Abstract
Acrolein is a common air pollutant that is present in high concentrations in wood, cotton, and tobacco smoke, automobile exhaust and industrial waste and emissions. Exposure to acrolein containing environmental pollutants such as tobacco smoke and automobile exhaust has been linked to the activation of the coagulation and hemostasis pathways and thereby to the predisposition of thrombotic events in human. To examine the effects of acrolein on platelets, adult male C57Bl/6 mice were subjected acute (5 ppm for 6 h) or sub-chronic (1 ppm, 6h/day for 4 days) acrolein inhalation exposures. The acute exposure to acrolein did not cause pulmonary inflammation and oxidative stress, dyslipidemia or induce liver damage or muscle injury. Platelet GSH levels in acrolein-exposed mice were comparable to controls, but acrolein-exposure increased the abundance of protein-acrolein adducts in platelets. Platelets isolated from mice, exposed to both acute and sub-chronic acrolein levels, showed increased ADP-induced platelet aggregation. Exposure to acrolein also led to an increase in the indices of platelet activation such as the formation of platelet-leukocyte aggregates in the blood, plasma PF4 levels, and increased platelet-fibrinogen binding. The bleeding time was decreased in acrolein exposed mice. Plasma levels of PF4 were also increased in mice exposed to environmental tobacco smoke. Similar to inhalation exposure, acrolein feeding to mice also increased platelet activation and established a pro-thrombotic state in mice. Together, our data suggest that acrolein is an important contributing factor to the pro-thrombotic risk in human exposure to pollutants such as tobacco smoke or automobile exhaust, or through dietary consumption.
Keywords: Acrolein, Platelets, Platelet Factor 4, fibrinogen binding
Introduction
Aldehydes are ubiquitous pollutants that are present in the air and in the drinking water. Large amounts of these chemicals are generated by the burning of fossil fuels and cooking. Aldehydes such as acrolein, malonaldehyde, and 4-hydroxy trans-2-nonenal (HNE) are generated endogenously during lipid peroxidation in biological systems (Adams and Klaidman, 1993; Uchida, 1999). Although these aldehydes are considered to be end-products of lipid peroxidation, they are highly reactive. They combine readily with nucleophilic sites in proteins, phospholipids and DNA to form covalent adducts that can induce structural damage or cause functional changes. An increase in endogenous production of aldehydes by oxidized lipids has been linked to the pathogenesis of cardiovascular, neuronal, neoplastic, and autoimmune diseases (Uchida, 1999). Of the several aldehydes present in the environment or generated in situ, acrolein is the most reactive. It is an unsaturated aldehyde and a common environmental and industrial pollutant and toxin which is present in high concentration in automobile exhaust, cigarette and wood smoke. Acrolein is also generated during the metabolism of several drugs (e.g., cyclophosphamide), toxins (e.g. allylamine), or atmospheric transformation of pollutants such as 1,3-butadiene (Kehrer and Biswal, 2000; Bhatnagar, 2006). Large amounts of acrolein are synthesized for industrial use in the production of acrylic acid, polymers and herbicides and it is used widely as a biocide against aqueous organisms and as a slimicide in the manufacture of paper (DeWoskin et al., 2003).
Previous studies have shown that acrolein is a potent respiratory tract and eye irritant. Inhalation of acrolein induces asthma-like symptoms, pulmonary edema and respiratory distress and the presence of acrolein in inhaled air exacerbates asthma (Leikauf, 2002). In contrast to the well-described pulmonary effects of acrolein, less is known in regard to its toxicity in the vasculature. In vitro studies show that acrolein is cytotoxic to pulmonary artery endothelial cells (Kachel and Martin, 1994) and cardiac fibroblasts (Toraason et al., 1989). Acrolein evokes NO- and prostacyclin-dependent relaxation in isolated rat aortic rings (Tsakadze et al., 2003). In medium resistance arteries, acrolein caused vasodilatation of rodent mesenteric bed via an increase in endothelium-derived hyperpolarizaton factor (EDHF) (Awe et al., 2006). In vivo exposure to low doses of acrolein has been associated with vasopressor effects suggesting changes in systolic blood pressure (Egle and Hudgins, 1974; Green and Egle, 1983). Continuous 1 year oral exposure to acrolein in dogs revealed little toxicity except for a mild and persistent depression of serum albumin, calcium, and a decrease in serum protein levels (Parent et al., 1992). Significantly, variable changes in coagulation times were observed, but the relevance of these findings to the systemic toxicity of acrolein remains unclear. Given that exposure to pollutant mixtures containing high levels of acrolein such as tobacco smoke or car exhaust are associated with increased thrombosis (Rahman and Laher, 2007), we studied the effects of acrolein on hemostasis. We focused on changes in blood platelets, because they are the primary cells that initiate coagulation to prevent excessive blood loss due to vessel injury. In addition, aberrant activation of platelets can increase thrombus formation, disrupt the lining of the arterial wall, and accelerate atherogenesis. Therefore, an increase in thrombosis is strongly associated with increased risk of ischemic heart disease (Law and Wald, 2003). In this study we have examined the effect of acrolein exposure on platelet activation in mice.
Methods
Animals
Male C57BL/6 mice (16–20 week old), obtained from The Jackson Laboratory (Bar Harbor, ME), were housed under pathogen-free conditions in the University of Louisville vivarium under controlled temperature and 12 h light/12 h dark cycle. The mice were fed a standard chow diet (PicoLab Rodent Chow 20 containing 4.5 % fat by weight and 0.02 % cholesterol).
Exposure to Acrolein
For acrolein inhalation exposures, acrolein atmospheres were generated from liquid acrolein (Sigma) diluted in dH2O (1:10) in a custom vapor system (Teague Inc.) using a primary chamber as a constant source. Acrolein vapors were diluted with HEPA-filtered room air in secondary chamber. Acrolein exposure concentration (ppm) was continuously monitored using an in-line photoionization detector (ppbRAE+, Rae Industries, Sunnyvale, CA) prior to delivery via a cage insert vapor delivery unit into a standard polycarbonate rat cage (16”×8.75”×13.5”) for exposures. Acrolein was distributed through a fine mesh screen at 3 liters per min by delivery units with a cyclone-type top that distributes air within 10% of the mean concentration at six locations in the cage. Exposure cages were placed partially over heating pads (~24 °C). In the first protocol, mice were exposed to 5 ppm acrolein for 6 h (4,944±44 ppb) and in the other to 1 ppm acrolein for 4 days (1,053±22 ppb). Mice exposed to filtered air served as controls. These exposure levels were in concordance with recently reported studies on acrolein (Kasahara et al., 2008). For oral exposures, mice were gavage-fed acrolein (1–5 mg/kg) or tap water (vehicle; controls) as described before (Conklin et al., 2010) and euthanized at 4 or 24 h after exposure.
Exposure to Tobacco Smoke
Mice (n=6) were exposed to environmental tobacco smoke (ETS) for 5h as described previously (Conklin et al., 2009). Tobacco smoke was generated from Kentucky 2R4F reference cigarettes (Tobacco Research Institute, University of Kentucky, Lexington, KY), using a smoke chamber (model TE-10, Teague Enterprises, Woodland, CA) and a mixture of sidestream (89%) and mainstream (11%) CS was used. Each smoldering cigarette was puffed for 2 s once every minute for a total of 8 puffs at a flow rate of 1.05 l/min to provide a standard puff of 35 cm3. Ten 2R4F cigarettes were burned at one time for 5 h. Mice (n=6) exposed to filtered air for 5h served as controls.
Isolation of Platelets
Mice were anesthetized with sodium pentobarbital (0.1 ml; 50 mg/kg, i.p) immediately after acrolein exposures - 5 ppm for 6 h or 1ppm for 6h/day for 4 days. Blood was collected from the heart using a tuberculin syringe with a 20 gauge needle using 1:9 (v/v) trisodium citrate (4%) as anti-coagulant (Srivastava et al., 1994). Blood was centrifuged at 180xg for 20 min at 22 °C. The supernatant - platelet rich plasma (PRP) - was aspirated and centrifuged at 1000xg for 10 min to sediment platelets. The supernatant - platelet poor plasma (PPP) - was transferred to a fresh tube. Platelet count in the PRP was adjusted to 3 × 108 cells/ml using PPP. To obtain washed platelets (WP), the platelets were washed three times with Tyrode buffer (137mM NaCl; 20mM HEPES, 1mM MgCl2, 2.7mM KCl, 3.3 mM NaH2PO4, 5.6mM glucose, 0.1% BSA; pH 7.4,) and reconstituted in Tyrode buffer at a density of 3 × 108 cells/ml.
Platelet Aggregation
Platelet aggregation was measured using a four-channel platelet aggregometer (Chrono-log Corp, Havertown, PA). PRP (adjusted to 3 × 108 cells/ml) was incubated for 2 min at 37 °C with continuous stirring at 1,000 rpm, and then stimulated with adenosine 5’-diphosphate (ADP; 10μM). The aggregation was measured for 5 min by following change in absorbance (Srivastava et al., 1994). For measuring platelet aggregation in washed platelets, CaCl2 (2mM) and fibrinogen (200μg/ml) were added to the platelet suspension prior to the stimulation with ADP.
Platelet-Leukocyte Aggregates
Blood was collected in Na4·EDTA (0.2M; 16 μl/ml blood). Aliquots of whole blood (100 μl) were diluted with 4 volume of HEPES-Tyrode solution and the samples were fixed in 1% formaldehyde for 30 min at 4°C. The red cells were lysed by dilution in water. Cells were then collected by centrifugation at 400xg for 8 min, washed with HEPES-Tyrode solution containing 1% BSA, and stained with FITC-labeled anti-CD41 and APC-labeled anti-CD11b or isotype matched controls for 30 min on ice (Harding et al., 2007). The stained cells were analyzed on a Moflo flow cytometer (Dako) or a BD LSR II Flow Cytometer (BD Biosciences). For each sample, a minimum of 10,000 events were collected. Platelet-leukocyte aggregates were defined as those events which were positive for both platelets and leukocytes and expressed as a percentage of total events.
Protein-Acrolein Adducts and Glutathione Measurement
Levels of protein-acrolein adducts accumulated in washed platelets were measured by Western blotting, using the anti-KLH-acrolein antibody (Conklin et al., 2009). Intracellular glutathione levels in platelets were measured as described by Staal et al (Staal et al., 1990). Briefly, washed platelets (3×108 platelets/mL) were labeled with anti-CD41-PE antibody and then incubated with monochlorobimane (MCB; 20 μM) for 15 min at 37°C. Cells were excited at 394 nm and emission was acquired at 490 nm. Data are presented as mean fluorescence intensity of MCB.
Platelet-Fibrinogen Binding
Aliquots of blood (100μl) were incubated with PE-labeled rat-anti-mouse CD41 antibody for 20 min at room temperature. Samples were then diluted with 4 volumes of HEPES-Tyrode solution and incubated with Alexa-flour488 fibrinogen (20μg/ml) for 10 min at room temperature. Samples were fixed with paraformaldehyde (2%) for 20 min. Erythrocytes were lysed by adding 4.6 volumes of distilled water. Samples were centrifuged at 1000xg for 8 min and the pellet was washed with HEPES-Tyrode solution containing 0.1% D-glucose and 0.1% BSA (FACS buffer). The pellet was re-suspended in FACS buffer (1.0 ml) and analyzed by flow cytometry.
Platelet Factor 4 Assay
Plasma PF-4 levels were assayed by a sandwich ELISA using DeoSet Mouse PF4/CXCL4 ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Briefly, 96-well ELISA plates were coated with a rat anti-mouse PF4 capture antibody (2μg/mL in PBS) for 16h at room temperature. Wells were washed free of the unbound antibody and blocked with 1% BSA for 1h at room temperature. Plasma samples or the PF-4 standards were incubated in the coated wells for 2h at room temperature. Wells were washed three times and then incubated with a biotinylated goat anti-mouse PF-4 antibody (100ng/ml) for 2h at room temperature.Wells were washed again and then incubated with streptavidin conjugated to horseradish-peroxidase for 20 min at room temperature. The washing step was repeated and the substrate, tetramethylbenzidine(TMB) was added and the reaction was incubated for 15 min. The reaction was stopped with 1N H2SO4and the color developed was measured using a microplate reader at 450 nm.
Expression of Cytokines
Expression of pro-inflammatory cytokines was measured in the lungs as described before (Srivastava et al., 2009). Lungs were snap-frozen immediately after harvesting. RNA was isolated using Trizol reagent and 2.0 μg RNA from each was reverse transcribed with AMV reverse transcriptase (Promega Corp., Madison, WI) at 42°C for 60 min, followed by PCR amplification. Quantitative RT-PCR was carried out in a BioRad Real Time PCR thermocycler using SYBR green/Fluorescein PCR master mix (SuperArray Biosciences Corp., Frederick, MD). Following primers were used: TNF-α-F:GCATGATCCGCGACGTGGAA and R: AGATCCATGCCGTTGGCCAG; IL-1β - F:CTCCATGAGCTTTGTACAAGG and R: TGCTGATGTACCAGTTGGGG; MCP-1-F: ATGCAGGTCCCTGTCATG and R: GCTTGAGGTGGTTGTGGA and CSF-2- F:TCGAGCAGGGTCTACGGGGC and R: AGCTGGCCTGGGCTTCCTCA. For IL-6 measurement a commercial set of primers from Super Array Biosciences was used. HPRT1 was used as a house keeping gene (Super Array Biosciences, Frederick, MD).
Measurements of Malonyldialdehyde by Gas Chromatography-Mass Spectrometry
Levels of malonyldialdehyde (MDA) in the lung were measured by gas chromatography-mass spectrometry as described before (Srivastava et al., 2009).
PATHSCREEN
The following parameters of PATHSCREEN were measured in the plasma to examine systemic toxicity: Levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) for liver damage, creatine kinase (CK) level to assess muscle and total cholesterol and lipoproteins for dyslipidemia. Lipoprotein subclasses in the plasma were analyzed by NMR spectroscopy as described before (Srivastava et al., 2009; Conklin et al., 2010).
Bleeding Time Measurement
Mice were anesthesized with an intraperitoneal injection of avertin (250 mg/kg) to measure tail bleeding time (Tranholm et al., 2003). Briefly, 5 mm of the distal tip of the tail was briskly cut by a scalpel blade and immediately immersed into a pre-warmed (37 °C) tube of 0.9 % saline. The tail was held near the base to avoid any tourniquet effect and the time for the clotting of venous blood was recorded.
Statistical Analysis
Values are reported as mean ± standard error (SEM). For statistical comparison between two groups, Student’s paired or unpaired t-test was used where appropriate. For comparing more than two groups, one-way ANOVA and Student-Newman-Keuls post-hoc test was used. For all statistics, type I error rate was controlled at α=0.05. P <0.05 was considered statistically significant.
Results
Exposure to Acrolein Increases Platelet Aggregation
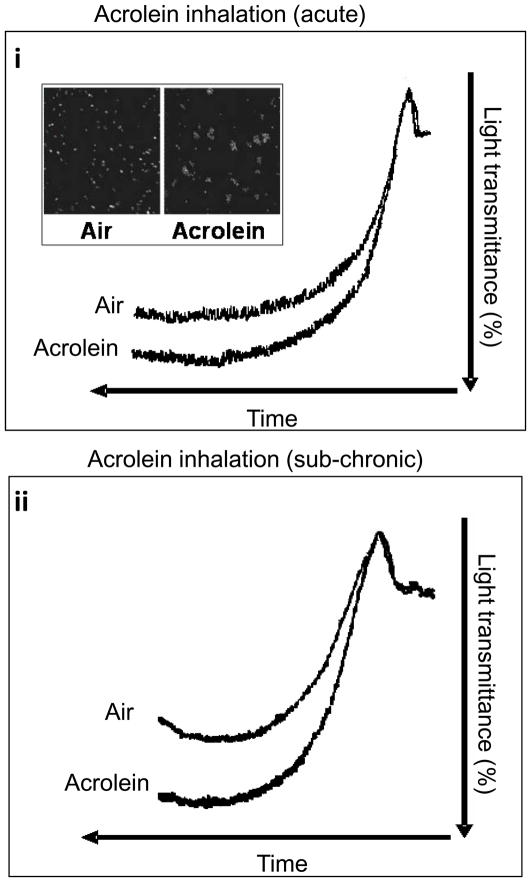
Initially, platelet aggregation studies were performed on PRP from mice (n=6) exposed to acute inhalation exposure of acrolein vapor (5ppm, 6h) (Fig. 1A). Mice (n=6) exposed to filtered air served as controls. No spontaneous platelet aggregation was detected in PRP isolated from acrolein or air-exposed mice. In response to ADP (10 μM), however, platelets harvested from acrolein-exposed mice showed a significant (P<0.01) increase in platelet aggregation than those from air-exposed mice (Fig. 1Ai). Microscopic examination indicated larger platelet aggregates were formed in acrolein exposed platelets when stimulated with ADP (Fig 1Ai insert). To assess whether this effect would persist at lower levels of acrolein exposure, mice (n=6) were exposed to 1 ppm acrolein 6h/day for 4 days (sub-chronic exposure). PRP isolated from mice exposed to acrolein under these conditions also hyper-aggregated in response to ADP, when compared with PRP from air-exposed mice (n=6; Fig. 1Aii). Collectively, these data indicate that acrolein inhalation increases platelet aggregation. The effect was evident when the mice were exposed to either a high-acute dose or a low sub-chronic dose of acrolein.
Figure 1. Acrolein exposure promotes platelet aggregation.
Representative tracings of ADP-induced platelet aggregation in platelet rich plasma (PRP) in mice following the acute (5 ppm for 6h; n=6; i) or sub-chronic (1 ppm for 6h/day for four days; n=6; ii) exposure to acrolein by inhalation are illustrated. Mice exposed to filtered air (n=6 for each group) served as controls. The insert in i shows a representative phase contrast image of aggregate size following ADP-induced platelet aggregation in washed platelets obtained from acrolein inhaled mice (1ppm for 6h/day for 4 days) and air inhaled controls.
Protein-Acrolein Adducts in Platelets
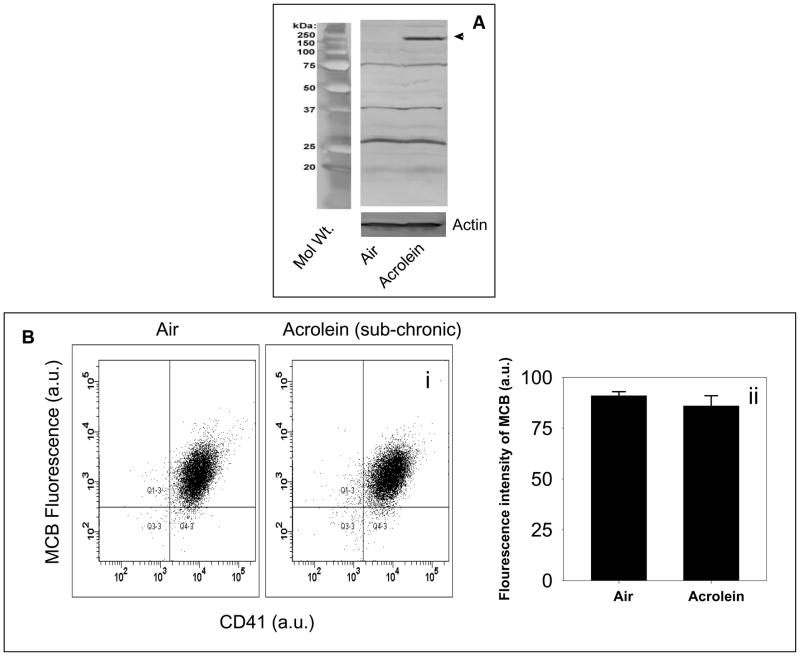
Acrolein is a highly reactive aldehyde and, therefore, it has a short half-life in the plasma. However, acrolein readily forms covalent adducts with protein and the presence of these adducts are a sensitive index of acrolein exposure, because these adducts are cleared more slowly than free acrolein (Conklin et al., 2009). The observations that the increase in platelet aggregation by acrolein was not mediated by plasmatic factors, suggested the possibility that the increase in aggregation was due to a direct effect of acrolein on the platelets. To test whether acrolein exposure leads to direct delivery of acrolein to the platelets, we measured the formation of protein-acrolein adducts in platelets from acrolein-inhaled (1ppm for 6h/day for four days; n=3) mice. Western blot analysis showed that exposure to acrolein resulted in the formation of a covalent adduct with a 150 kDa protein (Fig. 2A). Acrolein adducts were also observed with several other proteins, but the levels of these adducts were not affected by exposure to exogenous acrolein. These observations indicate that acrolein is delivered directly to platelets where it forms covalent adducts with a specific protein.
Figure 2. Increased protein-acrolein adduct formation and no change in GSH levels in acrolein-inhaled mice.
Panel A shows the representative Western blot of lysates of washed platelets obtained from mice exposed to acrolein (1 ppm/6h/day for four days; n=3) or filtered air (n=3), and probed with anti-acrolein-KLH antibody. Panel B shows the flow cytometric analysis of intracellular reduced glutathione levels in the platelets of mice exposed to acrolein (1 ppm/6h/day for four days; n=5) or filtered air (n=5). Washed platelets were labeled with monochlorobiamine (MCB) and mean fluorescence intensity of MCB was measured at excitation and emission wavelengths of 394 and 490 nm respectively. Representative illustration of fluorescence intensity of MCB in platelets isolated from control (air-exposed) and acrolein-exposed (1 ppm for 6h/day for four days) mice is shown in panel (i) and the group data is shown in panel (ii) as percent CD41-MCB double positive cells from total events. Values are mean ± SEM.
In addition to reacting with nucleophilic side chain of proteins, cytosolic acrolein reacts with reduced glutathione to form GS-acrolein conjugate. Glutathione conjugates of unsaturated aldehydes are actively extruded from the cell leading to a net depletion of glutathione. To examine whether platelet GSH is depleted in acrolein-exposed animals, washed platelets were labeled with MCB and GSH concentration was quantified by flow cytometry. As shown in Fig. 2B, GSH was present at similar levels in platelets isolated from mice exposed to filtered air (n=5) or those exposed to acrolein (n=5), 1ppm for 6h/day for 4 days. Therefore, as exposure to acrolein by inhalation does not deplete platelet GSH, this data suggest that the appearance of protein-acrolein adducts in acrolein-exposed mice is not secondary to GSH depletion and that these adducts arise primarily due to the modification of membrane protein by acrolein in the plasma.
Platelet-Fibrinogen Binding
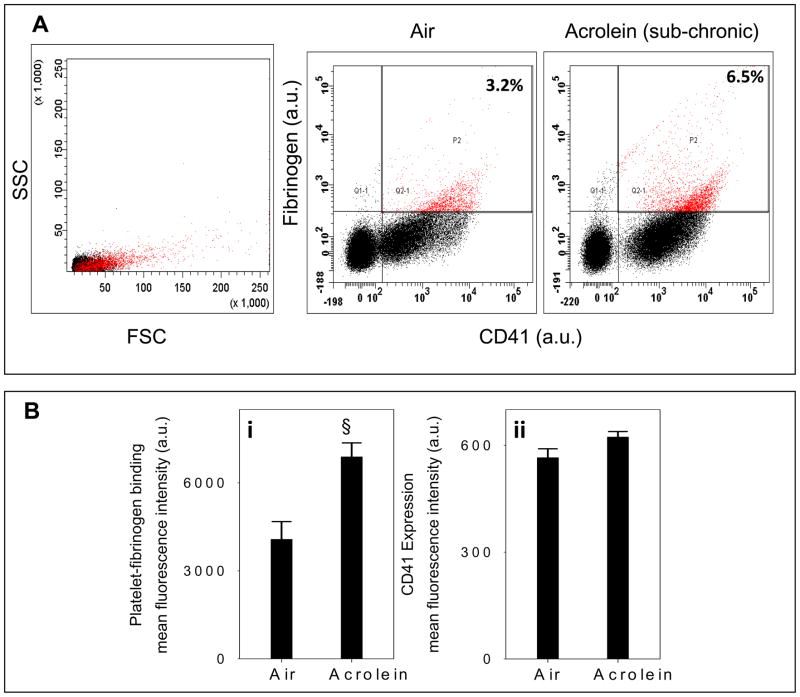
To examine whether acrolein exposure leads to platelet activation in vivo, we measured platelet-fibrinogen binding. CD41 is the α-subunit of the CD41/CD61 complex (GPllb-llla), a non-covalently associated heterodimer. The activated CD41/CD61 complex is a receptor for soluble fibrinogen and plays a central role in platelet aggregation and vascular hemostasis (D’Souza et al., 1990; D'Souza et al., 1991). To examine the effect of acrolein on platelet-fibrinogen binding, mice were exposed to acrolein (1 ppm 6h/day for 4 days; n=6) or filtered air (n=6). Isolated washed platelets were then labeled with the anti-PE-CD41 antibody, followed by incubation with a FITC-labeled fibrinogen and analyzed by flow cytometry with appropriate gating. In comparison with controls, platelets from acrolein-exposed mice showed significantly (P<0.02) greater binding to fibrinogen (Fig. 3A&Bi). There was, however, no change in the surface expression of CD41 due to acrolein exposure (Fig. 3Bii). These results indicate that acrolein inhalation results in in situ activation of platelets in exposed mice.
Figure 3. Acrolein exposure increases platelet-fibrinogen binding.
Panel A showsflow cytometric analysis of platelet-fibrinogen binding ex vivo in whole blood in mice following sub chronic exposure to acrolein (1 ppm for 6h/day for four days; n=6) or filtered air (n=6). For these assays PE-labeled anti-CD41 and FITC labeled fibrinogen were used. Forward scatter (FSC) and side scatter (SSC) of cells were used to distinguish platelets from other cells and cell debris. Panel B shows group data, presented as mean ± SEM of fluorescence intensity (arbitrary units) of fibrinogen bound to CD41-positive platelets (i) and surface expression of CD41(ii). §P ≤ 0.02 versus control.
Acrolein Exposure Increases the Release of Platelet Granular Contents
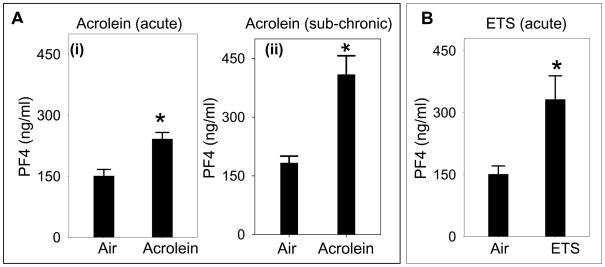
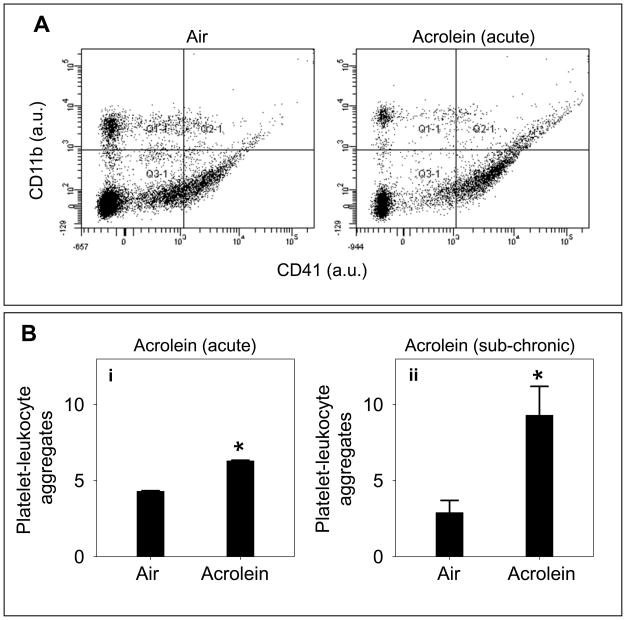
In addition to fibrinogen binding, release of granular contents is another hallmark of platelet activation. Platelets contain three types of granules - the α-granules (containing fibrinogen, platelet factor-4 [PF4], PDGF, β-thromboglobulin etc), dense granules (containing ADP, serotonin, calcium etc) and lysosomal granules (containing acid proteases, acid phospha’tases etc) and these granules are released upon activation (Lane et al., 1984). To examine whether acrolein exposure induces the release of platelet granules, we measured the plasma levels of PF4 in mice that were exposed to acrolein (n=8) or air (n=8; controls). Acute acrolein exposure (5ppm for 6h; Fig. 4Ai) led to a 1.6 fold increase (P<0.01) in plasma PF4 levels when compared with controls whereas sub-chronic exposure to acrolein (1 ppm, 6h/day for 4 days; Fig. 4Aii) led to 2.7-fold increase (P<0.01) in PF4 levels. A similar increase in plasma PF4 levels was observed in mice acutely exposed to ETS (Fig. 4B; n=6) as compared with air-exposed controls. Together, these results suggest that the activation of platelets in acrolein or ETS-exposed mice results in a greater release of PF4 from the α-granules.
Figure 4. Exposure to acrolein increases the release of platelet granular contents.
Panel A shows the plasma platelet factor 4 (PF4) levels in acrolein-treated mice following acute (5ppm, 6h; n=8; Ai) or sub-chronic (1 ppm, 6h/day for 4 days; n=8; panel; Aii) inhalation exposure. Mice exposed to filtered air (n=8 for each protocol) served as controls. Panel B shows plasma PF4 levels in environmental tobacco smoke (ETS; n=6) treated mice exposed by inhalation. The PF4 concentration in the plasma was measured by sandwich ELISA. Values are mean ± SEM. *P ≤0.01 versus controls.
Platelet-Leukocyte Aggregate Formation
Activated platelets interact with leukocytes in vivo to form platelet-leukocyte aggregates in the blood (Freedman and Loscalzo, 2002). The interaction between platelets and leukocytes is primarily through the mobilization of P-selectin, an adhesive protein, on the surface of activated platelets (Michelson et al., 2001; Freedman and Loscalzo, 2002). Increased formation of platelet-leukocyte aggregates is a validated marker of increased thrombosis in vivo (Sarma et al., 2002). Therefore, to assess whether platelets are activated in situ in animals exposed to acrolein, we measured the formation of platelet-leukocyte aggregates by flow cytometry. For this, the mice were exposed to air or acrolein (5ppm for 6h). Platelets in blood were detected by staining with anti-CD41 antibody and the monocytes were detected with the anti-CD11b antibody. The gating for platelets and monocytes was established by their respective staining and size in specific quadrants. High levels of platelet-leukocyte aggregates were observed in mice that were exposed to acrolein via inhalation (Fig. 5A). The level of these aggregates was nearly 40 % higher in mice exposed acutely to high dose acrolein (5 ppm for 6 h; n=8) than air-exposed mice (Fig. 5A; n=8). These adducts were even higher when the mice were sub-chronically exposed to low-dose (1 ppm, 6h/day for 4 days; n=8) acrolein. As shown in Fig. 5Bii, platelet-leukocyte aggregates were more than 2-fold higher in acrolein exposed than air-exposed mice. These data suggest that exposure to acrolein, increases the formation of platelet-leukocyte aggregates in the blood, indicating in situ platelet activation.
Figure 5. Acrolein exposure increases platelet-leukocyte adduct formation.
Panel A shows representative tracings of flow cytometric analysis of platelet-leukocyte aggregate formation in the whole blood of acrolein-exposed mice. Mice were exposed to acrolein (5 ppm for 6h) or filtered air and platelet-leukocyte aggregate formation was measured in the whole blood by FACS analysis, using PE-labeled anti-CD41 and PerCP-cy5.5-labeled anti CD11b antibodies. FSC versus SSC scales are shown to distinguish platelets from other cells and cell debris. Representative illustration in quadrent 2.1 shows platelet-leukocyte aggregates of double positive cells in control (air) and acrolein-exposed mice. Group data (n=8/group) for the platelet-leukocyte aggregates in acrolein-inhaled mice are shown in panel Bi. Panel Bii shows the group data for platelet-leukocyte aggregate formation in mice following sub-chronic (1 ppm for 6h/day for 4 days; n=8) exposure to acrolein or filtered air (controls; n=8). Values are mean ± SEM. *P ≤ 0.01 versus controls.
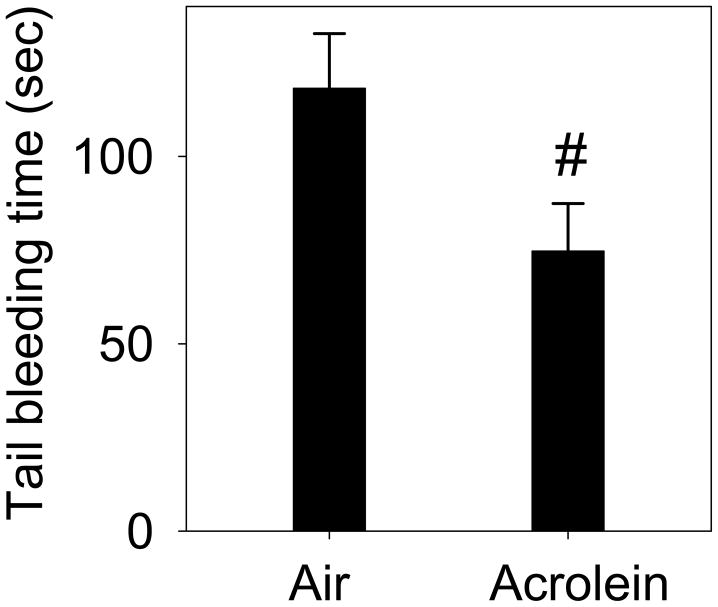
Tail Bleeding Time
Given that platelet activation was increased upon exposure to acrolein, we next studied the functional consequences of this effect. Activation of platelets in vivo affects hemostasis and blood clotting, hence an in vivo increase in platelet activation, if functionally meaningful, should lead to an accelerated in blood clotting. Accordingly, the tail bleeding time measured in mice exposed to acrolein (1 ppm. 6h/day for 4 days; n=6) was found to be decreased as compared to air-exposed controls(Fig. 6; n=6). Collectively, these data suggest that exposure to acrolein accelerates the overall hemostatic process in mice.
Figure 6. Exposure to acrolein decreases clotting time.
Mice were exposed to acrolein (sub-chronic exposure: 1 ppm, 6h/day for 4 days; n=6; ii) or filtered air (controls; n=6). Subsequently, the mice were anesthetized by avertin and distal tip of the tail (5 mm) was cut and immediately immersed in the pre-warmed saline. The duration between the start and stoppage of bleeding was recorded as clotting time. Values are mean ± SEM. #P≤ 0.05 versus controls.
Systemic Toxicity
To examine whether acute or sub-chronic exposure to acrolein causes systemic toxicity, we performed PATHSCREEN. Plasma levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured as indices for liver damage and plasma creatine kinase (CK) level was measured to assess muscle toxicity. Plasma levels of total cholesterol, HDL, LDL and triglycerides were also measured to examine whether the changes in platelet activation were accompanied by alterations in the levels of circulating lipoproteins. As shown in Table I, the levels of AST, ALT, CK and lipoproteins in both acute (n=8) and sub-chronically (n=8) acrolein-exposed mice were not statistically different from those in the plasma of mice exposed to filtered air acutely (n=8) or sub-chronically (n=8). Together these observations suggest that under our experimental conditions exposure to acrolein does not induce overt systemic toxicity or dyslipidemia.
Table 1.
Parameters measured in C57BL/6 mice exposed to air or acrolein.
| Acute | Sub-Chronic | |||
|---|---|---|---|---|
| Air | Acrolein (5ppm for 6h) |
Air | Acrolein (1ppm, 6h/day for 4 days) |
|
| Blood | ||||
| Cholesterola | 97±4 | 90±2 | 117±4 | 101±6* |
| HDLa | 73±3 | 68±2 | 88±3 | 69±5* |
| LDLa | 18±1 | 15±1 | 23±2 | 29±3 |
| Triglyceridesa | 28±3 | 27±3 | 38±5 | 20±4* |
| TPb | 4.81±0.06 | 4.61±0.05* | 4.99±0.18 | 4.97±0.12 |
| ALBb | 3.05±0.05 | 2.94±0.04 | 3.19±0.03 | 3.18±0.04 |
| LDHc | 165±16 | 154±13 | 181±11 | 202±17 |
| CKc | 164±11 | 202±34 | 166±22 | 196±22 |
| ALTc | 19±2 | 19±2 | 20±2 | 20±2 |
| ASTc | 56±2 | 62±6 | 48±6 | 62±6 |
| Lung | ||||
| TNF-αd | 1±0.08 | 1.01±0.15 | 1±0.16 | 0.84±0.18 |
| IL1-βd | 1±0.24 | 0.67±0.18 | 1±0.48 | 0.69±0.17 |
| IL-6d | 1±0.14 | 0.68±0.13 | 1±0.32 | 1.25±0.36 |
| MCP-1d | 1±0.21 | 1.46±0.30 | 1±0.17 | 0.91±0.19 |
| CSF-2d | 1±0.22 | 0.91±0.25 | 1±0.01 | 0.81±0.07 |
| MDAe | 0.35±0.03 | 0.43±0.07 | 0.38±0.07 | 0.43±0.03 |
Male, 12-14 week old mice were exposed to filtered air or acrolein. Mice were euthanized immediately after exposure. Units,
- mg/dL;
- g/L;
- units/L;
Values for TNF-α, IL-1β, IL-6, MCP-1 and CSF-2 are expressed as fold change;
p moles/mg tissue weight.
Values are mean ± SEM.
P<0.05 vs. air-exposed controls; n=6–8 mice/group.
Abbreviations: HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; TP, total protein; ALB, albumin; LDH, lactate dehydrogenase; CK, creatine kinase; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Pulmonary Inflammation and Oxidative Stress
Chronic exposure to acrolein in mice has been reported to increase the expression of pro-inflammatory cytokines in the lungs (Borchers et al., 2007) and acute exposure to acrolein in rats has been reported to cause oxidative stress in the lungs (Arumugam et al., 1999). To examine the potential effect of acrolein inhalation on pulmonary inflammation in mice under our experimental conditions, we measured the expression of pro-inflammatory cytokines in the lungs. Oxidative stress in the lungs of acrolein-exposed mice was examined by measuring the concentration of MDA in the lungs. As shown in Table 1 both acute (n=6) and sub-chronic (n=6) exposure to acrolein did not affect the mRNA expression of pro-inflammatory cytokines TNF-α, IL-1b and IL-6; chekmokine MCP-1 and colony stimulating factor 2 (CSF2) in the lungs. Similarly, the plasma levels of these cytokines in acrolein-exposed mice were comparable to controls (data not shown). Exposure to acrolein (n=6) also had no effect on the levels of MDA in the lungs suggesting that under our experimental conditions, acrolein-inhalation does not cause oxidative stress in mice lungs.
Oral Exposure to Acrolein and Platelet Activation
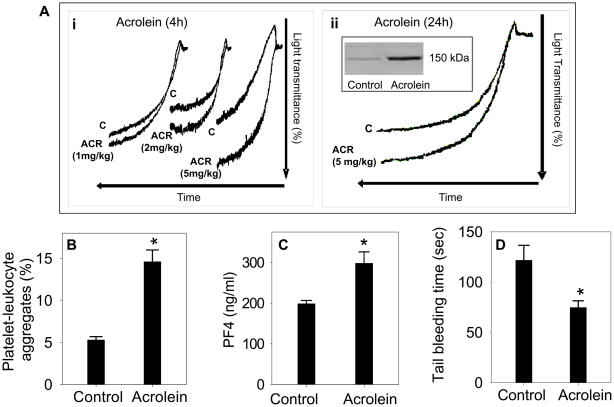
Data presented in Fig. 1–6 suggest that acute or sub-chronic exposure to acrolein by inhalation increases platelet activation without causing oxidative stress and inflammation in the lung and any overt systemic toxicity. To further examine that acrolein-induced platelet activation is independent of secondary pulmonary toxicity, we examined the effect of oral exposure to acrolein on platelet activation. Mice (n=8) were gavage-fed 1–5 mg/kg acrolein, which is in the dose-range of expected human consumption of total unsaturated aldehydes (Conklin et al., 2010). Control mice (n=8) were gavage-fed water. Four hours after gavage, the mice were euthanized and platelet aggregation was measured in washed platelets. As shown in Fig. 7Ai, platelets from acrolein-fed mice showed a dose-dependent increase in ADP-induced aggregation as compared with water-fed mice. The effects of acrolein were sustained and a significant increase in platelet aggregation was observed in washed platelets even when the platelets were isolated 24 h after a single gavage of acrolein (Fig. 7Aii). Western blot analysis of the washed platelets showed that similar to inhaled acrolein, oral exposure to acrolein (5 mg/kg, 24 h) resulted in the formation of a covalent adduct with a 150 kDa protein (inset Fig. 7Aii). Platelet-leukocyte adduct formation (Fig. 7B; n=6) and plasma levels of PF4 (Fig. 7C; n=8) were also significantly increased and tail bleeding time (Fig. 7D; n=6) was significantly decreased in acrolein (5 mg/kg, 24 h) - fed mice as compared with the water-fed controls. Together, these data suggest that similar to inhalation, oral exposure to acrolein also increases platelet activation. Therefore, the observed increase in platelet activation in acrolein-fed mice are independent of any pulmonary toxicity.
Figure 7. Oral exposure to acrolein promotes platelet aggregation.
Panel A shows representative tracings of ADP-induced platelet aggregation in washed platelets in mice following different doses of acute oral exposure of acrolein (Ai; 1-5 mg/kg by gavage; n=8). Mice gavage-fed with water served as controls (n=8). Panel Aii shows ADP-induced platelet aggregation in washed platelets of mice exposed to acrolein (5 mg/kg) after 24 h. Inset shows the protein-acrolein adduct formation in the washed platelets of acrolein (5 mg/kg) - fed mice. Panel B shows the group data of platelet-leukocyte aggregate formation in the whole blood of acrolein (5mg/kg, 24 h; n=6) - exposed mice. Panel C shows the levels of PF4 in the plasma of acrolein (5 mg/kg, 24 h; n=8) exposed mice. Panel D shows the effect of oral exposure to acrolein (5 mg/kg, 24 h; n=6) on tail bleeding time.
Discussion
The major finding of this study is that acute (5ppm for 6h) and sub-chronic (1ppm for6h/day for 4 days) exposure to acrolein exposure causes platelet activation in mice. This conclusion is supported by the observation that exposure to acrolein increases platelet-fibrinogen binding, PF4 levels, and platelet-leukocyte aggregates. Also, platelets isolated from acrolein-exposed mice showed an increase in aggregation ex vivo and the tail bleeding time was decreased in the mice exposed to acrolein, indicating that acrolein exposure creates and sustains a pro-thrombotic state. The increase in platelet activation was not associated with overt systemic toxicity, liver injury or changes in plasma lipoproteins, indicating that platelets are highly sensitive to exogenous acrolein. This is consistent with the observation that the formation of protein-acrolein adducts was increased in platelets upon exposure to acrolein. This evidence points to the possibility that despites its high reactivity and efficient metabolism, acrolein survives transportation from the site of exposure to the blood to directly affect circulating cells such as platelets. The observation that inhalation exposure to acrolein increased platelet activation supports the notion that direct effects of acrolein appearing in the blood could be responsible for the pro-thrombotic effects. Taken together, these data suggest the possibility that environmental or occupational exposure to acrolein or related aldehydes could have a pro-thrombotic effect in humans.
In the process of primary hemostasis, platelets play a crucial role in wound healing and in arresting blood loss. However, undue or excessive activation of platelets can result in thrombotic activity within the vasculature, which could lead to occlusion of blood vessels. Extensive evidence supports the view that the acute cardiovascular effects of cigarette smoke have been linked to thromboembolic events (Rahman and Laher, 2007). Human exposure to second-hand smoke been shown to increase platelet activation and aggregation (Burghuber et al., 1986; Davis et al., 1989) and bleeding time is decreased in animals exposed to tobacco smoke (Zhu et al., 1993; Zhu et al., 1994). In addition, the high cardiovascular toxicity of environmental pollutants has been linked to an increase in thrombosis and several investigators suggest that the strong epidemiological link between exposure to air pollution and cardiovascular disease is driven in most part, by the ability of environmental pollutants to promote a pro-coagulant state and to precipitate acute thrombotic events leading to acute myocardial infarction and stroke (Ware, 2000; Hoek et al., 2001; Bhatnagar, 2006; Pope and Dockery, 2006). Nevertheless, the in vivo effects of acrolein, which is a common component of tobacco smoke and car exhaust (Bhatnagar, 2006), on platelet have not been studied before. Hence, our studies showing for the first time that acrolein induces platelet activation suggest that the pro-thrombotic effects of acrolein containing pollutants may be related, at least in part, to the effects of acrolein described here.
Platelets derived from acrolein-exposed mice have a greater propensity to aggregate ex-vivo in the presence of a weak agonist (ADP) compared with platelets obtained from control, non-exposed mice (Fig 1). Acrolein-induced augmentation of platelet aggregation was observed in the PRP and with washed platelets. The observation that platelet aggregation was increased even in the absence of plasma indicates that acrolein directly affects platelets and that the effects of acrolein are unlikely to be mediated by changes in the plasma. No significant changes in plasma lipoproteins were observed in mice exposed to acrolein via inhalation, suggesting that acrolein-induced platelet activation is independent of the dyslipidemic effects of acrolein. The selective and independent effect of acrolein on platelets is similar to that of tobacco smoke. Even low dose exposure to tobacco smoke significantly and rapidly increase platelet activation and much of the ischemic heart disease risk of smoking could be ascribed to an increase in platelet activation (Law and Wald, 2003). Moreover, exposure to tobacco smoke is an independent risk factor for heart disease, which means that the effects of tobacco smoke are not mediated by other risk factors such as an increase in cholesterol or hypertension. Although smokers have higher levels of LDL-cholesterol and low levels of HDL than non-smokers, dyslipidemia accounts for <9 % of the cardiovascular risk of smoking (Craig et al., 1989). Similarly, cholesterol levels do not affect the risk association between air pollutant exposure and cardiovascular disease (Miller et al., 2007). Thus, the effects of acrolein bear a striking resemblance to those of tobacco smoke and air pollutants and appear to be driven by a direct increase in pro-thrombotic indices rather than indirect changes in blood lipoproteins.
We found that exposure to acrolein and ETS increased the plasma concentration of PF4. PF4 is mainly synthesized in megakaryocytes, and then stored in the α-granules of platelets. Upon platelet activation PF4, released from the α-granules of platelets, binds to heparin-like molecules and prevents the activation of antithrombin (Denton et al., 1983); thereby facilitating thrombus formation at the site of vessel injury. Our data complement recent studies showing that acrolein in vitro inhibits plasma antithrombin activity by inhibiting heparin affinity (Gugliucci, 2008; Martinez-Martinez et al., 2009). Lower antithrombin activity levels could potentially increase thrombin availability in the circulation, adding another pro-thrombotic component to the effects of acrolein exposure. PF4 has also been reported to affect the anticoagulant activity of Activated Protein C (Preston et al., 2009). Apart for the well documented role of platelets in thrombosis and hemostasis, emerging data also suggest that platelet activation could be involved in the development of atherogenesis (Massberg et al., 2002; Massberg et al., 2003). Significantly, PF4 has been shown to modulate the atherogenic process. ApoE-null mice lacking PF4 show a significant decrease in aortic lesion formation as compared with ApoE (−/−) mice with PF4 (Sachais et al., 2007). The high levels of PF4 that we have observed in acrolein and ETS exposed mice in conjunction with the augmented activation of platelets suggests that acrolein exposure is not only thrombogenic, but could also be considered pro-atherogenic. In this regard it is important to note that the sub-chronic exposure to acrolein had a more pronounced effect on PF4 formation than the acute exposure, suggesting that the sustained exposure to lower levels of acrolein could be more deleterious than acute exposure.
Platelet activation leads to the mobilization of the granular protein, P-selectin, on the cell membrane. Activated platelets expressing P-selectin interact with circulating leukocytes, through P-selectin glycoprotein ligand (PSGL) to form platelet-leukocyte aggregates. We observed a 1.5–3-fold increase in the number of platelet-leukocyte aggregates in the blood of acrolein-exposed mice, suggesting that acrolein exposure mediates the activation of platelets in situ in the vasculature of the mice. Similar to PF4, sub-chronic exposure to acrolein had a more pronounced effect on platelet-leukocyte adduct formation than acute exposure, suggesting that continous exposure to lower levels of acrolein is more pro-thrombotic. The shortening of tail bleeding times observed in acrolein-exposed mice further suggests that the exposure to acrolein causes pro-thrombotic shift in the platelet hemostatic parameters, affecting the in vivo coagulation process. Together, multiple parameters of platelet activation measured in this study clearly show that acrolein exposure is pro-thrombotic in mice. In humans markers of platelet activation such as PF4 and platelet-leukocyte aggregate formation are associated with increased thrombotic disorders (Dole et al., 2005; McGregor et al., 2006; Kowalska et al., 2010), suggesting that human exposure to acrolein could be pro-thrombotic. Similar increase in platelet activation has been observed in the individuals exposed to ultrafine particles (Ruckerl et al., 2007), diesel exhaust (Lucking et al., 2008), or tobacco smoke (Harding et al., 2004).
High concentrations of acrolein are generated in cigarette smoke (Bhatnagar, 2006). In mainstream tobacco smoke, 100–600 μg of acrolein are generated per cigarette (50–70 ppm) (Ghilarducci and Tjeerdema, 1995; Smith and Fischer, 2001; Dong and Moldoveanu, 2004). Due to incomplete combustion, side-stream smoke contains 12-fold more acrolein than mainstream smoke (Ghilarducci and Tjeerdema, 1995); consistent with the high cardiovascular risk of second-hand tobacco smoke (Barnoya and Glantz, 2005). In smoky environments such as bars, restaurants, automobiles and trains where smoking is permitted, 20–300 μg/m3 (0.04 to 0.6 ppm) acrolein has been detected (Badre et al., 1978). Along with other aldehydes acrolein is also generated in high concentrations in car exhaust and it accounts for 1 mole % of the total aldehydes generated by car exhausts (20 to 60 mg/mile).Acrolein levels of 3 ppm have been measured in 10 % of the personal monitors of firefighters and 0.1 to 1.3 ppm acrolein has been measured in kitchen ventilator outlets (Ghilarducci and Tjeerdema, 1995). Hence, acrolein at concentrations present in mainstream or sidestream tobacco smoke could increase thrombosis in exposed humans and contribute to the atherogenic effects of environmental exposure to tobacco smoke or traffic-generated pollutants. In this regard it is significant to point out that transient exposure to traffic pollutants is associated with an increase in the risk of myocardial infarction (Peters et al., 2004). Because myocardial infarction is usually precipitated by the formation of an occlusive thrombus, it could be a reflection of an increase in thrombosis due to exposure to traffic-related pollutants which include acrolein.
Our data suggest that increased platelet activation in mice exposed to acrolein by inhalation are not due to pulmonary oxidative stress or inflammation. Exposure to acrolein under the experimental conditions did not affect the levels of MDA, measured as an index of oxidative stress. On the contrary, it has been reported that in rats, 4h exposure to acrolein (1 and 2 ppm) significantly increases oxidative stress in lungs (Arumugam et al., 1999). This could most likely due to species-dependent sensitivity to acrolein. The LD50 for 4h exposure to acrolein in rats is 8 ppm (Carpenter et al., 1949), whereas the LD50 for 6h exposure of acrolein to mice is 66 ppm (Philippin et al., 1970; WHO, 1992). Also, we did not observe any change in the expression of pro-inflammatory cytokines in the lungs of mice acutely or sub-chronically exposed to acrolein by inhalation. On the contrary, a previous report showed that chronic inhalation to acrolein (2 ppm for 6h/day, 5 days per week for 12 weeks) increases the expression of cytokines IL-10, IFN-g, IL-12p40, RANTES and MCP-1 in the lungs (Borchers et al., 2007). Together these observations suggest that acrolein-induced platelet activation precedes pulmonary inflammation.
Moreover we also observed that in addition to inhaled exposure, oral exposure to acrolein increases platelet activation and thrombosis. These data further strengthen the notion that acrolein-induced platelet activation is likely to be independent of secondary pulmonary toxicity. Unsaturated aldehydes such as acrolein, crotonaldehyde, and hexenal are also natural constituents of several foods and their concentration in food and drink increases upon cooking, heating or frying (Ghilarducci and Tjeerdema, 1995). Consequently, acrolein consumption via food exceeds that due to inhalation even in smokers (Wang et al., 2008). Hence, our observation that ingestion of acrolein increases platelet activation and thrombosis, suggests that components of diet and food can be significant determinants of cardiovascular disease risk. Importantly, the observation that both oral and inhalation exposure to acrolein increased thrombosis supports the notion that direct effects of acrolein appearing in the blood could be responsible for the pro-thrombotic effects of acrolein. This is consistent with the observation that the formation of protein-acrolein adducts was increased in platelets upon exposure to acrolein. Similarly, it is also possible that acrolein generated endogenously during oxidative stress (Uchida, 1999) can increase platelet activation. Collectively, this evidence points to the possibility that despites its high reactivity and efficient metabolism, acrolein survives transportation from the site of exposure to the blood to directly affect circulating cells such as platelets.
In summary, our data show that exposure to acrolein, either via inhalation or ingestion induces platelet activation and that this results in an pro-thrombotic state. We speculate that human exposure to acrolein might have pro-thrombotic effects similar to those observed in mice. Moreover, our data also support the idea that acrolein may be responsible for the well-documented pro-thrombotic effects of direct or second-hand tobacco smoke exposure or exposure to polluted environments rich in combustion products.
Acknowledgments
The authors are thankful to Mr. David Young and Ms. Erica Werkman for expert technical assistance. This work was supported in part by NIH grants ES17260, ES11594, ES11860, HL 95593, HL55477, HL59378, HL89380 and RR 24489.
Abbreviations
- ADP
Adenosine 5′-diphosphate
- PRP
platelet rich plasma
- WP
washed platelets
- PF4
platelet factor 4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JD, Jr, Klaidman LK. Acrolein-induced oxygen radical formation. Free Radic Biol Med. 1993;15:187–193. doi: 10.1016/0891-5849(93)90058-3. [DOI] [PubMed] [Google Scholar]
- Arumugam N, Sivakumar V, Thanislass J, Pillai KS, Devaraj SN, Devaraj H. Acute pulmonary toxicity of acrolein in rats--underlying mechanism. Toxicol Lett. 1999;104:189–194. doi: 10.1016/s0378-4274(98)00370-1. [DOI] [PubMed] [Google Scholar]
- Awe SO, Adeagbo AS, D’Souza SE, Bhatnagar A, Conklin DJ. Acrolein induces vasodilatation of rodent mesenteric bed via an EDHF-dependent mechanism. Toxicol Appl Pharmacol. 2006;217:266–276. doi: 10.1016/j.taap.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre R, Guillerm R, Abran N, Bourdin M, Dumas C. Atmospheric pollution by smoking (author’s transl) Ann Pharm Fr. 1978;36:443–452. [PubMed] [Google Scholar]
- Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res. 2006;99:692–705. doi: 10.1161/01.RES.0000243586.99701.cf. [DOI] [PubMed] [Google Scholar]
- Borchers MT, Wesselkamper SC, Harris NL, Deshmukh H, Beckman E, Vitucci M, Tichelaar JW, Leikauf GD. CD8+ T cells contribute to macrophage accumulation and airspace enlargement following repeated irritant exposure. Exp Mol Pathol. 2007;83:301–310. doi: 10.1016/j.yexmp.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghuber OC, Punzengruber C, Sinzinger H, Haber P, Silberbauer K. Platelet sensitivity to prostacyclin in smokers and non-smokers. Chest. 1986;90:34–38. doi: 10.1378/chest.90.1.34. [DOI] [PubMed] [Google Scholar]
- Carpenter CP, Smyth HF, Jr, Pozzani UC. The assay of acute vapor toxicity, and the grading and interpretation of results on 96 chemical compounds. J Ind Hyg Toxicol. 1949;31:343–346. [PubMed] [Google Scholar]
- Conklin DJ, Barski OA, Lesgards JF, Juvan P, Rezen T, Rozman D, Prough RA, Vladykovskaya E, Liu S, Srivastava S, Bhatnagar A. Acrolein consumption induces systemic dyslipidemia and lipoprotein modification. Toxicol Appl Pharmacol. 2010;243:1–12. doi: 10.1016/j.taap.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin DJ, Haberzettl P, Prough RA, Bhatnagar A. Glutathione-S-transferase P protects against endothelial dysfunction induced by exposure to tobacco smoke. Am J Physiol Heart Circ Physiol. 2009;296:H1586–1597. doi: 10.1152/ajpheart.00867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig WY, Palomaki GE, Haddow JE. Cigarette smoking and serum lipid and lipoprotein concentrations: an analysis of published data. BMJ. 1989;298:784–788. doi: 10.1136/bmj.298.6676.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza SE, Ginsberg MH, Burke TA, Plow EF. The ligand binding site of the platelet integrin receptor GPIIb-IIIa is proximal to the second calcium binding domain of its alpha subunit. J Biol Chem. 1990;265:3440–3446. [PubMed] [Google Scholar]
- D’Souza SE, Ginsberg MH, Matsueda GR, Plow EF. A discrete sequence in a platelet integrin is involved in ligand recognition. Nature. 1991;350:66–68. doi: 10.1038/350066a0. [DOI] [PubMed] [Google Scholar]
- Davis JW, Shelton L, Watanabe IS, Arnold J. Passive smoking affects endothelium and platelets. Arch Intern Med. 1989;149:386–389. [PubMed] [Google Scholar]
- Denton J, Lane DA, Thunberg L, Slater AM, Lindahl U. Binding of platelet factor 4 to heparin oligosaccharides. Biochem J. 1983;209:455–460. doi: 10.1042/bj2090455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWoskin RSGMW, Pepelko W, Strickland J. Toxicological Review of Acrolein. Environmental Protection Agency; Washington, DC: US: 2003. pp. 1–99. (CAS No. 107–102–108) [Google Scholar]
- Dole VS, Bergmeier W, Mitchell HA, Eichenberger SC, Wagner DD. Activated platelets induce Weibel-Palade-body secretion and leukocyte rolling in vivo: role of P-selectin. Blood. 2005;106:2334–2339. doi: 10.1182/blood-2005-04-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JZ, Moldoveanu SC. Gas chromatography-mass spectrometry of carbonyl compounds in cigarette mainstream smoke after derivatization with 2,4-dinitrophenylhydrazine. J Chromatogr A. 2004;1027:25–35. doi: 10.1016/j.chroma.2003.08.104. [DOI] [PubMed] [Google Scholar]
- Egle JL, Jr, Hudgins PM. Dose-dependent sympathomimetic and cardioinhibitory effects of acrolein and formaldehyde in the anesthetized rat. Toxicol Appl Pharmacol. 1974;28:358–366. doi: 10.1016/0041-008x(74)90221-x. [DOI] [PubMed] [Google Scholar]
- Freedman JE, Loscalzo J. Platelet-monocyte aggregates: bridging thrombosis and inflammation. Circulation. 2002;105:2130–2132. doi: 10.1161/01.cir.0000017140.26466.f5. [DOI] [PubMed] [Google Scholar]
- Ghilarducci DP, Tjeerdema RS. Fate and effects of acrolein. Rev Environ Contam Toxicol. 1995;144:95–146. doi: 10.1007/978-1-4612-2550-8_2. [DOI] [PubMed] [Google Scholar]
- Green MA, Egle JL., Jr The effects of acetaldehyde and acrolein on blood pressure in guanethidine-pretreated hypertensive rats. Toxicol Appl Pharmacol. 1983;69:29–36. doi: 10.1016/0041-008x(83)90115-1. [DOI] [PubMed] [Google Scholar]
- Gugliucci A. Antithrombin activity is inhibited by acrolein and homocysteine thiolactone: Protection by cysteine. Life Sci. 2008;82:413–418. doi: 10.1016/j.lfs.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Harding SA, Din JN, Sarma J, Jessop A, Weatherall M, Fox KA, Newby DE. Flow cytometric analysis of circulating platelet-monocyte aggregates in whole blood: methodological considerations. Thromb Haemost. 2007;98:451–456. [PubMed] [Google Scholar]
- Harding SA, Sarma J, Josephs DH, Cruden NL, Din JN, Twomey PJ, Fox KA, Newby DE. Upregulation of the CD40/CD40 ligand dyad and platelet-monocyte aggregation in cigarette smokers. Circulation. 2004;109:1926–1929. doi: 10.1161/01.CIR.0000127128.52679.E4. [DOI] [PubMed] [Google Scholar]
- Hoek G, Brunekreef B, Fischer P, van Wijnen J. The association between air pollution and heart failure, arrhythmia, embolism, thrombosis, and other cardiovascular causes of death in a time series study. Epidemiology. 2001;12:355–357. doi: 10.1097/00001648-200105000-00017. [DOI] [PubMed] [Google Scholar]
- Kachel DL, Martin WJ., 2nd Cyclophosphamide-induced lung toxicity: mechanism of endothelial cell injury. J Pharmacol Exp Ther. 1994;268:42–46. [PubMed] [Google Scholar]
- Kasahara DI, Poynter ME, Othman Z, Hemenway D, van der Vliet A. Acrolein inhalation suppresses lipopolysaccharide-induced inflammatory cytokine production but does not affect acute airways neutrophilia. J Immunol. 2008;181:736–745. doi: 10.4049/jimmunol.181.1.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- Kowalska MA, Rauova L, Poncz M. Role of the platelet chemokine platelet factor 4 (PF4) in hemostasis and thrombosis. Thromb Res. 2010;125:292–296. doi: 10.1016/j.thromres.2009.11.023. [DOI] [PubMed] [Google Scholar]
- Lane DA, Ireland H, Wolff S, Ranasinghe E, Dawes J. Detection of enhanced in vivo platelet alpha-granule release in different patient groups--comparison of beta-thromboglobulin, platelet factor 4 and thrombospondin assays. Thromb Haemost. 1984;52:183–187. [PubMed] [Google Scholar]
- Law MR, Wald NJ. Environmental tobacco smoke and ischemic heart disease. Prog Cardiovasc Dis. 2003;46:31–38. doi: 10.1016/s0033-0620(03)00078-1. [DOI] [PubMed] [Google Scholar]
- Leikauf GD. Hazardous air pollutants and asthma. Environ Health Perspect. 2002;110(Suppl 4):505–526. doi: 10.1289/ehp.02110s4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucking AJ, Lundback M, Mills NL, Faratian D, Barath SL, Pourazar J, Cassee FR, Donaldson K, Boon NA, Badimon JJ, Sandstrom T, Blomberg A, Newby DE. Diesel exhaust inhalation increases thrombus formation in man. Eur Heart J. 2008;29:3043–3051. doi: 10.1093/eurheartj/ehn464. [DOI] [PubMed] [Google Scholar]
- Martinez-Martinez I, Ordonez A, Guerrero JA, Pedersen S, Minano A, Teruel R, Velazquez L, Kristensen SR, Vicente V, Corral J. Effects of acrolein, a natural occurring aldehyde, on the anticoagulant serpin antithrombin. FEBS Lett. 2009;583:3165–3170. doi: 10.1016/j.febslet.2009.07.062. [DOI] [PubMed] [Google Scholar]
- Massberg S, Brand K, Gruner S, Page S, Muller E, Muller I, Bergmeier W, Richter T, Lorenz M, Konrad I, Nieswandt B, Gawaz M. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196:887–896. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S, Schulz C, Gawaz M. Role of platelets in the pathophysiology of acute coronary syndrome. Semin Vasc Med. 2003;3:147–162. doi: 10.1055/s-2003-40673. [DOI] [PubMed] [Google Scholar]
- McGregor L, Martin J, McGregor JL. Platelet-leukocyte aggregates and derived microparticles in inflammation, vascular remodelling and thrombosis. Front Biosci. 2006;11:830–837. doi: 10.2741/1840. [DOI] [PubMed] [Google Scholar]
- Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation. 2001;104:1533–1537. doi: 10.1161/hc3801.095588. [DOI] [PubMed] [Google Scholar]
- Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- Parent RA, Caravello HE, Long JE. Two-year toxicity and carcinogenicity study of acrolein in rats. J Appl Toxicol. 1992;12:131–139. doi: 10.1002/jat.2550120210. [DOI] [PubMed] [Google Scholar]
- Peters A, von Klot S, Heier M, Trentinaglia I, Hormann A, Wichmann HE, Lowel H. Exposure to traffic and the onset of myocardial infarction. N Engl J Med. 2004;351:1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- Philippin C, Gilgen A, Grandjean E. Toxicological and physiological investigation on acroleine inhalation in the mouse. Int Arch Arbeitsmed. 1970;26:281–305. [PubMed] [Google Scholar]
- Pope CA, 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Preston RJ, Tran S, Johnson JA, Ainle FN, Harmon S, White B, Smith OP, Jenkins PV, Dahlback B, O’Donnell JS. Platelet factor 4 impairs the anticoagulant activity of activated protein C. J Biol Chem. 2009;284:5869–5875. doi: 10.1074/jbc.M804703200. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Laher I. Structural and functional alteration of blood vessels caused by cigarette smoking: an overview of molecular mechanisms. Curr Vasc Pharmacol. 2007;5:276–292. doi: 10.2174/157016107782023406. [DOI] [PubMed] [Google Scholar]
- Ruckerl R, Phipps RP, Schneider A, Frampton M, Cyrys J, Oberdorster G, Wichmann HE, Peters A. Ultrafine particles and platelet activation in patients with coronary heart disease--results from a prospective panel study. Part Fibre Toxicol. 2007;4:1. doi: 10.1186/1743-8977-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachais BS, Turrentine T, Dawicki McKenna JM, Rux AH, Rader D, Kowalska MA. Elimination of platelet factor 4 (PF4) from platelets reduces atherosclerosis in C57Bl/6 and apoE-/- mice. Thromb Haemost. 2007;98:1108–1113. [PubMed] [Google Scholar]
- Sarma J, Laan CA, Alam S, Jha A, Fox KA, Dransfield I. Increased platelet binding to circulating monocytes in acute coronary syndromes. Circulation. 2002;105:2166–2171. doi: 10.1161/01.cir.0000015700.27754.6f. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Fischer TH. Particulate and vapor phase constituents of cigarette mainstream smoke and risk of myocardial infarction. Atherosclerosis. 2001;158:257–267. doi: 10.1016/s0021-9150(01)00570-6. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Joshi CS, Sethi PP, Agrawal AK, Srivastava SK, Seth PK. Altered platelet functions in non-insulin-dependent diabetes mellitus (NIDDM) Thromb Res. 1994;76:451–461. doi: 10.1016/0049-3848(95)90177-h. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Vladykovskaya E, Barski OA, Spite M, Kaiserova K, Petrash JM, Chung SS, Hunt G, Dawn B, Bhatnagar A. Aldose reductase protects against early atherosclerotic lesion formation in apolipoprotein E-null mice. Circ Res. 2009;105:793–802. doi: 10.1161/CIRCRESAHA.109.200568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal FJ, Roederer M, Herzenberg LA. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990;87:9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraason M, Luken ME, Breitenstein M, Krueger JA, Biagini RE. Comparative toxicity of allylamine and acrolein in cultured myocytes and fibroblasts from neonatal rat heart. Toxicology. 1989;56:107–117. doi: 10.1016/0300-483x(89)90216-3. [DOI] [PubMed] [Google Scholar]
- Tranholm M, Kristensen K, Kristensen AT, Pyke C, Rojkjaer R, Persson E. Improved hemostasis with superactive analogs of factor VIIa in a mouse model of hemophilia A. Blood. 2003;102:3615–3620. doi: 10.1182/blood-2003-05-1369. [DOI] [PubMed] [Google Scholar]
- Tsakadze NL, Srivastava S, Awe SO, Adeagbo AS, Bhatnagar A, D’Souza SE. Acrolein-induced vasomotor responses of rat aorta. Am J Physiol Heart Circ Physiol. 2003;285:H727–734. doi: 10.1152/ajpheart.00269.2003. [DOI] [PubMed] [Google Scholar]
- Uchida K. Current status of acrolein as a lipid peroxidation product. Trends Cardiovasc Med. 1999;9:109–113. doi: 10.1016/s1050-1738(99)00016-x. [DOI] [PubMed] [Google Scholar]
- Wang GW, Guo Y, Vondriska TM, Zhang J, Zhang S, Tsai LL, Zong NC, Bolli R, Bhatnagar A, Prabhu SD. Acrolein consumption exacerbates myocardial ischemic injury and blocks nitric oxide-induced PKCepsilon signaling and cardioprotection. J Mol Cell Cardiol. 2008;44:1016–1022. doi: 10.1016/j.yjmcc.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Ware JH. Particulate air pollution and mortality--clearing the air. N Engl J Med. 2000;343:1798–1799. doi: 10.1056/NEJM200012143432409. [DOI] [PubMed] [Google Scholar]
- WHO. Environmental Health Criteria 127, acrolein. Geneva: World Health Oranization; 1992. [Google Scholar]
- Zhu BQ, Sun YP, Sievers RE, Glantz SA, Parmley WW, Wolfe CL. Exposure to environmental tobacco smoke increases myocardial infarct size in rats. Circulation. 1994;89:1282–1290. doi: 10.1161/01.cir.89.3.1282. [DOI] [PubMed] [Google Scholar]
- Zhu BQ, Sun YP, Sievers RE, Isenberg WM, Glantz SA, Parmley WW. Passive smoking increases experimental atherosclerosis in cholesterol-fed rabbits. J Am Coll Cardiol. 1993;21:225–232. doi: 10.1016/0735-1097(93)90741-i. [DOI] [PubMed] [Google Scholar]