Abstract
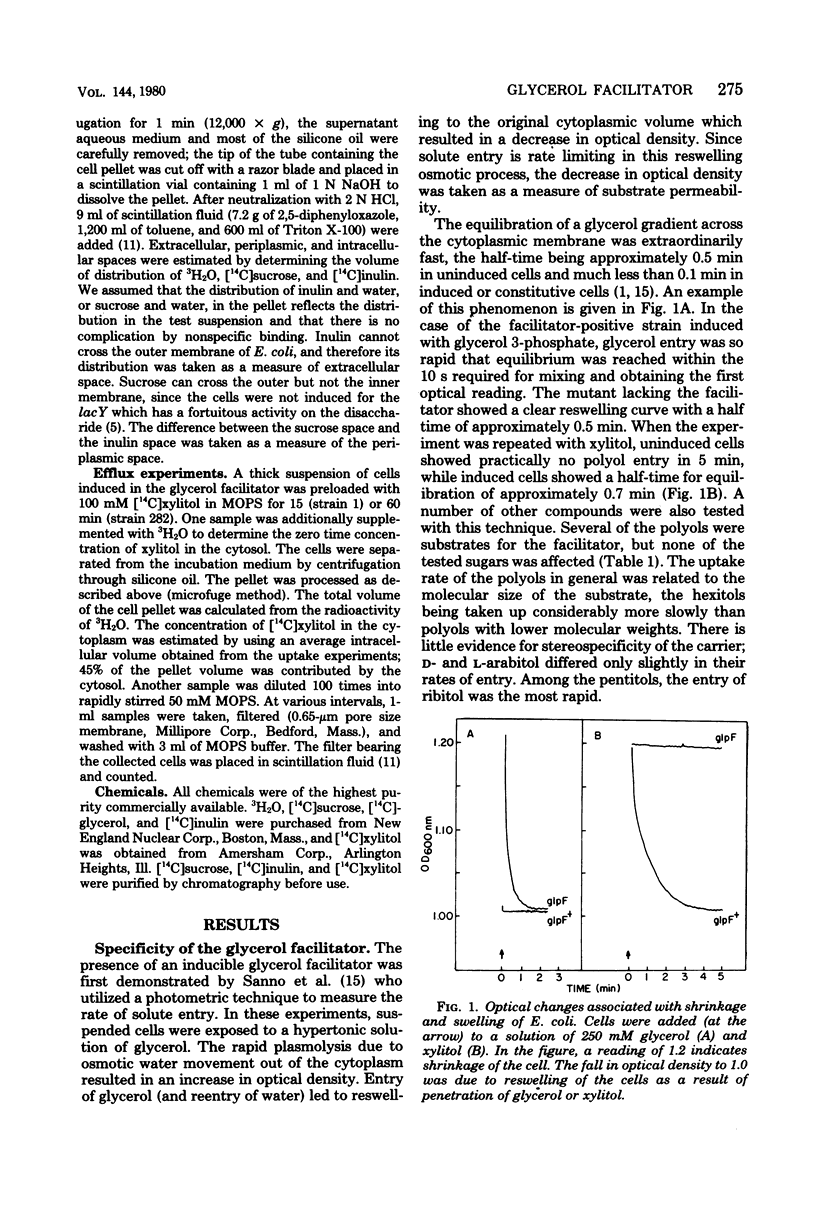
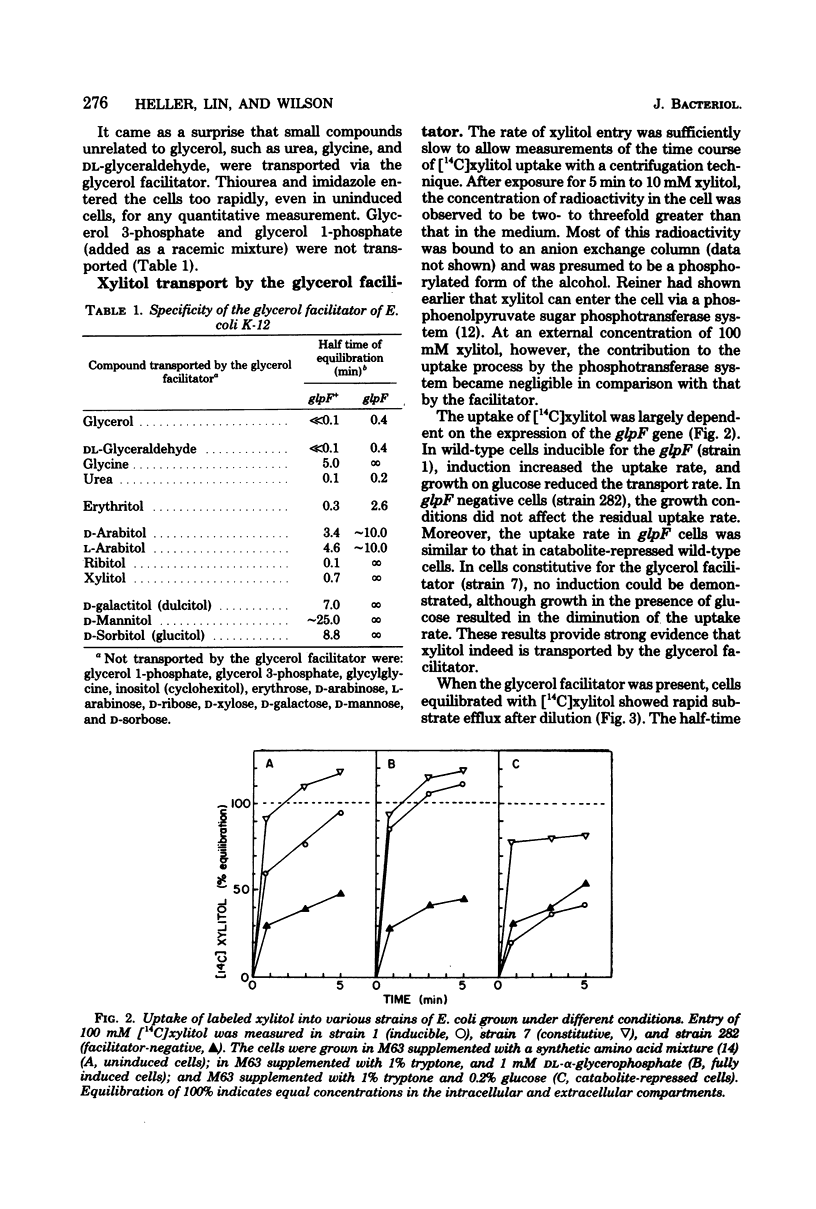
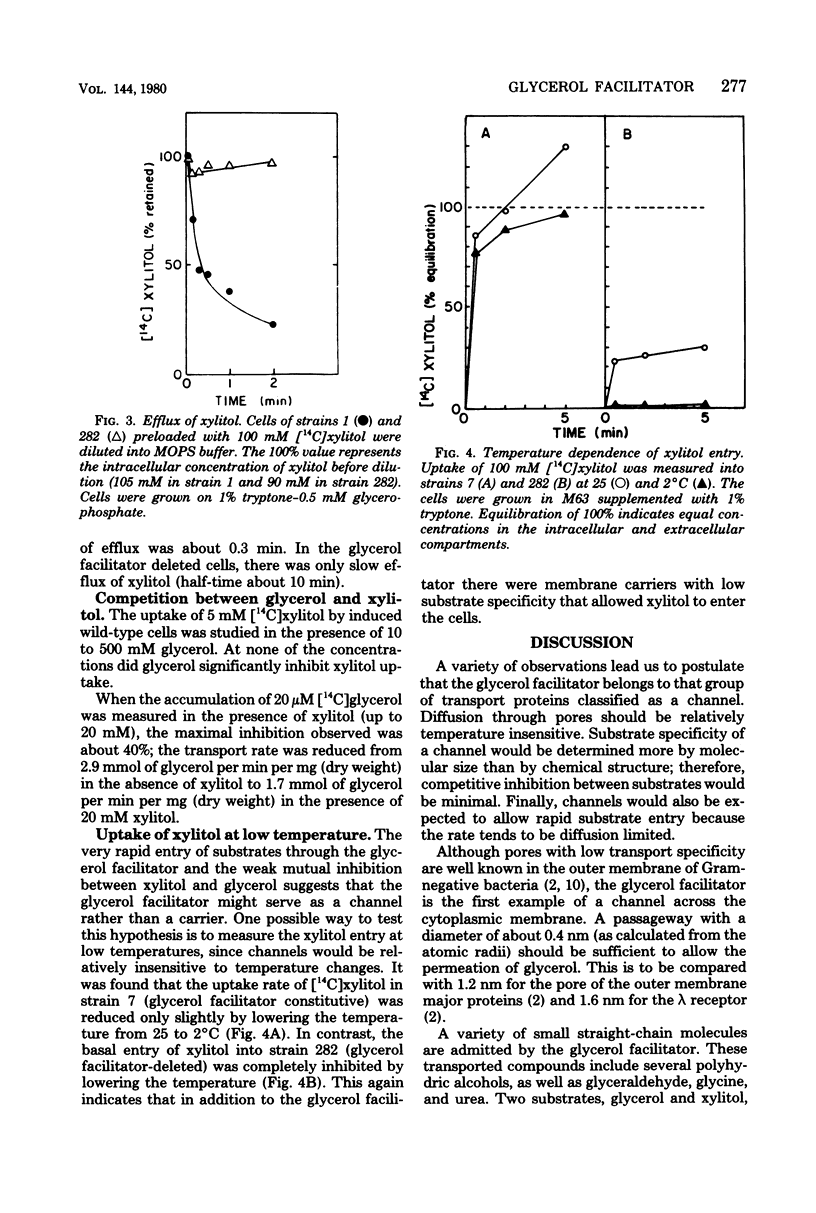
The specificity of the glycerol facilitator (glpF) of Escherichia coli was studied with an osmotic method. This transport system allowed the entry of polyols (glycerol and erythritol), pentitols, and hexitols. The analogous sugars were not transported. However, urea, glycine, and DL-glyceraldehyde could use this pathway to enter the cell. The glpF protein allowed the rapid efflux of preequilibrated xylitol. Glycerol surprisingly did not inhibit the uptake of xylitol, and xylitol only slightly reduced the uptake of glycerol. The observation and the insensitivity of the xylitol transport to low temperature suggest that the facilitator behaves as a membrane channel.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alemohammad M. M., Knowles C. J. Osmotically induced volume and turbidity changes of Escherichia coli due to salts, sucrose and glycerol, with particular reference to the rapid permeation of glycerol into the cell. J Gen Microbiol. 1974 May;82(1):125–142. doi: 10.1099/00221287-82-1-125. [DOI] [PubMed] [Google Scholar]
- Boehler-Kohler B. A., Boos W., Dieterle R., Benz R. Receptor for bacteriophage lambda of Escherichia coli forms larger pores in black lipid membranes than the matrix protein (porin). J Bacteriol. 1979 Apr;138(1):33–39. doi: 10.1128/jb.138.1.33-39.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Decad G. M., Nikaido H. Outer membrane of gram-negative bacteria. XII. Molecular-sieving function of cell wall. J Bacteriol. 1976 Oct;128(1):325–336. doi: 10.1128/jb.128.1.325-336.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K. B., Wilson T. H. Sucrose transport by the Escherichia coli lactose carrier. J Bacteriol. 1979 Nov;140(2):395–399. doi: 10.1128/jb.140.2.395-399.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuzenroeder M. W., Reeves P. Periplasmic maltose-binding protein confers specificity on the outer membrane maltose pore of Escherichia coli. J Bacteriol. 1980 Feb;141(2):431–435. doi: 10.1128/jb.141.2.431-435.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C., KOCH J. P., CHUSED T. M., JORGENSEN S. E. Utilization of L-alpha-glycerophosphate by Escherichia coli without hydrolysis. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2145–2150. doi: 10.1073/pnas.48.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E. C. Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol. 1976;30:535–578. doi: 10.1146/annurev.mi.30.100176.002535. [DOI] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Specificity of diffusion channels produced by lambda phage receptor protein of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):167–171. doi: 10.1073/pnas.77.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Reiner A. M. Xylitol and D-arabitol toxicities due to derepressed fructose, galactitol, and sorbitol phosphotransferases of Escherichia coli. J Bacteriol. 1977 Oct;132(1):166–173. doi: 10.1128/jb.132.1.166-173.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey D. P., Lin E. C. Importance of facilitated diffusion for effective utilization of glycerol by Escherichia coli. J Bacteriol. 1972 Nov;112(2):784–790. doi: 10.1128/jb.112.2.784-790.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanno Y., Wilson T. H., Lin E. C. Control of permeation to glycerol in cells of Escherichia coli. Biochem Biophys Res Commun. 1968 Jul 26;32(2):344–349. doi: 10.1016/0006-291x(68)90392-6. [DOI] [PubMed] [Google Scholar]
- Teather R. M., Müller-Hill B., Abrutsch U., Aichele G., Overath P. Amplification of the lactose carrier protein in Escherichia coli using a plasmid vector. Mol Gen Genet. 1978 Feb 27;159(3):239–248. doi: 10.1007/BF00268260. [DOI] [PubMed] [Google Scholar]
- Wandersman C., Schwartz M., Ferenci T. Escherichia coli mutants impaired in maltodextrin transport. J Bacteriol. 1979 Oct;140(1):1–13. doi: 10.1128/jb.140.1.1-13.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


