Abstract
An increase in PCO2 in the arterial blood triggers immediate release of ATP from the ventral chemosensory site(s) on the surface of the medulla oblongata. Systemic hypoxia in anesthetized rats was also associated with increased ATP release on the ventral medullary surface. During both hypoxia and hypercapnia, ATP and possibly other gliotransmitters released in the ventral medulla seemed to enhance cardiorespiratory responses to these stressors, and some of this ATP was proposed to be derived from astrocytes. Astrocytes also play a vital role controlling local blood flow. Astrocytes are activated by neurotransmitter release - especially glutamate and ATP. The astrocytic activation is manifest as a rise in intracellular Ca2+ that is closely coupled to the metabolic activity of neurons in the active area. The activation of astrocytes spreads as a wave from astrocyte to astrocyte and causes release of ATP, adenosine, and other gliotransmitters that may alter neuronal function in the region of astrocytic activation. In addition, ATP, adenosine and other vasoactive substances, when released at the endfeet of astrocytes, interact with vascular receptors that may either dilate or constrict the vessels in the region closely adjacent to the site of neuronal activity. Thus, astrocytes seem to integrate neuronal metabolic needs by responding to the level of neuronal activity to regulate local blood flow and cardiorespiratory responses to hypoxia and hypercapnia to match substrate need (oxygen and glucose) with substrate availability and with the removal of CO2. In so doing, astrocytes assume a larger role in information processing and in the regulation of neuronal activity and homeostasis of the entire organism than has been ascribed to them in the past.
1. Introduction
ATP, astrocytes and respiratory function are the focus of this review. There are two purinergic signaling-related phenomena in the central nervous system that fall under the broad heading of respiratory function that are relevant to this review. The first is chemosensory responses, especially responses to CO2, and the second is the control of blood flow in the brain. Both of these topics have been reviewed extensively (Gordon et al., 2009; Gourine, 2005; Koehler et al., 2006; Spyer et al., 2004; Spyer and Gourine, 2009; Xu and Pelligrino, 2007), and rather than reviewing these reviews, we have opted to develop a synthetic hypothesis in which the effects of ATP on ventilation and brain blood flow may be seen as two facets of a common homeostatic process aimed at matching glucose and oxygen consumption with glucose and oxygen delivery (and CO2 removal). Astrocytes seem to be at the center of a complex network of neuronal and vascular interactions that regulate metabolic homeostasis. As the study of astrocytes has expanded, the number of known neurotransmitters and neuromodulators released by astrocytes has grown significantly (Perea et al., 2009). Even though there are a variety of mediators of respiratory and vascular reactivity released from astrocytes, only purines - ATP and its degradation product, adenosine - will be discussed in this review.
2. Respiratory effects: ATP release from the ventral surface of the medulla in response to chemosensory stimulation
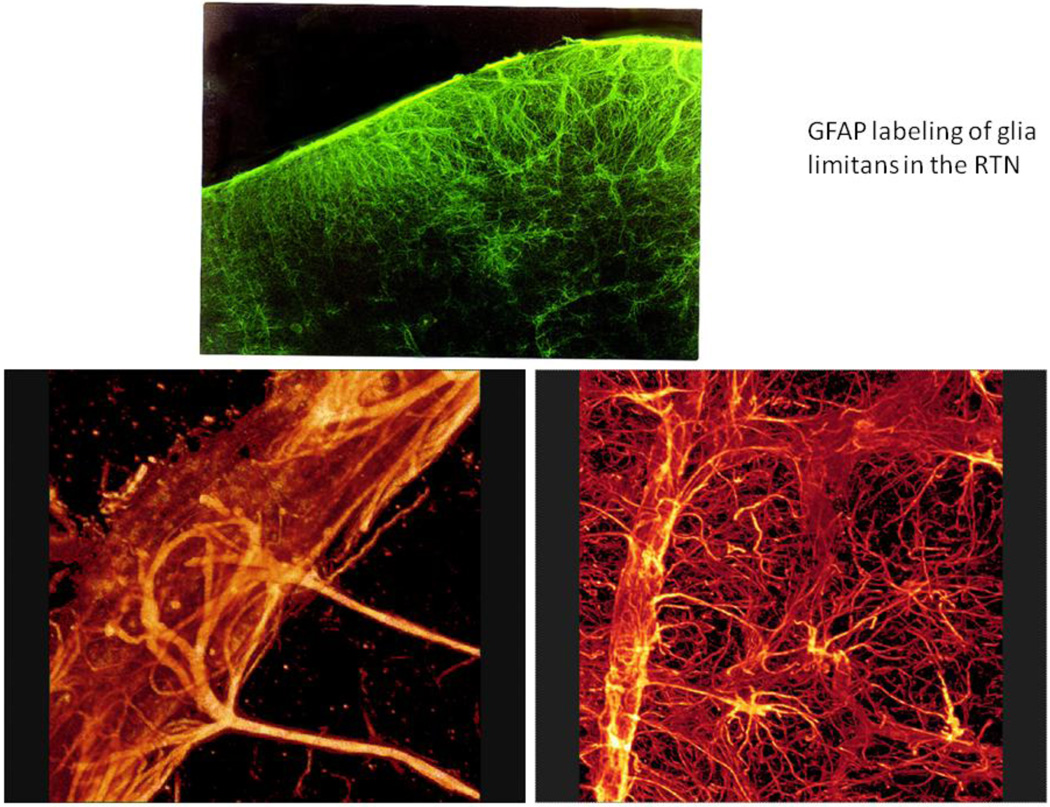
An increase in PCO2 in the arterial blood triggers immediate release of ATP from the ventral medullary surface chemosensitive regions (Gourine et al., 2005a). This was shown using amperometric enzymatic biosensors placed into direct contact with the ventral medullary surface pia matter in anaesthetized, peripherally chemodenervated, vagotomized and artificially ventilated rats. ATP release detected by the biosensors in response to an increase in inspired CO2 always preceded the development of the adaptive respiratory response (Gourine et al., 2005a). The “ATP releasing mechanism” is preserved in vitro in horizontal slices of the medulla oblongata, and biosensors failed to detect any significant release of ATP in response to CO2 elsewhere in the brainstem, apart from the ventral medullary surface (Gourine et al., 2005a). Moreover, removal of pia matter eliminates CO2-evoked ATP release irreversibly, indicating the importance of the structural integrity of the marginal glial layer of the ventral medullary surface. On the basis of these observations, the marginal glia appear to be the likely source of ATP release in response to increases in PCO2/[H+] (Spyer et al., 2004). The marginal glia are particularly dense along the surface of the ventral medulla as shown in Fig. 1. Moreover, the astrocytes in the glia limitans invest blood vessels with their endfeet and arborize extensively in the neuropil to make close contact with neurons (Fig 1).
Figure 1.
In the upper panel, the glia limitans on the ventral surface of the medulla (up) has been stained immunohistochemically using an antibody directed against glial fibrillary acid protein (GFAP). Note the density of astrocytes near the ventral surface. The lower panels demonstrate the dense investment of blood vessels by astrocytic endfeet and the extensive filling of the neuropil in the ventral medulla by astrocytic processes. Astrocytes are more sparsely distributed in the dorsal medulla; for example, in the NTS there are relatively few astrocytes (Erlichman et al., 2004).
The exact mechanisms and cellular sources of ATP release in response to an increase in PCO2/[H+] are as yet unclear. Initially, it seemed possible that pH sensitivity of purinergic receptor channels themselves might act as a chemosensitive mechanism in respiratory neurons (Thomas et al., 1999; Thomas and Spyer, 2000). Subsequent studies demonstrated that in vitro pH-sensitivity of neurons in the retrotrapezoid nucleus (RTN) did not depend on P2 receptor activation (Mulkey et al., 2004). Moreover, mice in which the P2X2 and P2X3 receptor subunits were knocked out had normal ventilatory responses to hypercapnia; the hypoxic response of these mice, on the other hand, was significantly attenuated (Rong et al., 2003). The role of P2 receptors within the RTN deserves further comment since it highlights the multiplicity of effects these receptors may have. Activation of P2X receptors in the RTN seemed to augment inhibition of RTN neurons (see Fig. 2) although the actual source of this inhibitory GABAergic or glycinergic input was not identified (Mulkey et al., 2006). In contrast, activation of P2Y receptors stimulated RTN neurons directly. These studies, however, were not exhaustive either in terms of the possible multiple subtypes of P2 receptors expressed by chemoreceptor neurons or in terms of the multiple chemosensory nuclei of the brainstem that express different P2 receptors. So it remains possible that there are P2 receptors of subtypes not yet tested that may contribute to neuronal pH sensitivity in the RTN and other chemosensory brainstem areas.
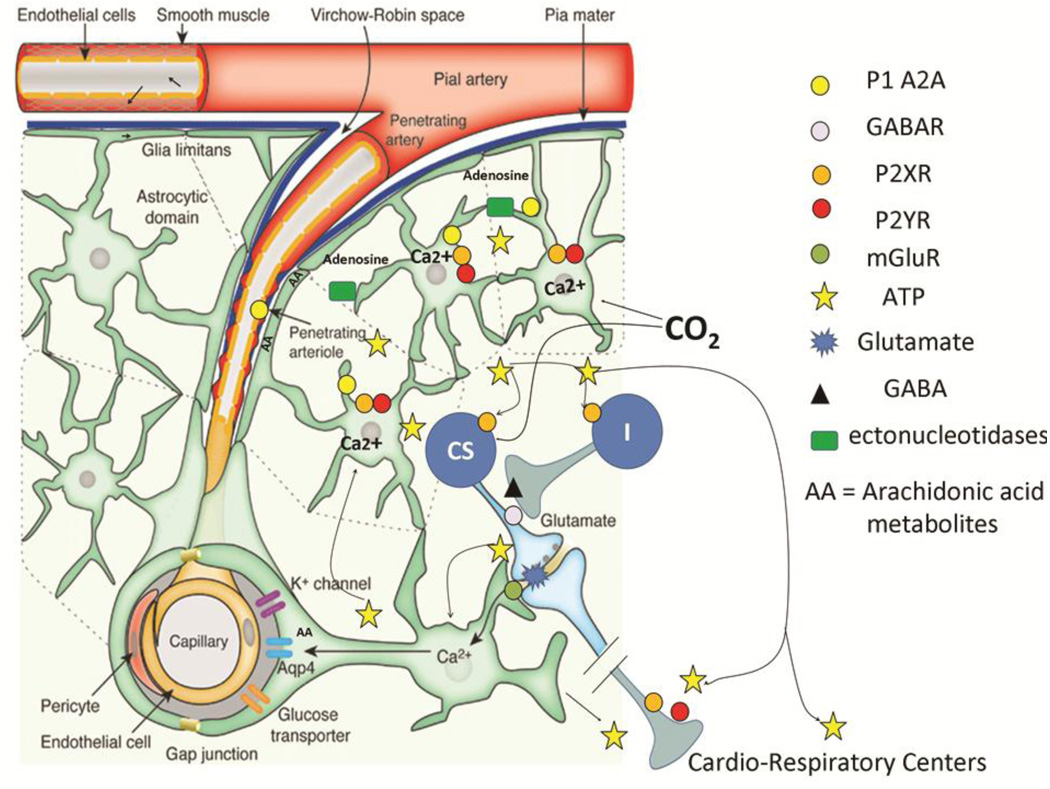
Figure 2.
A schematic drawing showing the effects of hypercapnia on ATP release and the ramification of ATP throughout the ventral medulla (adapted from (Iadecola and Nedergaard, 2007) with permission). One of the critical cellular events associated with glial ATP release is an increase in intracellular calcium that precedes vesicular fusion and release. Preliminary studies from one of our labs using the calcium indicator, calcium crimson, in GFP-expressing astrocytes suggest that intracellular calcium is increased in glia limitans in the RTN during exposure to CO2 (Erlichman, J.S.; unpublished findings). The mechanisms of ATP release appear to be varied. Recent work suggests that vesicular ATP release is via exocytotic release by lysosomes; however release may also be mediated by connexins, pannexins, or other transport mechanisms in Ca2+ independent manner (Cotrina et al., 1998; Iglesias et al., 2009; Zhang et al., 2007). Ectonucleotidases in the extracellular space generates adenosine that binds to the A2A subtype of P1 receptors, thereby augmenting dilation of pial/penetrating arteries (Langer et al., 2008; Stefan et al., 2006; Xu and Pelligrino, 2007). Purinergic activation results in the generation of vasoactive arachadonic acid metabolites (AA) (Haydon and Carmignoto, 2006; Takano et al., 2006; Zonta et al., 2003a). As the ATP/calcium cascade traverses down the vascular tree to smaller vessels, the mechanisms responsible for the dilation may rely more heavily on AA generation as opposed to the actions of adenosine.
As glial ATP migrates deeper into the neuropil, P2Y receptors on some chemosensory cells (CS) may be activated by ATP release (Mulkey et al., 2006). These cells appear to receive projections from unidentified cells that release GABA/glycine in response P2X2 receptor activation (I). ATP released in cardiovascular control centers in the ventral medulla also seems to augment the activity of these centers via P2X and P2Y receptors during hypoxia and hypercapnia.
2.1 Respiratory effects of ATP
Regardless of the exact cellular source(s), ATP released in response to an increase in PCO2/[H+] is perfectly capable of transmitting chemosensory stimuli (i.e. changes in either pH or PCO2 or both) into a modified pattern of breathing. Indeed, CO2-evoked release of ATP occurs in the areas of the ventral medullary surface located just underneath and in a close proximity to the brainstem respiratory network – exactly where the marginal glia are (Fig. 1). ATP has been shown to activate the rhythm-generating circuits and individual medullary respiratory neurons via its actions at certain P2 receptors (Gourine et al., 2003; Huxtable et al., 2009; Lorier et al., 2007; Lorier et al., 2008; Thomas and Spyer, 2000). Blockade of ATP receptors following microinjections of P2 antagonists into the ventral medulla or direct application of the antagonists on the ventral medullary surface markedly reduced CO2-evoked increases in breathing (Gourine et al., 2005a; Thomas et al., 1999). At the level of individual respiratory units, blockade of P2 receptors abolished CO2-evoked excitation of all tested inspiratory neurons (Thomas and Spyer, 2000).
2.2 ATP Release Arising from Central or Peripheral Respiratory Drive
Systemic hypoxia (10% oxygen in the inspired air) in anesthetized rats was also associated with an increase in ATP release on the ventral medullary surface, which appeared ~25 seconds after the onset of the hypoxic ventilatory response. A smaller, though still significant, increase in ATP concentration on the ventral medullary surface occurred even after sinoaortic denervation and vagotomy. Blocking P2 receptors in these studies augmented the late slowing of respiratory frequency seen after the initial increase in respiratory activity during the hypoxic challenge. ATP released in the medulla seemed to reduce the magnitude of hypoxic depression that occurs as the hypoxic stress persists (Gourine et al., 2005b). This observation is consistent with the enhanced hypoxic respiratory depression seen in P2X2 receptor subunit deficient mice (Rong et al., 2003).
2.3 ATP and cardiorespiratory control
ATP injected into the pressor region of the rostral ventrolateral medulla increased arterial blood pressure, heart rate and renal sympathetic nerve activity. On the other hand, ATP injection into the depressor regions of the rostral ventrolateral medulla decreased blood pressure, heart rate and renal sympathetic nerve activity, but antagonists of P2 receptors did not alter the baseline values of these variables (Horiuchi et al., 1999). Therefore, ATP may be active only when centrally released during hypoxia (Gourine et al., 2005b) or hypercapnia (Gourine et al., 2005a).
There are two additional points to be made that apply to both cardiac and respiratory responses to ATP released within the medulla. First, both P2X and P2Y receptors are involved in these cardiorespiratory responses. But beyond recognizing that both receptor types are present, it is premature to form any general conclusions about how responses segregate on the basis of these receptor types; there are not that many studies on this topic. Second, there is no consistent evidence that ATP release is functional at rest. The data available at this time suggest that ATP contributes to cardiorespiratory responses to significant stressors such as hypercapnia and hypoxia. Thus, ATP is released from the ventral surface of the medulla when homeostasis is significantly perturbed. The role of ATP in the control of respiration when, for example, PCO2 and pH of the brain parenchyma fluctuate near the physiological levels has not yet been examined.
2.4 Where does ATP come from?
ATP is co-released with a variety of neurotransmitters in the CNS. The Cl−-dependent vesicular nucleotide transporter (VNUT), which transports ATP into vesicles and secretory granules, is widely expressed in the CNS (Sawada et al., 2008), and all synaptic vesicles and secretory granules may contain some level of ATP that is co-released with other neurotransmitters. Some vesicles may contain only ATP (Abbracchio et al., 2009). ATP is also released by activated astrocytes (see below), and neurotransmitters released from neurons, especially glutamate and ATP, are potent activators of astrocytes.
Which of these possible mechanisms contributes to ATP release during hypercapnia and hypoxia is being evaluated currently. Glial responses and ATP release during hypercapnia may be secondary to activation of local neurons (for example pH-sensitive neurons of the RTN) or reflect intrinsic chemosensitivity of the ventral surface astrocytes leading to the release of ATP or intrinsic astrocytic Ca2+ oscillations and ATP release (Fiacco and McCarthy, 2006). Many glutamatergic neurons co-release ATP, and it is possible that ATP is released from neuronal sources such as glutamatergic RTN neurons, and this neuronally derived ATP may then initiate a cascade of astrocytic activation and further ATP release. On the other hand, glia near the ventral surface may be pH-sensitive. Fukuda et al. (1978) first observed that cells in the ventral medulla with the electrophysiological characteristics of astrocytes were depolarized during hypercapnic conditions. Moreover, about half of the astrocytes in the RTN were depolarized by hypercapnia (J.S. Erlichman, unpublished observations). These cells were identified as astrocytes by the expression of enhanced green fluorescent protein under control of the glial fibrillary acid protein (GFAP) promoter in mice or the presence of depolarization induced alkalosis in rats. To the extent that these pH-sensitive astrocytes are depolarized by hypercapnia, they may not require neuronal input to release gliotransmitters. Hypercapnia may depolarize some astrocytes by inhibiting potassium channels, and this depolarization might lead directly to astrocytic release of ATP. Finally, elevated CO2 may activate and open connexin hemichannels in astrocytes and facilitate ATP release by this mechanism (Huckstepp et al., 2010). Whatever the mechanism that initiates the process, astrocytic release of ATP serves to distribute ATP through the ventral medulla were it may amplify both cardiovascular and respiratory response to CO2. Thus, the increased level of ATP may reflect, in part, that aggregate neuronal and astrocytic activity present in any particular area of the brain.
3. Coupling Blood Flow with Neural Activity
It is difficult to study blood flow either in arterioles or capillaries within the matrix of the brainstem; the vessels are not easily accessible. Three approaches to study vascular reactivity in the brain have been used: confocal microscopy to study vessels just under the brain surface, studies of pial vessels on the brain surface and studies of unpressurized vessels in brain slices. All three of these approaches provide strong evidence that local blood flow is tightly coupled to local neuronal activity – the process called neurovascular coupling.
Confocal microscopy has been used to infer changes in blood flow from the motion of red blood cells just below the pial surface (Kleinfeld et al., 1998). In this study, activation of the somatosensory cortex by stimulating vibrissae or the hind limb resulted in local changes in red blood cell speed and the flux of red blood cells through capillaries per unit time. In addition, spectroscopic measurements of hemoglobin and hemoglobin oxygenation were used with multiunit recordings in the barrel cortex of rats, and here also, whisker stimulation produced changes in hemoglobin concentration and oxygenation that were temporally correlated. The amplitude of the hemodynamic response, however, followed a power law; the increase in the hemodynamic response was not linearly correlated with the increase in neuronal activity as had been described previously (Devor et al., 2003). While these studies confirm that neuronal activity is tightly coupled to blood flow, they require sophisticated methods, and they do not provide any insight into the chemical and cellular mechanisms whereby neurovascular coupling is achieved.
For studies of cellular mechanisms, investigators have studied more easily accessible pial vessels on the surface of the brain or they have used acute brain slices. Pial vessels do not have direct contact with deeper neurons, but pial vessels dilate as neuronal activity increases. The pial vessels sit adjacent to the glia limitans and are invested by an extensive network of astrocytic foot processes (Mulligan and MacVicar, 2004; Zonta et al., 2003a). Moreover, the control of upstream, pial resistance seems to depend on the glia limitans (Xu et al., 2008). Astrocytes, which form the glia limitans (and substance of the brain), are well positioned to interpret neuronal signals and integrate and communicate this information to blood vessels (Simard et al., 2003). Mediators of vascular control released by astrocytes within the glia limitans have ready access to blood vessels coursing over the brain surface. Studies of pial vessel have revealed a variety of ATP- and non-ATP-dependent astrocytic regulators of cerebral blood flow (Koehler et al., 2006), and it seems likely that similar processes control vascular reactivity within the substance of the brain where the blood vessels are also invested by astrocytic endfeet.
3.1 ATP and the control of vascular resistance in pial vessels
Increased neuronal activity elicited by local application of bicuculline or contralateral sciatic nerve stimulation induced pial vessel dilation. Suppression of neuronal activity by treatment with tetrodotoxin in the presence of these stimuli prevented pial vessel dilation – neuronal activity was essential to see pial vessel dilation (Xu et al., 2008). Disruption of the astrocytes within the glia limitans using L-α-adipic acid prevented pial vessel dilation even though electrophysiological activity of the neurons persisted (Xu et al., 2004). Blocking gap junctions and/or hemichannels containing connexin 43 also prevented pial vessel dilation (Xu et al., 2008). On the other hand, disruption of endothelial function did not alter the neuronal activity-driven pial vessel dilation (Xu et al., 2004). Astrocytes are electrically coupled through gap junctions containing connexin 43 and form local syncytia. Calcium waves may propagate through adjacent astrocytes over tens of microns on a time scale of seconds (Simard et al., 2003). In this way, glial calcium waves are propagated along the vessels leading to the release of vasoactive substances at the astrocytic endfeet. These calcium waves can propagate some 50 mm along a vessel (Simard et al., 2003). Thus, astrocytes appear to communicate a signal related to the intensity of local neuronal activity via inter-astrocytic mechanisms that inhibit vascular smooth muscle contraction so as to increase pial blood flow in the region of the active neurons (Xu et al., 2008).
In cortical brain slices, electrical stimulation within the slice, which was associated with increased intracellular astrocytic calcium, was followed by an increase in the diameter of small arteries (Zonta et al., 2003a). Metabotropic glutamate receptors (mGluR) are expressed by astrocytes, and blocking mGluR reduced the elevation in astrocytic intracellular Ca2+ and reduced vascular responses to electrical stimulation. Moreover, mGluR antagonists blunted the hyperemic response in the somatosensory cortex following paw stimulation in intact animals. Neuronal glutamate may stimulate mGluRs on astrocytes to activate phospholipase C and increase inositol 3-phosphate (IP3) levels in astrocytes (Carmingoto et al., 1998). Inositol 3-phosphate receptor mediated Ca2+ waves communicate the temporal and spatial distribution of neuronal activity to the endfeet of astrocytes. Electrical stimulation in cortical brain slices activated astrocytes through mGluRs and increased Ca2+ oscillations in astrocytes, which in turn lead to decreased Ca2+ oscillations in vascular smooth muscle and vasodilation (Filosa et al., 2004). Although mGluR agonists inhibited Ca2+ oscillations in vascular smooth muscle, mGluR antagonists did not prevent the activation of astrocytic Ca2+ oscillations during electrical stimulation of brains slices; implying that there are also non-mGLuR mechanisms that may activate astrocytes and promote vasodilation.
Autocrine activation of P2 purinergic receptors (likely involving P2Y subtype receptors) on astrocytes by ATP leads to increased intracellular Ca2+ (just as glutamate acting through mGluR receptors did) and further ATP release, which stimulates P2 receptors on adjacent astrocytes and elevates intracellular Ca2+ in these adjacent cells as well. ATP may be released from astrocytes by a variety of mechanisms. ATP may be co-released from neurons or may be released alone by synaptic mechanisms (Abbracchio et al., 2009; Pankratov et al., 2006), but it may also be released through gap junction hemichannels (Blum et al., 2008; Cotrina et al., 1998), P2X7 channels or volume-sensitive channels. Vesicular release of ATP seems to be the primary intercellular mediator of calcium waves propagating among astrocytes; connexins and glutamate receptors are not required (Bowser and Khakh, 2007; Guthrie et al., 1999). On the other hand, the presence of gap junctions enhances the spread of Ca2+ waves from cell to cell (Blum et al., 2008), but it appears that the connexins facilitate ATP release rather than intercellular diffusion of Ca2+ and IP3 (Cotrina et al., 1998; Cotrina et al., 2000).
3.2 What is the vasodilator released at astrocytic endfeet?
Electrical stimulation or mGluR agonists applied to cortical brain slices increased astrocytic Ca2+ and suppressed calcium oscillations in blood vessels and promoted vasodilation (Filosa et al., 2004). The vasodilator response in brain slices was blunted by aspirin administration, a cyclooxygenase inhibitor. Thus, synaptically derived glutamate activated mGluRs on astrocytes, which increased intracellular Ca2+ in astrocytes, which distributed this neuronally-derived information to other astrocytes and to the endfeet of individual astrocytes where the synthesis and release of a vasoactive prostaglandin was increased (Zonta et al., 2003a). There may be, however, other vasodilatory mechanisms mediated by astrocytes. Calcium-activated channels in the endfeet may be activated by the arrival of intracellular Ca2+ waves through astrocytes. Increased intracellular Ca2+ may enhance the activity of Ca2+-activated potassium channels (BK channels), and the elevation in extracellular potassium associated with greater K+ conductance through the BK channels in the region of astrocytic endfeet and smooth muscle cells appeared to activate an inward rectifying K+ channel on vascular smooth muscles cells, which hyperpolarized the vascular smooth muscle cells, reduced spontaneous Ca2+ oscillations and caused vasodilation (Filosa et al., 2006). This mechanisms works in concert with the cyclooxygenase-sensitive mechanism, which presumably synthesizes a vasodilating prostanoid, probably PGE2 (Zonta et al., 2003b).
ATP itself may be the source of additional vasodilating agents. Sciatic nerve stimulation and the associated somoatosensory cortical activation mediates local cortical vasodilation by adenosinergic mechanisms (Meno et al., 2001) in addition to the mechanisms described above. ATP is broken down by ectonucleotidases (which are ubiquitous in the central nervous system) to ADP, AMP and adenosine (Langer et al., 2008). Adenosine binds to P1 receptors, specifically the adenosine A2A receptor in the pial and penetrating arteries, and augments dilation of these vessels (Ngai et al., 2001). In the more superficial regions of pial vessels, much of the ATP is converted to adenosine by the ectonucleotidase alkaline phosphatase, which is highly expressed in these regions (Koehler et al., 2006). Consistent with this hypothesis, the adenosine receptor antagonists caffeine and theophylline both attenuate the activity-dependent vasodilation of pial vessels in the somatosensory cortex that accompanies sciatic nerve stimulation (Meno et al., 2005).
Adenosine has at least two additional effects that are relevant to matching metabolic activity to glucose and oxygen utilization. First, adenosine reduces the metabolic activity of neurons and inhibits neuronal activity through A1 receptors (Cunha, 2001). Activation of A1 receptors seems to increase potassium conductance and decrease Ca2+ conductance, thereby inhibiting neuronal activity (Cunha, 2005; Newman, 2003). It is interesting that the vasodilating substance elicited by increased neuronal activity may both increase blood flow and glucose and oxygen delivery locally to meet the increased neuronal metabolic demand while simultaneously decreasing that metabolic demand directly by inhibiting neuronal metabolism and by suppressing neuronal activity. As is so often true in physiological control systems, the stimulus and response (increased neuronal activity and metabolism) elicited is associated with a parallel and often less potent negation of the original stimulus and response (inhibition of neuronal activity and metabolism).
4. Unifying cardiorespiratory and vascular effects of ATP
In this review, we have developed the hypothesis that ATP is at the nexus of a variety of processes that match glucose and oxygen delivery to glucose and oxygen demand at a macroscopic, whole body level and at a microscopic tissue level. It seems that the same signaling pathway has been adapted to control multiple levels of metabolic demand/coupling mechanisms. Support for such an idea is more fully developed at the microscopic scale where neurovascular coupling is well-established. The essential element in this hypothesis is that astrocytes are activated by neurotransmitter release - especially glutamate and ATP. The astrocytic activation is manifest as a rise in intracellular Ca2+ that is closely coupled to the metabolic activity of neurons in the active area. Astrocytic activation is propagated locally via release of ATP. ATP diffuses to and activates additional astrocytes in the area and generates calcium waves in these astrocytes that then propagate the ‘activation’ internally and cause additional astrocytic release of ATP so that there is regenerative release of ATP and sequential activation of calcium waves in astrocytes as this ATP ‘signal’ spreads locally from the initial site of increased neuronal activity. ATP, adenosine, and other vasoactive substances released at the endfeet of astrocytes interact with vascular receptors that may either dilate or constrict the vessels in the region closely adjacent to the site of neuronal activity. Through these mechanisms, the extent of neuronal activation is correlated with the extent of astrocytic activation, which then determines the degree of vasodilation. Thus, the metabolic demand related to neuronal activation is appropriately matched by an increase in blood flow and the supply of glucose and oxygen. Support for this hypothesis comes from the observation that the extent of astrocytic activation is proportional to the extent of neuronal activation – there is a dose-response relationship between neuronal activity and changes in intracellular Ca2+ in astrocytes (Pasti et al., 1997; Porter and McCarthy, 1996), and the increase in astrocytic Ca2+ is spatially and temporally related to changes in neuronal activity and blood flow (Winship et al., 2007).
Can the same argument be made for macroscopic changes in ventilation and cardiac function? First, the changes in extracellular ATP levels in the medulla oblongata measured during hypercapnia and hypoxia are temporally associated with the stimulus. The CO2-evoked ATP release develops rapidly, while the ATP release triggered by hypoxia is more delayed. Ventilatory responses to both hypoxia and hypercapnia are blunted following application of P2 receptor antagonists. In a similar way, activation of P2 receptors seems to augment the cardiovascular response to hypoxia and hypercapnia (Horiuchi et al., 1999).
In addition, there appears to be a specific pattern to the spatial distribution of ATP release during hypoxia and hypercapnia. In response to elevated CO2, ATP release is greatest at the ventral medullary surface closely associated with the glia limitans. This is clearest during CO2 challenge, but even during hypoxia, the amount of ATP released is smaller in the areas located deeper in the brainstem more distant from the ventral surface and glia limitans and also smaller in the dorsal medulla. Moreover, one does not see similar patterns in the spatial distribution of the release of other neurotransmitters, such as glutamate (Gourine AV and Dale N, unpublished observations). Finally, many of the neurons in the ventral medulla with respiratory-related activity are activated by ATP. Purinergic regulation of respiratory-related neuronal activity may be quite complicated since P2X-dependent pre-synaptic inhibition and post-synaptic P2Y-dependent excitation have been found in RTN neurons (Mulkey et al., 2006). Presumably the same mechanisms of neuronal control exist for many neurons within the areas of the brainstem controlling cardiovascular function since ATP injected into the ventral medullary sites augments cardiovascular responses during hypoxia and hypercapnia. Thus, the spatial and temporal distribution of ATP release is closely associated with neurons involved in cardiorespiratory control. As was true for neurovascular coupling, the ATP release is closely associated with the glia limitans, and activated astrocytes are a likely source of this ATP. However, the detailed mechanisms of astrocytic ATP release and associated astrocytic Ca2+ waves have not yet been demonstrated for the ATP release detected on the ventral surface of the medulla. Moreover, a clear dose-response relationship between the level of hypercapnia or hypoxia and the amount of ATP release and ventilatory stimulation from ATP has not yet been established.
Presumably all glia near pia vessels, even those outside the ventral medulla, participate in neurovascular coupling through ATP-dependent mechanisms, yet CO2-evoked ATP release only occurs near the ventral surface of the medulla. It seems unlikely that the mere presence of pH-sensitive neurons in the ventral medulla gives rise to elevated ATP concentrations in this region. CO2 sensitivity of neurons is too widespread to be the sole explanation, at least as we understand the relatively restricted region of elevated ATP levels in the medulla and the wide distribution of chemosensory nuclei. On the other hand, the amount of ATP co-released by pH-sensitive neurons might be heterogeneous among chemosensory sites and within the medulla. Moreover, the amplification of ATP levels that occurs by activation of astrocytes will be greatest in the areas of greatest density of the astrocytes, such as the ventral medullary surface glial layer. Differences in the density of astrocytes seem most likely to contribute to the different levels of ATP released within the medulla.
The heterogeneous distribution of ATP release is an example of a more general phenomenon. The concentration of astrocytes varies within the medulla, and to the extent astrocytes modify both the stimulus and the response of neurons, then the density of astrocytes will modify or amplify the neuronal responses to what might otherwise have been equivalent chemosensory stimuli in different nuclei. Astrocytes are yet one more factor that contributes to heterogeneous responses from different chemosensory nuclei (Erlichman and Leiter, 2010).
That ATP released in the ventral medulla should augment the ventilatory and cardiovascular response to hypoxia and hypercapnia is appealing because the same need to match oxygen and glucose delivery to neuronal activity at a local level via neurovascular coupling exists at a global, whole organ level during hypoxia and hypercapnia. This ‘neuro-cardiorespiratory coupling’ mirrors the mechanisms involved in neurovascular coupling. In some sense, there is no benefit to increasing blood flow by neurovascular coupling if the blood delivered to a specific location has not been conditioned by the cardiorespiratory system to enhance oxygen delivery and CO2 removal. Moreover, astrocytes seem to play an essential role in metabolic control or support of neuronal activity at the local and the whole organ level. Astrocyte-to-neuron communication is dominated by diffusion, and for this reason, astrocytic processes usually work at a local level within the brain. We are hypothesizing that the local effects of ATP released by astrocytes in the ventral medulla may participate in integrated control of cardiorespiratory function beyond the level of the central nervous system. Other evidence of astrocytic involvement in homeostasis of the whole organism may be found in the role of astrocytes in sleep regulation (Halassa et al., 2009).
In summary, astrocytes seem to integrate neuronal metabolic need by responding to the level of neuronal activity to regulate local blood flow and cardiorespiratory responses to hypoxia and hypercapnia to match substrate need (oxygen and glucose) with substrate availability and with the removal of CO2. In so doing, astrocytes assume a much larger role in information processing and in the regulation of neuronal activity and homeostasis of the entire organism than has been ascribed to them in the past.
Acknowledgement
AVG research is supported by the Wellcome Trust. This work was also supported by grants NIH HL-71001and HL56683 (JCL) and NSF IOB-0517698 (JSE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Abbracchio MP, Burnstock G, Verkhrasky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. TINS. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Blum AE, Joseph SM, Pryzbylski RJ, Dubyak GR. Rho-family GTPases modulate Ca2+-dependent ATP release from astrocytes. Am. J. Physiol. 2008;295:231–241. doi: 10.1152/ajpcell.00175.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser DN, Khakh BS. Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J. Gen. Physiol. 2007;129:485–491. doi: 10.1085/jgp.200709780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmingoto G, Pasti L, Pozzan T. On the role of voltage-dependent calcium channels in calcium signaling of astrocytes in situ. J. Neurosci. 1998;18:4637–4645. doi: 10.1523/JNEUROSCI.18-12-04637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc. Natl. Acad. Sci. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, López-García JC, Naus CC, Nedergaard M. ATP-mediated glia signaling. J. Neurosci. 2000;20:2835–2844. doi: 10.1523/JNEUROSCI.20-08-02835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem. Int. 2001;38:107–125. doi: 10.1016/s0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- Cunha RA. Neuroprotection by adenosine in the brain: From A1 receptor activation to A2A receptor blockade. Purinergic Signalling. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Dunn AK, Andermann ML, Ulbert I, Boas DA, Dale AM. Coupling of total hemoglobin concentration, oxygenation, and neural activity in rat somatosensory cortex. Neuron. 2003;39:353–359. doi: 10.1016/s0896-6273(03)00403-3. [DOI] [PubMed] [Google Scholar]
- Erlichman JS, Cook A, Schwab MC, Budd TW, Leiter JC. Heterogeneous patterns of pH regulation in glial cells in the dorsal and ventral medulla. Am. J. Physiol. 2004;286:R289–R302. doi: 10.1152/ajpregu.00245.2003. [DOI] [PubMed] [Google Scholar]
- Erlichman JS, Leiter JC. Glial modulation of the extracellular mileau as a factor in central CO2 chemosensitivity and respiratory control. J. Appl. Physiol. 2010 doi: 10.1152/japplphysiol.01321.2009. doi:10.1152/japplphysiol.01321.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiacco TA, McCarthy KD. Astrocyte calcium elevations: Properties, propagation, and effects on brain signalling. Glia. 2006;54:676–690. doi: 10.1002/glia.20396. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ. Res. 2004;95:e73–e81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat. Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Honda Y, Schläfke ME, Loeschke HH. Effect of H+ on the membrane potential of silent cells in the ventral and dorsal surface layers of the rat medulla in vitro. Pflugers Arch. 1978;376:229–235. doi: 10.1007/BF00584955. [DOI] [PubMed] [Google Scholar]
- Gordon GRJ, Mulligan SJ, MacVicar BA. Astroctye control of blood flow. In: Parpura V, Haydon PG, editors. Astrocytes in (Patho)Physiology of the Nervous System. Springer Science+Business Media, LLC; 2009. pp. 461–486. [Google Scholar]
- Gourine AV. On the peripheral and central chemoreception and control of breathing: an emerging role of ATP. J. Physiol. 2005;568.3:715–724. doi: 10.1113/jphysiol.2005.095968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Atkinson L, Deuchars J, Spyer KM. Purinergic signalling in the medullary mechanisms of respiratory control in the rat: respiratory neurones express the P2X2 receptor subunit. J. Physiol. 2003;552.1:197–211. doi: 10.1113/jphysiol.2003.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005a;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. Release of ATP in the ventral medulla during hypoxia in rats: Role in hypoxic ventilatory response. J. Neurosci. 2005b;25:1211–1218. doi: 10.1523/JNEUROSCI.3763-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie PB, Knappenberger J, Segal M, Bennett MVL, Charles AC, Kater SB. ATP released from astrocytes mediates clial calcium waves. J. Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee S-Y, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Horiuchi J, Potts PD, Tagawa T, Dampney RAL. Effects of activation and blcokade of P2X receptors in the ventrolateral medulla on arterial pressure and sympathetic activity. J. Autonomic Nerv. Sys. 1999;76:118–126. doi: 10.1016/s0165-1838(99)00019-3. [DOI] [PubMed] [Google Scholar]
- Huckstepp RTR, Eason R, Id Bihi R, Dicke N, Willecke K, Spyer KM, Gourine AV, Dale NE. Connexin 26 is responsible for ATP release underlying central CO2 chemosensitivity. Exp. Biol. 2010 doi: 10.1113/jphysiol.2010.192088. abstract, prog. No. 1026.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable AG, Zwicker JD, Poon BY, Pagliardini S, Vroue SQ, Greer JJ, Funk GD. Tripartite purinergic modulation of central respiratory networks during perinatal development: The influence of ATP, ectonucleotidases, and ATP metabolites. J. Neurosci. 2009;29:14713–14725. doi: 10.1523/JNEUROSCI.2660-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte "hemichannels". J. Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D, Mitra PP, Helmchen F, Denk W. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc. Natl. Acad. Sci. 1998;95:15741–15746. doi: 10.1073/pnas.95.26.15741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J. Appl. Physiol. 2006;100:307–317. doi: 10.1152/japplphysiol.00938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer D, Hammer K, Koszalka P, Schrader J, Robson S, Zimmermann H. Distribution of ectonucleotidases in the rodent brain revisited. Cell Tissue Res. 2008;334:199–217. doi: 10.1007/s00441-008-0681-x. [DOI] [PubMed] [Google Scholar]
- Lorier AR, Huxtable AG, Robinson DM, Lipski J, Housley GD, Funk GD. P2Y1 receptor modulation of the pre-Bötzinger complex inspiratory rhythm generating network in vitro. J. Neurosci. 2007;27:993–1005. doi: 10.1523/JNEUROSCI.3948-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorier AR, Lipski J, Housley GD, Greer JJ, Funk GD. ATP sensitivity of preBötzinger complex neurones in neonatal rat in vitro: mechanism underlying a P2 receptor-mediated increase in inspiratory frequency. J. Physiol. 2008;586.5:1429–1446. doi: 10.1113/jphysiol.2007.143024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meno JR, Crum AV, Winn HR. Effect of adenosine receptor blockade on pial arteriolar dilation during sciatic nerve stimulation. Am. J. Physiol. 2001;281:H2018–H2027. doi: 10.1152/ajpheart.2001.281.5.H2018. [DOI] [PubMed] [Google Scholar]
- Meno JR, Nguyen TK, Jensen EM, West GA, Groysman L, Kung DK, Ngai AC, Britz GW, Winn HR. Effect of caffeine on cerebral blood flow response to somatosensory stimulation. J. Cereb. Blood Flow Metab. 2005;25:775–784. doi: 10.1038/sj.jcbfm.9600075. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Mistry AM, Guyenet PG, Bayliss DA. Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J. Neurosci. 2006;26:7230–7233. doi: 10.1523/JNEUROSCI.1696-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat. Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Newman EA. Glial cell inhibition of neurons by release of ATP. J. Neurosci. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai AC, Coyne EF, Meno JR, West A, Winn HR. Receptor subtypes mediating adenosine-induced dilation of cerebral arterioles. Am. J. Physiol. 2001;280:H2329–H2335. doi: 10.1152/ajpheart.2001.280.5.H2329. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Verkhratsky A, North RA. Vesicular relase of ATP at central synapses. Pflugers Arch.-Eur. J. Physiol. 2006;452:589–597. doi: 10.1007/s00424-006-0061-x. [DOI] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: A highly plastic bidirectional form of communication between neurons and astrocytes in situ. J. Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J. Neurosci. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong W, Gourine AV, Cockayne DA, Xiang A, Ford APDW, Spyer KM, Burnstock G. Pivotal role of nucleotide P2X2 receptor subunit of ATP-Gated ion channel mediating ventilatory responses to hypoxia. J. Neurosci. 2003;23:11315–11321. doi: 10.1523/JNEUROSCI.23-36-11315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc. Natl. Acad. Sci. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M, Arcuino G, Takano T, Liu QS, Nedergard M. Signaling at the gliovascular interface. J. Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyer KM, Dale N, Gourine AV. ATP is a key mediator of central and peripheral chemosensory transduction. Exp. Physiol. 2004;98.1:53–59. doi: 10.1113/expphysiol.2003.002659. [DOI] [PubMed] [Google Scholar]
- Spyer KM, Gourine AV. Chemosensory pathways in the brainstem controlling cardiorespiratory activity. Phil. Trans. R. Soc. B. 2009;364:2603–2610. doi: 10.1098/rstb.2009.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan C, Jansen S, Bollen M. Modulation of purinergic signaling by NPP-type ectoectophsphodiesterases. Purinergic Signal. 2006;2:361–370. doi: 10.1007/s11302-005-5303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Tian G-F, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 2006;9:159–161. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Thomas T, Ralevic V, Gadd C, Spyer K. Central CO2 chemoreception: a mechanism involving P2 purinoreceptors localized in the ventrolateral medulla of the anesthetized rat. J. Physiol. (London) 1999;517.3:899–905. doi: 10.1111/j.1469-7793.1999.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Spyer KM. ATP as a mediator of mammalian central CO2 chemoreception. J. Physiol. 2000;523.2:441–447. doi: 10.1111/j.1469-7793.2000.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship IR, Plaa N, Murphy TH. Rapid astrocyte calcium signals correlate with neuronal activity and onset of hemodynamic response in vivo. J. Neurosci. 2007;27:6268–6272. doi: 10.1523/JNEUROSCI.4801-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H-L, Mao L, Ye S, Paisansathan C, Vetri F, Pelligrino DA. Astrocytes are a key conduit for upstream signaling of vasodilation during cerebral cortical neuronal activation in vivo. Am. J. Physiol. 2008;294:H622–H632. doi: 10.1152/ajpheart.00530.2007. [DOI] [PubMed] [Google Scholar]
- Xu H-L, Pelligrino DA. ATP release and hydrolysis contribute to rat pial arteriolar dilation elicited by neuronal activation. Exp. Physiol. 2007;92.4:647–651. doi: 10.1113/expphysiol.2006.036863. [DOI] [PubMed] [Google Scholar]
- Xu HL, Koenig HM, Ye S, Feinstein DL, Pelligrino DA. Influence of glia limitans on pial arteriolar relaxation in the rat. Am. J. Physiol. 2004;287:H331–H339. doi: 10.1152/ajpheart.00831.2003. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu X, Duan S. Regulated ATP release from astrocytes through lysosome exocytosis. Nat. Cell Biol. 2007;9:945–953. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossman K-A, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat. Neurosci. 2003a;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- Zonta M, Sebelin A, Gobbo S, Fellin T, Pozzan T, Carmignoto G. Glutamate-mediated cytosolic calcium oscillations regulate a pulsatile prostaglandin release from cultured rat astrocytes. J. Physiol. 2003b;553.2:407–414. doi: 10.1113/jphysiol.2003.046706. [DOI] [PMC free article] [PubMed] [Google Scholar]