Scholar]
- Lytton J., Westlin M., Hanley M. R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991 Sep 15;266(26):17067–17071. [PubMed] [Google Scholar]
- Lyu R. M., Smith L., Smith J. B. Ca2+ influx via Na(+)-Ca2+ exchange in immortalized aortic myocytes. I. Dependence on [Na+]i and inhibition by external Na+. Am J Physiol. 1992 Sep;263(3 Pt 1):C628–C634. doi: 10.1152/ajpcell.1992.263.3.C628. [DOI] [PubMed] [Google Scholar]
- Lyu R. M., Smith L., Smith J. B. Sodium-calcium exchange in renal epithelial cells: dependence on cell sodium and competitive inhibition by magnesium. J Membr Biol. 1991 Oct;124(1):73–83. doi: 10.1007/BF01871366. [DOI] [PubMed] [Google Scholar]
- Minta A., Tsien R. Y. Fluorescent indicators for cytosolic sodium. J Biol Chem. 1989 Nov 15;264(32):19449–19457. [PubMed] [Google Scholar]
- Ng L. L., Fennell D. A., Dudley C. Kinetics of the human leucocyte Na(+)-H+ antiport in essential hypertension. J Hypertens. 1990 Jun;8(6):533–537. doi: 10.1097/00004872-199006000-00006. [DOI] [PubMed] [Google Scholar]
- Ng L. L., Simmons D., Frighi V., Garrido M. C., Bomford J., Hockaday T. D. Leucocyte Na+/H+ antiport activity in type 1 (insulin-dependent) diabetic patients with nephropathy. Diabetologia. 1990 Jun;33(6):371–377. doi: 10.1007/BF00404642. [DOI] [PubMed] [Google Scholar]
- Oshima T., Matsuura H., Kido K., Matsumoto K., Fujii H., Masaoka S., Okamoto M., Tsuchioka Y., Kajiyama G., Tsubokura T. Intralymphocytic sodium and free calcium and plasma renin in essential hypertension. Hypertension. 1988 Jul;12(1):26–31. doi: 10.1161/01.hyp.12.1.26. [DOI] [PubMed] [Google Scholar]
- Parekh A. B., Terlau H., Stühmer W. Depletion of InsP3 stores activates a Ca2+ and K+ current by means of a phosphatase and a diffusible messenger. Nature. 1993 Aug 26;364(6440):814–818. doi: 10.1038/364814a0. [DOI] [PubMed] [Google Scholar]
- Philipson K. D., Bersohn M. M., Nishimoto A. Y. Effects of pH on Na+-Ca2+ exchange in canine cardiac sarcolemmal vesicles. Circ Res. 1982 Feb;50(2):287–293. doi: 10.1161/01.res.50.2.287. [DOI] [PubMed] [Google Scholar]
- Pijuan V., Zhuang Y., Smith L., Kroupis C., Condrescu M., Aceto J. F., Reeves J. P., Smith J. B. Stable expression of the cardiac sodium-calcium exchanger in CHO cells. Am J Physiol. 1993 Apr;264(4 Pt 1):C1066–C1074. doi: 10.1152/ajpcell.1993.264.4.C1066. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr The capacitative model for receptor-activated calcium entry. Adv Pharmacol. 1991;22:251–269. doi: 10.1016/s1054-3589(08)60037-x. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Tsien R. Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993 Aug 26;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Reeves J. P. Measurement of sodium-calcium exchange activity in plasma membrane vesicles. Methods Enzymol. 1988;157:505–510. doi: 10.1016/0076-6879(88)57099-4. [DOI] [PubMed] [Google Scholar]
- Reusch H. P., Reusch R., Rosskopf D., Siffert W., Mann J. F., Luft F. C. Na+/H+ exchange in human lymphocytes and platelets in chronic and subacute metabolic acidosis. J Clin Invest. 1993 Aug;92(2):858–865. doi: 10.1172/JCI116660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohowsky-Kochan C., Eiman D., Denny T., Oleske J., Cook S. D. Induction of autologous mixed lymphocyte culture responses by myelin basic protein-reactive T cell clones. J Neuroimmunol. 1994 Feb;50(1):59–70. doi: 10.1016/0165-5728(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Rosskopf D., Düsing R., Siffert W. Membrane sodium-proton exchange and primary hypertension. Hypertension. 1993 May;21(5):607–617. doi: 10.1161/01.hyp.21.5.607. [DOI] [PubMed] [Google Scholar]
- Rosskopf D., Frömter E., Siffert W. Hypertensive sodium-proton exchanger phenotype persists in immortalized lymphoblasts from essential hypertensive patients. A cell culture model for human hypertension. J Clin Invest. 1993 Nov;92(5):2553–2559. doi: 10.1172/JCI116865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff O., Foder B., Thastrup O., Hofmann B., Møller J., Ryder L. P., Jacobsen K. D., Langhoff E., Dickmeiss E., Christensen S. B. Effect of thapsigargin on cytoplasmic Ca2+ and proliferation of human lymphocytes in relation to AIDS. Biochim Biophys Acta. 1988 Dec 9;972(3):257–264. doi: 10.1016/0167-4889(88)90200-5. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Dwyer S. D., Smith L. Decreasing extracellular Na+ concentration triggers inositol polyphosphate production and Ca2+ mobilization. J Biol Chem. 1989 Jan 15;264(2):831–837. [PubMed] [Google Scholar]
- Smith J. B., Dwyer S. D., Smith L. Decreasing extracellular Na+ concentration triggers inositol polyphosphate production and Ca2+ mobilization. J Biol Chem. 1989 Jan 15;264(2):831–837. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trosper T. L., Philipson K. D. Effects of divalent and trivalent cations on Na+-Ca2+ exchange in cardiac sarcolemmal vesicles. Biochim Biophys Acta. 1983 May 26;731(1):63–68. doi: 10.1016/0005-2736(83)90398-x. [DOI] [PubMed] [Google Scholar]
- Ueda T. Na+-Ca2+ exchange activity in rabbit lymphocyte plasma membranes. Biochim Biophys Acta. 1983 Oct 12;734(2):342–346. doi: 10.1016/0005-2736(83)90133-5. [DOI] [PubMed] [Google Scholar]
- Wacholtz M. C., Cragoe E. J., Jr, Lipsky P. E. A Na(+)-dependent Ca2+ exchanger generates the sustained increase in intracellular Ca2+ required for T cell activation. J Immunol. 1992 Sep 15;149(6):1912–1920. [PubMed] [Google Scholar]
- Wacholtz M. C., Cragoe E. J., Jr, Lipsky P. E. Delineation of the role of a Na+/Ca2+ exchanger in regulating intracellular Ca2+ in T cells. Cell Immunol. 1993 Mar;147(1):95–109. doi: 10.1006/cimm.1993.1051. [DOI] [PubMed] [Google Scholar]
- Wacholtz M. C., Lipsky P. E. Anti-CD3-stimulated Ca2+ signal in individual human peripheral T cells. Activation correlates with a sustained increase in intracellular Ca2+1. J Immunol. 1993 Jun 15;150(12):5338–5349. [PubMed] [Google Scholar]
- Wakabayashi S., Goshima K. Kinetic studies on sodium-dependent calcium uptake by myocardial cells and neuroblastoma cells in culture. Biochim Biophys Acta. 1981 Mar 20;642(1):158–172. doi: 10.1016/0005-2736(81)90146-2. [DOI] [PubMed] [Google Scholar]