Summary
Antagonists of H3-type histamine receptors exhibit cognitive-enhancing properties in various memory paradigms as well as evidence of antipsychotic activity in normal animals. The present study determined if a prototypical H3 antagonist, ciproxifan, could reverse the behavioral effects of MK-801, a drug used in animals to mimic the hypoglutamatergic state suspected to exist in schizophrenia. Four behaviors were chosen for study, locomotor activity, ataxia, prepulse inhibition (PPI), and delayed spatial alternation, since their modification by dizocilpine (MK-801) has been well characterized. Adult male Long-Evans rats were tested after receiving a subcutaneous injection of ciproxifan or vehicle followed twenty minutes later by a subcutaneous injection of MK-801 or vehicle. Three doses of MK-801 (0.05, 0.1, & 0.3 mg/kg) increased locomotor activity. Each dose of ciproxifan (1.0 & 3.0 mg/kg) enhanced the effect of the moderate dose of MK-801, but suppressed the effect of the high dose. Ciproxifan (3.0 mg/kg) enhanced the effects of MK-801 (0.1 & 0.3 mg/kg) on fine movements and ataxia. Deficits in PPI were observed after treatment with MK-801 (0.05 & 0.1 mg/kg), but ciproxifan did not alter these effects. Delayed spatial alternation was significantly impaired by MK-801 (0.1 mg/kg) at a longer delay, and ciproxifan (3.0 mg/kg) alleviated this impairment. These results indicate that some H3 antagonists can alleviate the impact of NMDA receptor hypofunction on some forms of memory, but may exacerbate its effect on other behaviors.
Keywords: Histamine, glutamate, locomotor activity, prepulse inhibition, ataxia, working memory, NMDA receptor
Introduction
Ciproxifan is an imidazole-containing compound that was originally described by Ligneau and colleagues (1998) as a potent antagonist at histamine H3 receptors, and it remains a useful tool for disseminating the role of H3 receptors in behavior and brain function. The H3 receptor acts as an autoreceptor and is located on synaptic terminals where it modifies the release of histamine (Ligneau et al., 1998) along with several other neurotransmitters. H3 antagonists, such as ciproxifan, have been shown to increase the release of acetylcholine in the hippocampus, entorhinal cortex, and prefrontal cortex (Clapham and Kilpatrick, 1992; Bacciottini et al., 2002; Fox et al., 2005; Medhurst et al., 2007; Ligneau et al., 2007b) and dopamine and norepinephrine in the prefrontal cortex (Fox et al., 2005; Medhurst et al., 2007; Ligneau et al., 2007b).
Since increased cholinergic and dopaminergic tone in these brain regions may enhance memory, attention, and wakefulness, there has been considerable interest in the behavioral effects of H3 antagonists. Improvements in attention, social memory, inhibitory avoidance, rotorod performance, and object recognition (Ligneau et al., 1998; Fox et al., 2003; Medhurst et al., 2007) have been observed in normal rats and mice after the administration of H3 antagonists. In models of memory impairment, H3 antagonists ameliorate the amnesic effects of aging (Medhurst et al., 2007) and the muscarinic receptor antagonist, scopolamine (Medhurst et al., 2007; Ligneau et al., 2007b; Galici et al., 2009). H3 receptors have also been considered a target for new antipsychotic compounds since H3 antagonists have been shown to reverse the deficits in prepulse inhibition (PPI) observed in DBA mice (Browman et al., 2004) and apomorphine-treated mice (Ligneau et al., 2007a), as well as psychostimulant-induced increases in locomotor activity (Clapham and Kilpatrick, 1994, Morisset et al., 2002; Fox et al., 2005; Ligneau et al., 2007a).
The behavioral sequelae observed in rats and mice after the administration of drugs that block N-methyl-D-aspartate (NMDA) receptors has been viewed by many researchers as a valid animal model of schizophrenia. Drugs, such as dizocilpine (MK-801), that block NMDA receptors in a use-dependent manner (Homayoun and Moghaddam 2007), produce deficits in memory and PPI that may emulate the cognitive and sensorimotor gating deficits observed in people with schizophrenia. Moreover, the hyperlocomotion produced by MK-801 in rats has been used to screen potential antipsychotic drugs (Bardgett, 2004). It is important to note that the use of NMDA antagonists in animals as a neurochemical model of schizophrenia is not perfect; among several issues, higher doses of non-competitive antagonists such as MK-801 or PCP produce ataxia and motor impairment (Wozniak et al., 1990; Brosnan-Watters et al., 1996) that have not been associated with the disorder, and may be related to some of the behavioral deficits that they produce in animals.
Despite these limitations, characterization of the behavioral activity of H3 antagonists in animals treated with NMDA antagonists could, at the very least, yield fundamental insights into the modulation of NMDA-dependent behavioral processes by H3 receptors. Along these lines, Bernearts et al., (2004) demonstrated that the H3 antagonist, thioperamide, reverses the adverse effects of MK-801 on passive avoidance memory in mice. Other studies in mice (Faucard et al., 2006; Ligneau et al., 2007a) have shown that newer, non-imidazole H3 antagonists alter the effects of MK-801 on locomotor behavior. However, research in rats regarding the interactive effects of H3 and NMDA antagonists is lacking, not only in the area of locomotor activity, but especially in the domains of PPI and memory. Given this paucity of research, the present study sought to ascertain the effects of the H3 antagonist, ciproxifan, on the locomotor changes, ataxia, PPI deficits, and delayed spatial alternation impairment produced by acute MK-801 treatment in rats.
Methods
Subjects
Fifty-nine adult male Long-Evans rats (200-225 grams) were purchased from Harlan Bioproducts (Indianapolis, IN). The rats were group-housed three per cage with free access to food and water except where noted. Lighting in the animal colony was maintained on a 12-hour light/dark schedule with lights on at 07:00. All experimental procedures were performed according to the Current Guide for the Care and Use of Laboratory Animals (USPHS) under a protocol approved by the Northern Kentucky University Institutional Animal Use and Care Committee.
Drugs and treatment procedure
Doses of ciproxifan were chosen (1.0 & 3.0 mg/kg) based on their behavioral activity in tests of arousal (Le et al., 2008), attention (Ligneau et al., 1998), and memory (Fox et al., 2003). Moreover, the selected doses bind to 75% and 90%, respectively, of H3 receptors as reported in rat cortex receptor binding studies (Le et al., 2007). Doses of MK-801 (0.05 & 0.1 mg/kg) were based on their activity in our previous research on locomotor activity, PPI and delayed spatial alternation (Bardgett et al., 1997; 2008; 2009; Jacobs et al., 2000). Additional experiments were performed to assess the effects of a higher dose of MK-801 (0.3 mg/kg) on locomotor activity and ataxia, since this dose has been used in locomotor studies involving H3 antagonists and MK-801 in mice (Faucard et al., 2006; Ligneau et al., 2007a). The 0.3 mg/kg dose of MK-801 was not used in the PPI or delayed spatial alteration experiments due to its ataxic effects (Wozniak et al, 1990).
Ciproxifan (free base) was kindly provided by the National Institute of Mental Health’s Chemical Synthesis and Drug Supply program, and (+)-MK-801 hydrogen maleate was purchased from Sigma (St. Louis, MO). In each experiment, ciproxifan or saline was injected 40 minutes prior to testing, and MK-801 or saline was injected 20 minutes prior to testing, with the exception of the ataxia experiments where testing was conducted 45 minutes after the last injection. All injections were administered subcutaneously. All testing occurred with at least a 48-hour interval between treatments. Dosing for each group of rats was performed using a counterbalanced, within-subjects design. A different set of rats was used for the locomotor (n = 12), ataxia (n = 12), startle/PPI (n = 12), and delayed alternation tests (n = 11). A separate group of 12 rats were tested later to determine the interactive effect of each ciproxifan dose with a high dose of MK-801 (0.3 mg/kg) on locomotor activity.
Locomotor testing
The procedure for assessing the effects of MK-801 on locomotor activity was based on previous work from our lab (Bardgett et al., 1997; Jacobs et al., 2000; Bardgett et al., 2003; Bardgett et al., 2009) that demonstrated its sensitivity to the doses of MK-801 used in this study. Clear polypropylene cages that measured 25.9 cm wide × 47.6 cm long × 20.9 cm high were used to test locomotor activity. These cages were filled with wood chips to cover the bottom and were placed in Hamilton-Kinder (Poway, CA) Smart-Frame photocell-based, activity-monitoring racks. Rats were placed in the test cages for two hours a day for three consecutive days prior to any drug testing. The overhead lights were off during testing. On drug testing days, the rats were placed in the testing cages twenty minutes after the last injection (saline or MK-801). The number of consecutive photobeam breaks was recorded every five minutes for one hour, and this data was used to compare the effects of each pretreatment-treatment condition. The number of consecutive breaks at a single photobeam was used as a measure of fine movements, a metric that has been used to assess stereotyped behavior produced by NMDA antagonists (Swanson and Schoepp 2002). As mentioned above, a separate group of 12 rats were tested to determine the effects of ciproxifan on locomotor activity and fine movements elicited by a high dose (0.3 mg/kg) of MK-801.
Ataxia measures
Twelve rats were used to assess sensorimotor function in three tests of ataxia that are sensitive to MK-801 (Wozniak et al., 1990; Brosnan-Watters et al., 1996). Each test was conducted twice beginning at 45 minutes after the last drug injection. The test battery included measures of platform balance, walking initiation, and righting reflex. For the platform test, each rat was placed on a polypropylene platform that measured 17.5 × 26.5 cm approximately 90 cm above the ground. Rats were left on the platform until they fell off or 120 seconds had elapsed. For the walking initiation test, each rat was placed in the center of a 90 cm × 90 cm sheet of Plexiglas that was painted black. Lines of white tape were used to form a 51 × 51 cm square centered on the Plexiglas. The time for the rat to place all four paws outside of this square was recorded. For the righting reflex task, the rat was placed supine on a towel in a tabletop and the time to right itself and stand on its four paws was recorded. Rats were tested without drugs for two days. Rats were then tested every other day after drug treatments. All 12 rats were tested once in a single task, and then tested again after all 12 rats had been tested once. This was continued until all three tests were performed two times. The times for the two trials were averaged for each rat. Only the 3.0 mg/kg dose of ciproxifan was tested since the locomotor activity studies indicated equipotent effects of the 1.0 and 3.0 mg/kg doses of the drug on motor activity.
Auditory startle and prepulse inhibition
The acoustic startle and PPI studies used a standard procedure (Swerdlow et al., 2000; 2004) that is sensitive to NMDA antagonists (Swerdlow et al., 2004). A Hamilton-Kinder Startle Monitor (Hamilton-Kinder, Poway, CA) system was used to test startle and PPI after drug treatment. Prior to any drug testing, animals were first exposed to a baseline session of predominantly startle trials and two additional sessions of predominantly PPI trials. The first session consisted of a five-minute acclimation period in which the rat was exposed to continuous 70 dB background noise. The rat was then exposed to, in random order, seventeen startle trials (40 ms 120 dB tone), five PPI trials (20 ms tones that were 12 dB above background followed 80 ms later by a startle tone), and three no startle (i.e., no stimulus) trials. The average intertrial interval was 15 seconds. During the next two sessions, rats were acclimated as in the first baseline session and then exposed to four consecutive startle trials. Following these trials, rats were given two blocks of testing that included five random exposures to each of the following trial types within each testing block: startle, no startle, 5 dB PPI, 10 dB PPI, and 15 dB PPI. The last testing block was followed by four startle trials. In each trial, response to startle stimuli was measured in newtons for 250 ms after the presentation of the last stimulus and averaged across that time. Data from the first and last four startle trials were not included in the data analysis. On drug testing days, animals were tested using this procedure 20 minutes after the last drug treatment.
For data analyses, startle was defined as the response on the startle trials minus the response on the no startle trials. The percentage of PPI was calculated as follows: 100 x (1 - (response on the trials at a specific PPI level (e.g. 5 dB PPI) - response on the no startle trials)/(response on the startle trials - the response on the no startle trials)).
Discrete-trials rewarded delayed spatial alternation
A discrete-trial, rewarded delayed spatial alternation procedure was chosen for study since this procedure is sensitive to NMDA antagonists (Verma and Moghaddam 1996; Baron et al., 1998; Moghaddam and Adams 1998; Bardgett et al., 2008 & 2009; Watson et al., 2009), has been used to screen for cognitive enhancing drugs (Moghaddam and Adams 1998; Wedzony et al., 2000; Bardgett et al., 2008 & 2009), and may be analogous to procedures used to detect spatial working memory deficits in people with schizophrenia (Park and Holzman 1992). Furthermore, a discrete trial approach was used where each trial is composed of a forced and free run, and the location of the forced run arm is not dependent on the free run arm location from the previous trial. This approach is different from other rewarded delayed spatial alternation procedures where the rat simply has to alternate over consecutive free trials in order to obtain food reward (see Lipska et al., 2002 for comparison of the two procedures).
The T-maze used in these experiments was constructed of wire-mesh and wood, and painted black. The walls were 10 cm tall and arms were 9 cm wide. The start arm was 40 cm long, each goal arm was 45 cm long, and the entire maze had a wire mesh bottom. One week prior to habituation and testing, all rats were placed on a food-restricted diet and reduced to ~90% of their free-feeding weight. During habituation, all rats were placed on the T-maze until they ate two pieces of food or 90 seconds had elapsed. This was repeated three times a day with a five-minute intertrial interval for four days. During testing, rats received six trials a day with a five-minute intertrial interval. Each test trial consisted of two runs, a forced run and a free run. On the forced run, rats were forced to obtain a piece of food from one goal arm of the T-maze. Goal arm entries were defined as placing four paws in the arm. After a delay, the free run was conducted. At the beginning of the free run, the rats were returned to the start arm and allowed to choose either goal arm. If the rats returned to the same arm that they were forced into on the previous run, they received no food reward. If the rats chose the opposite arm, they received a food reward. Prior to drug testing, rats were given six 15 second delay trials on each testing day, with three forced runs to the right arm and three to the left arm. The sequence of forced run arm locations (left or right) was randomized each day, with the stipulation that the same forced arm location could not be used for three trials in a row. All animals were tested for at least two weeks before drug testing began. A criterion for stable baseline performance was not used in this study, although the average rate of alternation for the 11 rats on the final day of pretesting was 5 correct out of 6 total trials. During drug testing, three 10-second and three 40-second delay trials were used on each day of testing. On these days, the sequence of trial delays as well as the forced run arm locations (left or right) was randomized each day, with the stipulation that the same delay or forced arm location could not be used for three trials in a row. The number of correct choices at each delay and the latency to choose a goal arm (defined as the time the rat was placed on the maze until the rat placed all four paws into a goal arm) under each pretreatment-treatment condition were used in the data analysis.
Data analyses
For the locomotor and fine movement data, an overall three-way analysis of variance (ANOVA) was conducted that compared the effects of time (as a repeated measure), pretreatment (saline, 1.0, and 3.0 mg/kg of ciproxifan), and treatment (saline, 0.05, 0.1, and 0.3 mg/kg of MK-801) on the number of photobeam breaks and fine movements made every five minutes. Since different sets of rats were used to test the effects of ciproxifan on the two lower doses of MK-801 as opposed to the highest dose of MK-801, separate three-way ANOVAs comparing time, ciproxifan pretreatment, and MK-801 treatment were performed on the locomotor and fine movement data sets from each cohort of rats. These analyses were then followed by individual two-way ANOVAs for each MK-801 dose that compared the effects of time and ciproxifan pretreatment on photobeam breaks and fine movements.
A three-way ANOVA was also conducted on the auditory startle/PPI data to compare the effects of trial block (as a repeated measure), pretreatment, and treatment. In the ataxia and delayed spatial alternation experiments, each dependent measure, as defined above, was analyzed using a two-way ANOVA with pretreatment and treatment serving as independent variables. Significant differences were accepted if p < .05 (two-tailed). One-way ANOVA’s and post-hoc testing (Fishers Protected Least Squares (PLSD) test) were performed as indicated by significant interactions in the initial ANOVA’s.
Results
Effects of ciproxifan on hyperactivity induced by MK-801
A three-way ANOVA was conducted on the combined locomotor data from all experiments to assess the effects of ciproxifan pretreatment and MK-801 treatment (all three doses in addition to saline) as a function of time on locomotor activity. This analysis yielded a significant three-way interaction between ciproxifan, MK-801, and time (F(66, 1452) = 2.1, p < .0001). The analysis also indicated significant interactions between MK-801 treatment and time (F(33, 1452) = 20.7, p < .0001) and ciproxifan pretreatment and MK-801 treatment (F(6, 132) = 3.7, p < .0018), as well as significant main effects of MK-801 treatment (F(3, 132) = 138.3, p < .0001) and time (F(11, 1452) = 10.9, p < .0001) on locomotor activity.
A three-way analysis of variance was then performed on the locomotor data generated with the 12 rats treated with saline and the two lower doses of MK-801. This analysis indicated a significant effect of MK-801 treatment (F(2, 99) = 106.9, p < .0001) and a near significant effect of ciproxifan pretreatment (F(2, 99) = 2.5, p = .09). There were also significant effects of time (F(11, 1089) = 48.5, p < .0001) as well as significant MK-801 treatment x time (F(11, 1089) = 2.7, p < .0001), and ciproxifan pretreatment x time (F(11, 1089) = 8.9, p < .0001) interactions. Because of these latter interactions, two-way ANOVA’s examining pretreatment and time were performed as a function of MK-801 dose.
In rats treated with saline alone after ciproxifan (1.0 & 3.0 mg/kg) or saline pretreatment, ciproxifan produced a relatively marginal, yet significant effect on locomotor activity (Ciproxifan effect: F(2, 33) = 3.4, p < .05)(Figure 1a). A post-hoc comparison of the ciproxifan groups at each time point after saline treatment indicated that the higher dose of ciproxifan significantly decreased locomotor activity during the first five minutes of testing (Fishers PLSD, p < .05). The lowest dose of MK-801 tested (0.05 mg/kg) appeared to increase activity in comparison to treatment with saline (Figure 1b). The level of activity produced by this MK-801 dose was not altered by pretreatment with either dose of ciproxifan.
Figure 1.
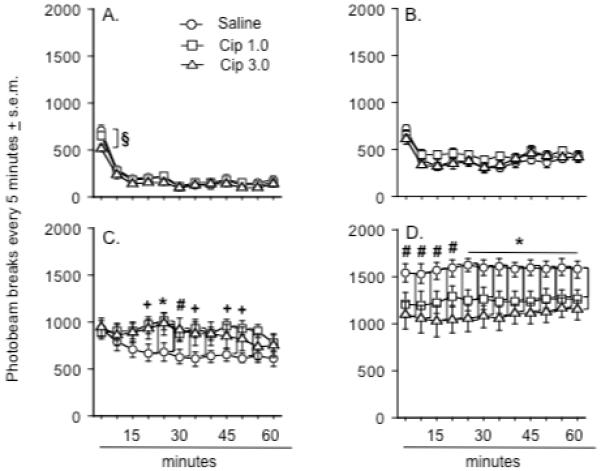
Effects of ciproxifan pretreatment on locomotor activity after A.) saline treatment, B.) MK-801 0.05 mg/kg, C.) MK-801 0.1 mg/kg, and D.) MK-801 0.3 mg/kg. An asterisk (*) indicates a significant difference between rats pretreated with either dose of ciproxifan and those pretreated with saline. A plus sign (+) indicates a significant difference between the saline and Cip 1.0 dose group. A number sign (#) indicates a significant difference between the saline and Cip 3.0 dose group. A § indicates a significant difference between the Cip 3.0 dose groups and the two other groups. Group differences refer to outcomes of post-hoc comparisons that were significant at p ≤ .05 (Fishers PLSD) and are indicated by connecting lines. Data represent the mean number of photobeam breaks every five minutes ± s.e.m. There were 12 animals in each group although a separate cohort of 12 rats was used to generate the data depicted in d.
Rats treated with 0.1 mg/kg of MK-801 were markedly more active than rats treated with the lowest dose of MK-801. Rats pretreated with ciproxifan at this dose of MK-801 were more active than rats pretreated with saline (Figure 1c) (Ciproxifan x time interaction: F(22, 363) = 1.7, p < .03). Rats pretreated with the low dose of ciproxifan were significantly more active than rats pretreated with saline at 20, 25, 35, 45, and 50 minutes and rats treated with the high dose of ciproxifan were significantly more active at 25 and 30 minutes than the saline pretreated rats (Fishers PLSD, p < .04 - .005).
A separate three-way ANOVA was performed on the locomotor data generated from the 12 rats used to test the interaction between ciproxifan and a high dose (0.3 mg/kg) of MK-801. This analysis revealed a significant effect of ciproxifan pretreatment (F(2, 66) = 4.2, p < .02), MK-801 treatment (F(1, 66) = 348.0, p < .0001), and significant interactions between pretreatment and treatment (F(2, 66) = 7.1, p < .002) and time and treatment (F(11, 726) = 1.8, p < .04)(Figure 1d). Treatment with the high dose of MK-801 (0.3 mg/kg) appeared to increase activity levels beyond those observed after treatment with the moderate dose (0.1 mg/kg) (Figure 1d). Using a two-way ANOVA to compare the effects of ciproxifan dose and time, it was found that ciproxifan pretreatment decreased locomotor activity levels in rats treated with the high dose of MK-801 relative to rats pretreated with saline (Ciproxifan effect: F(2, 33) = 5.7, p < .007)(Figure 1d). When compared to saline-pretreated rats, rats pretreated with the high dose of ciproxifan demonstrated significantly less activity at each time point, while those pretreated with lower dose of ciproxifan showed less activity from 25 minutes until the end of testing (Fishers PLSD, p < .05 - .001).
Because of the within-subject design used in these studies, one concern was that locomotor responses to MK-801 would change as a function of repeated exposure to the drug (Schulz et al., 2001). To address this concern, data from animals pretreated with saline and treated with one of the MK-801 doses were split into two test periods (n = 6 per period for each dose) according to the date of testing: the first days of testing vs. the last days of testing. A two-way ANOVA that compared the effects of MK-801 dose and test period revealed a significant dose effect (F(3, 52) = 173.2, p < .0001), but no significant effect of test period or dose x test period interaction (data not shown).
Effects of ciproxifan on the increased fine movements produced by MK-801
During the activity sessions, data on fine movements was also collected. These data represent repetitive breaks of the same photobeam, and it has been suggested that such data may reflect some aspects of stereotyped behavior (Swanson and Schoepp 2002). A three-way ANOVA was performed that included the combined data from the two locomotor studies and compared the effects of time within each testing session, ciproxifan pretreatment, and MK-801 treatment. This analysis indicated significant effects of treatment (F(3, 168) = 124.8, p < .0001) and time (F(11, 1848) = 8.6, p < .0001) and significant pretreatment x time (F(22, 1848) = 2.1, p < .002) and treatment x time (F(33, 1848) = 3.9, p < .0001) interactions.
Similar analyses were performed on the separate studies involving the lower doses of MK-801 (0.05 and 0.1 mg/kg) versus the higher dose of MK-801 (0.3 mg/kg). The analysis of the former data set indicated significant effects of treatment (F(2, 99) = 54.5, p < .0001) and time (F(11, 1089) = 18.0, p < .0001) and significant pretreatment x time (F(22, 1089) = 2.3, p < .0002) and treatment x time (F(22, 1089) = 7.6, p < .0001) interactions. MK-801 treatment increased fine movements in a dose-dependent manner (Figures 2a – 2c). Ciproxifan pretreatment did not alter the number of fine movements observed after treatment with saline (Figure 2a) or the low dose (0.05 mg/kg) of MK-801 (Figure 2b), but enhanced the number of fine movements produced by the moderate dose (0.1 mg/kg) of MK-801 (Ciproxifan x time interaction: F(22, 363) = 2.0, p < .005)(Figure 2c). After treatment with this dose of MK-801, rats pretreated with the low dose of ciproxifan demonstrated significantly more fine movements than rats pretreated with saline at 45 and 50 minutes, and rats pretreated with the high dose of ciproxifan exhibited significantly more fine movements at 20 and 25 minutes than the saline pretreated rats (Fishers PLSD, p ≤ .05).
Figure 2.
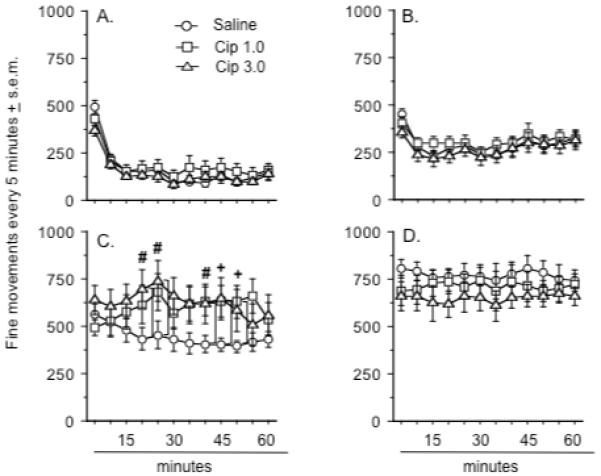
Effects of ciproxifan pretreatment on the number of fine movements seen after treatment with: A.) saline treatment, B.) MK-801 0.05 mg/kg, C.) MK-801 0.1 mg/kg, and D.) MK-801 0.3 mg/kg. A plus sign (+) indicates a significant difference between the saline and Cip 1.0 dose group. A number sign (#) indicates a significant difference between the saline and Cip 3.0 dose group. Group differences refer to outcomes of post-hoc comparisons that were significant at p ≤ .05 (Fishers PLSD) and are indicated by connecting lines. Data represent the mean number of photobeam breaks every five minutes ± s.e.m. There were 12 animals in each group although a separate cohort of 12 rats was used to generate the data depicted in d.
The analysis of the data set including the high dose of MK-801 only revealed a significant treatment effect (F(1, 66) = 259.2, p < .0001)(Figure 2d). The amount of fine movements observed at this dose was greater than the amount seen after treatment with 0.1 mg/kg of MK-801 (Fishers PLSD, p < .0003). Ciproxifan pretreatment did not alter the effects of the 0.3 mg/kg dose of MK-801.
A two-way ANOVA that compared the effects of test period (i.e., the first days of testing vs. the last days of testing) and MK-801 dose revealed a significant effect of dose (F(3, 52) = 73.4, p < .0001), but no significant effect of test period or a dose x test period interaction (data not shown).
Effects of ciproxifan on ataxia produced by MK-801
Three measures of sensorimotor function that are sensitive to MK-801 were used to assess the effects of ciproxifan on ataxia induced by MK-801. Only one dose of ciproxifan was studied (3.0 mg/kg) since there were no major dose-dependent effects of ciproxifan on the locomotor and fine movement activity induced by MK-801. There was a significant interaction between ciproxifan pretreatment and MK-801 treatment on the platform test (Ciproxifan x MK-801 interaction: F(2, 44) = 4.1, p < .02)(Figure 3a). Ciproxifan reduced the amount of time rats remained on the platform after treatment with the 0.1 mg/kg dose of MK-801 (Fishers PLSD, p < .01) but did not alter the effects of treatment with saline or the high dose of MK-801. Treatment with the high dose of MK-801 significantly delayed walking initiation (MK-801 effect: F(2, 44) = 18.8, p < .0001)(Figure 3b), regardless of pretreatment. Finally, there was a significant interaction between ciproxifan pretreatment and MK-801 dose on the righting reflex (Ciproxifan x MK-801 interaction: F(2, 44) = 8.3, p < .0009)(Figure 3c). The righting reflex was slightly but significantly delayed in rats treated with 0.1 mg/kg of MK-801, regardless of pretreatment (Fishers PLSD, p < .0001 & .04 for the respective comparisons of the saline and 0.1 mg/kg groups as a function of saline or ciproxifan pretreatment). In comparison to the effect of the 0.1 mg/kg of MK-801, the righting reflex was dramatically delayed in both groups of rats treated with 0.3 mg/kg of MK-801 (Fishers PLSD, p < .003 & .0006 for the respective comparisons between the 0.1 and 0.3 mg/kg groups as a function of saline or ciproxifan pretreatment), an effect made significantly worse by ciproxifan pretreatment (Fishers PLSD, p < .007 for the comparison of 0.3 mg/kg MK-801-treated rats following saline and ciproxifan pretreatment).
Figure 3.
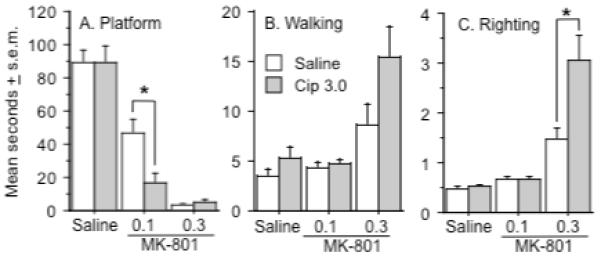
Effects of ciproxifan pretreatment on measures of ataxia recorded after treatment with MK-801. A.) Each dose of MK-801 decreased the time spent on the platform and ciproxifan (3.0 mg/kg) enhanced the effect of the 0.1 mg/kg dose as indicated by the asterisk. B). The 0.3 mg/kg dose of MK-801 significantly increased the latency for walking initiation. C.) Each dose of MK-801 increased the righting reflex and ciproxifan (3.0 mg/kg) enhanced the effect of the 0.3 mg/kg dose as indicated by the asterisk. Group differences refer to outcomes of post-hoc comparisons that were significant at p ≤ .05 (Fishers PLSD) and are indicated by connecting lines. Data represent mean seconds ± s.e.m. n = 12 rats per group.
Effects of ciproxifan on prepulse inhibition deficits induced by MK-801
In another series of experiments, auditory startle and PPI were measured in rats pretreated with ciproxifan and treated with MK-801. When responses to the 120 dB auditory startle stimulus were measured, it was found that each MK-801 dose (0.05 and 0.1 mg/kg) increased the startle response relative to the effects of saline treatment (MK-801 effect: F(2, 99) = 41.3, p < .0001)(Figure 4a). There was also a significant effect of ciproxifan pretreatment on auditory startle responses (Ciproxifan effect: F(2, 99) = 3.2, p < .05), although post-hoc testing did not reveal significant differences between individual pretreatment groups at the p < .05 level. Finally, startle responses during the second block of daily test trials were significantly lower than the responses observed during the first block of daily test trials (Block effect: F(1, 99) = 10.1, p < .002).
Figure 4.
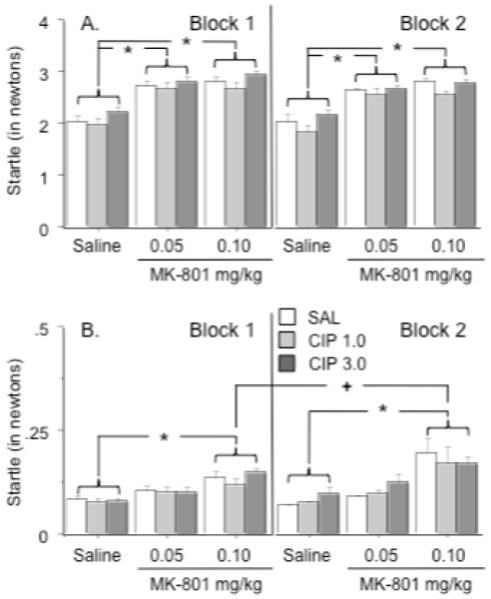
Effects of ciproxifan and MK-801 on startle responses to the auditory stimulus and activity during no startle trials during each block of sensorimotor testing. A.) Each dose of MK-801 significantly enhanced auditory startle responses regardless of ciproxifan pretreatment as indicated by asterisk. B.) The high dose of MK-801 significantly increased activity during the no startle trials relative to saline treatment as indicated by the asterisk. After treatment with this dose, activity was greater during test block 2 than test block 1 as indicated by a + sign. Group differences refer to outcomes of post-hoc comparisons that were significant at p ≤ .05 (Fishers PLSD) and are indicated by connecting lines. Data represent average force (expressed in newtons) ± s.e.m during startle and no startle trials. n = 12 rats per group.
An examination of activity during no startle trials revealed significant effects of treatment (F(2, 99) = 19.0, p < .0001), and trial block (Block 1 vs. Block 2: F(1, 99) = 4.9, p < .03) and a significant treatment x trial block interaction (F(2, 99) = 4.3, p < .02)(Figure 4b). Animals treated with the high dose of MK-801 were more active during the no stimulus trials in comparison to saline-treated rats, regardless of pretreatment (Fishers PLSD, p < .04 - .0002). Responses to saline or 0.05 mg/kg of MK-801 did not differ as a function of test block, but the rats treated with 0.1 mg/kg of MK-801 were more active during the second test block than the first one (Fishers PLSD, p < .02).
Exposure to each prepulse stimulus (5 dB, 10 dB, and 15 dB) inhibited the subsequent startle responses to the 120 dB tone in an intensity-dependent manner (Prepulse intensity effect: F(2, 198) = 205.8, p < .0001)(Figure 5). An effect of trial block was not observed at any prepulse intensity; therefore the data generated at each prepulse intensity have been combined across the two trial blocks for Figure 5. Prepulse inhibition was impaired by MK-801 in a dose-dependent manner (MK-801 overall effect: F(2, 99) = 34.1, p < .0001; specific MK-801 effects at the 5dB, 10 dB, and 15 dB tones: F(2, 99) = 28.1, 33.0, and 30.0, p < .0001, for each respective tone). Comparisons of individual treatment groups suggested that all of the groups treated with 0.05 mg/kg of MK-801 (with the exception of the group pretreated with 3.0 mg/kg of ciproxifan at the 10 dB PPI) displayed PPI deficits at all intensities (Fishers PLSD, p < .05 - .0001) relative to rats treated with saline. Most of the groups treated with the 0.1 mg/kg dose of MK-801 showed significantly greater PPI deficits than the groups treated with 0.05 mg/kg of MK-801 (Fishers PLSD, p < .05 - .0001). Ciproxifan pretreatment did not significantly alter PPI or the deficit in PPI produced by MK-801 at any of the prepulse intensities studied.
Figure 5.
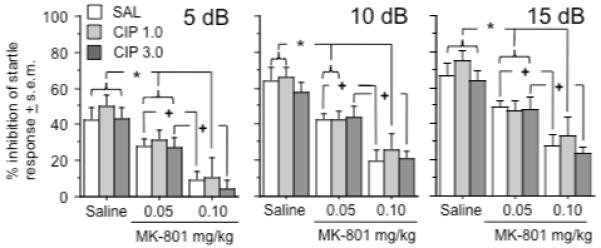
Effects of ciproxifan and MK-801 on PPI after presentation of a 5 dB (left graph), 10 dB (middle graph), and 15 dB prepulse auditory stimulus (right graph). Symbols indicate significant differences within each of the three ciproxifan pretreatment groups at each intensity – an asterisk (*) indicates a significant difference from saline treatment and a cross (+) indicates a significant difference from saline and MK-801 (0.05 mg/kg) treatments. Group differences refer to outcomes of post-hoc comparisons that were significant at p ≤ .05 (Fishers PLSD) and are indicated by connecting lines. Data represent average % of PPI (see text for further definition) ± s.e.m during prepulse inhibition trials. n = 12 rats per group.
Startle responses in rats pretreated with saline and treated with either saline, 0.05 mg/kg or 0.1 mg/kg of MK-801 did not differ as function of test period (i.e. the first block of test days vs. the second block of test days)(n = 6 rats per group) under any testing condition (startle, no startle, PPI5, PPI10, or PPI15).
Effects of ciproxifan on delayed spatial alternation deficits induced by MK-801
The last experiment examined the interaction between ciproxifan and MK-801 on performance in the delayed spatial alternation task. Rats were given six trials on each day of testing with three trials involving a 10 sec delay and three involving a 40 sec delay. There was a statistical trend towards a MK-801 effect on performance during the 10 sec delay trials (MK-801 effect: F (2, 60) = 2.8, p = .07)(Figure 6). The 0.1 mg/kg dose of MK-801 appeared to produce the largest mean deficit in performance relative to the rats that received saline and 0.05 mg/kg dose of MK-801. There was a significant interaction between the effects of ciproxifan and MK-801 on performance during 40 sec delay (Interaction term: F (4, 60) = 2.6, p < .05). Rats pretreated with saline and treated with 0.1 mg/kg of MK-801 performed significantly worse on the task when compared to rats pretreated and treated with saline (Fishers PLSD, p < .0001). But rats that were pretreated with 3.0 mg/kg of ciproxifan prior to treatment with 0.1 mg/kg of MK-801 made as many correct choices as did the rats pretreated and treated with saline. Moreover, the former rats performed significantly better than rats pretreated with saline and treated with 0.1 mg/kg of MK-801 (Fishers PLSD, p < .01). There were no significant main effects of pretreatment or treatment or interactive effects of these variables on the latency to choose a goal arm (data not shown). Finally, alternation performance did not differ as function of test period (i.e. the first block of test days vs. the second block of test days)(n = 6 rats per group) in the rats pretreated with saline and treated with saline, 0.05 mg/kg or 0.1 mg/kg of MK-801.
Figure 6.
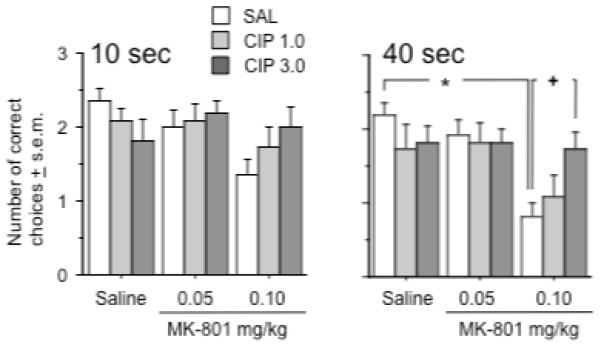
Effects of ciproxifan and MK-801 on delayed spatial alternation at a 10 second (left graph) or 40 second delay (right graph). Pretreatment with saline and treatment with 0.1 mg/kg of MK-801 caused a significant performance deficit at the 40 second delay relative to the effect of saline pretreatment and treatment as indicated by the asterisk. Animals treated with 0.1 mg/kg of MK-801 performed significantly better at the 40 s delay when they received pretreatment with the 3.0 mg/kg dose of ciproxifan as opposed to saline pretreatment, as indicated by the cross. Group differences refer to outcomes of post-hoc comparisons that were significant at p ≤ .05 (Fishers PLSD) and are indicated by connecting lines. Data represent average number of correct choices ± s.e.m. n = 11 rats per group.
Discussion
This set of studies characterized the effect of a prototypical H3 receptor antagonist, ciproxifan, on the behavioral dysfunction produced by MK-801, a use-dependent NMDA antagonist used in animals to model some aspects of schizophrenia. The results suggested that ciproxifan enhances the effect of MK-801 on locomotor activity and ataxia, without altering the changes in auditory startle and PPI induced by MK-801. On the other hand, ciproxifan alleviated the ability of MK-801 to disrupt delayed spatial alternation. These findings indicate that H3 receptor antagonism can worsen some aspects of the behavioral deficits caused by NMDA receptor hypofunction but relieves some of the cognitive impairment associated with such pathophysiology.
Previous work has shown that H3 receptors modulate the locomotor effects of psychostimulant drugs that act as dopamine agonists. In mice, H3 receptor antagonists, such as thioperamide, ciproxifan, and ABT-239 have been shown to reduce locomotor responses to amphetamine, apomorphine, cocaine, and methamphetamine (Clapham and Kilpatrick, 1994, Morisset et al., 2002; Fox et al., 2005) although exacerbation of the hyperactivity induced by dopamine agonists has also been reported (Brabant et al., 2009; Ferrada et al., 2008). The present study revealed biphasic effects of ciproxifan on the locomotor hyperactivity elicited by the NMDA antagonists, MK-801. Specifically, ciproxifan enhanced the hyperactivity elicited by a moderate dose of MK-801, but suppressed the hyperactivity generated by a higher dose of MK-801. Two previous studies in mice reported that H3 antagonists decrease the effects of high doses of MK-801 on locomotor activity (Faucard et al., 2007; Ligneau et al., 2007a), and the present results that are consistent with these previously reported data. The data reported here, however, are unique since lower doses of MK-801 were also studied, and ciproxifan was found to enhance their impact on locomotor activity. This pattern of results is consistent with the effects of another H3 antagonist, thioperamide, which also produces a biphasic effect on the hyperactivity induced by different doses of MK-801 (Bardgett et al., 2009).
The simplest explanation of ciproxifan’s biphasic effect on locomotor activity is that it enhances locomotor sensitivity to MK-801. Moderate to high doses of MK-801 cause hyperactivity, while even higher doses of MK-801 produce ataxia and stereotypy (Wozniak et al., 1990; Brosnan-Watters et al., 1996; Qi et al., 2008). Ciproxifan may allow these effects to become manifest at relatively lower doses of MK-801 than usual. To test this possibility, we assessed the effects of ciproxifan on two behavioral measures sensitive to higher doses of MK-801, ataxia (Wozniak et al., 1990; Brosnan-Watters et al., 1996) and fine movements, a possible measure of stereotyped behavior (Swanson and Schoepp 2002). As expected, ciproxifan increased the effect of the moderate dose of MK-801 on the platform test, a measure of motor coordination, and the effect of a high dose of MK-801 on the righting reflex. Moreover, ciproxifan enhanced the effect of a moderate dose of MK-801 on fine movements. These results support the idea that H3 antagonists may shift the dose-response curve for the motor effects of MK-801 to the left.
One mechanism that may account for ciproxifan’s enhancement of MK-801’s motor effects is that imidazole-containing H3 antagonists, such as ciproxifan, inhibit drug-metabolizing enzymes (Zhang et al., 2005). If ciproxifan were to inhibit the metabolism of MK-801, the effects of MK-801 on locomotor activity would be enhanced. However, this explanation is tenuous since ciproxifan reduced the effects of MK-801 on delayed spatial alternation and had no effect on the ability of MK-801 to enhance auditory startle and disrupt PPI. An alternative hypothesis regarding the effects of ciproxifan on MK-801-induced motor dysfunction is that H3 and NMDA antagonists work together within circuits that subserve motor behavior. In the case of locomotor behavior, it is notable that both H3 and NMDA antagonists elevate immediate-early gene expression in the nucleus accumbens (Chartoff et al., 2005; Southam et al., 2009), a brain region known to regulate locomotor activity. This common effect on immediate-early gene expression is also seen in the motor cortex (Jacobs et al., 2000; De Leonibus et al., 2002; Bonaventure et al., 2007) where the ability of MK-801 to increase expression has been correlated with its impact on locomotion (Jacobs et al., 2000) and where H 3receptors are known to exist in relative abundance (Pillot et al., 2002a).
Most studies have indicated that H3 antagonists do not alter auditory startle but may influence PPI. H3 antagonists improve PPI in DBA/2 mice, a mouse strain that demonstrates deficient PPI (Browman et al., 2004), and reverse the debilitating effects of the dopamine agonist, apomorphine, on PPI (Ligneau et al., 2007a). While the ability of MK-801 to increase auditory startle and decrease PPI have been reported in numerous studies (Bakshi and Geyer 1998; Schulz et al., 2001; Bardgett et al., 2009), the effects of ciproxifan on PPI deficits induced by NMDA antagonists have not been reported. Using methods identical to those described by Swerdlow et al., (2000) who used them to assess differences in PPI between rat strains, the present study found that ciproxifan does not alter the disruptive effects of MK-801 on acoustic startle and PPI. These findings are consistent with our previous work that showed that thioperamide did not alter the effects of MK-801 on either measure (Bardgett et al., 2009). The most parsimonious explanation for ciproxifan’s failure to modify MK-801’s action on PPI is that H3 and NMDA receptors do not fully interact in brain regions (Bakshi and Geyer 1998) that support startle and PPI. However, other approaches should be considered before this conclusion is accepted. For example, it is possible that studies using other H3 or NMDA antagonists or a greater range of prepulse intensities (Bakshi and Geyer 1998; Schulz et al., 2001; Jones et al., 2005) could reveal an effect of H3 antagonists on PPI deficits. Another approach would be to wait longer after drug injections before testing acoustic startle. The effects of ciproxifan on the locomotor hyperactivity induced by MK-801 seemed maximal at 40 – 80 minutes after MK-801 injection whereas PPI was tested at 20 – 40 minutes after MK-801 injection. A longer waiting period may have yielded a significant interaction between ciproxifan and MK-801 on PPI. This concern is allayed somewhat by the significant interaction observed in the delayed spatial alternation test which was also performed between 20 – 50 minutes after MK-801 injection, and by the results of the PPI study itself which did not reveal a greater effect of either ciproxifan or MK-801 on PPI during the last trial block.
Many studies have shown that H3 antagonists can improve learning and memory in normal rats, aged rats, and rats challenged with scopolamine (Ligneau et al., 1998; Fox et al., 2003; Medhurst et al., 2007; Ligneau et al., 2007b; Galici et al., 2009). Comparatively little work (Bernearts et al., 2004; Huang et al. 2004) has addressed the effects of H3 antagonists on learning and memory deficits produced by NMDA antagonists. Our results address this gap by demonstrating that ciproxifan alleviates deficits produced by MK-801 in the delayed spatial alternation task. These effects were more prominent at longer delays versus shorter ones, suggesting that ciproxifan provides greater assistance under conditions of more sustained memory load. We should note, however, that not all H3 antagonists produce such effects, since thioperamide does not alter the ability of MK-801 to disrupt delayed spatial alternation (Bardgett et al., 2009). Research is needed to identify the specific characteristics of H3 antagonists that enable some of them to improve alternation performance and perhaps other forms of memory. One simple issue to consider is drug dose. Le et al. (2007) reported that the dose of ciproxifan used for this study binds to a greater percentage of H3 receptors relative to the highest dose of thioperamide used in our previous study (Bardgett et al., 2009), suggesting that when H3 receptors are maximally bound by antagonists, then cognitive enhancing effects emerge.
There are several mechanisms that could account for the beneficial effects of ciproxifan in the delayed spatial alternation task. Performance in this task appears to be modulated by the hippocampus and prefrontal cortex, since lesions to or inactivation of either area leads to performance deficits (Bardgett et al., 2008; Mogensen et al., 2008; Yoon et al., 2008). In each region, H3 and non-competitive NMDA antagonists increase the release of neurotransmitters thought to be important in memory, such as acetylcholine, dopamine, and norepinephrine (Hasegawa et al., 1996; Etou et al., 1998; Moor et al., 1998; Kubota et al., 1999; Nelson et al., 2002; Lorrain et al., 2003; Medhurst et al., 2007; Southam et al., 2009). While the pattern of neurotransmitter release evoked by each class of compounds appears similar, the impact of H3 and NMDA antagonists on broad-scale electrophysiological activity in each region may differ. For example, theta rhythms in the hippocampus have long been implicated in cognitive processing (Berry and Seager, 2002; McNaughton et al., 2007; Lubenov and Siapas, 2009). In particular, the ability of treatments to augment hippocampal theta rhythms produced by midbrain stimulation may be indicative of their cognitive-enhancing potential (McNaughton et al., 2007). Ciproxifan has been shown to not only increase hippocampal theta, but to enhance theta elicited by midbrain stimulation or novelty (Hajos et al., 2008). The latter findings are notable since MK-801 and other NMDA antagonists reduce the ability of midbrain stimulation to generate hippocampal theta (Engin et al., 2009). Given the well-characterized ability of NMDA antagonists to disrupt neuronal firing patterns in the prefrontal cortex (Jackson et al., 2004; Homayoun and Moghaddam, 2007), it would be interesting to determine if ciproxifan and other H3 antagonists can correct the dysfunction caused by NMDA antagonists at an electrophysiological level.
Another issue that requires consideration is the affinity of ciproxifan for α2a and α2c adrenergic and 5HT3 receptors (Esbenshade et al., 2003), since these receptors that have been implicated in memory enhancement (Diez-Ariza et al., 2003; Marcus et al., 2005; Bardgett et al., 2008). It may be possible that ciproxifan’s combined activity at all three receptor types (H3, adrenergic, and 5HT3) allows it to overcome the adverse effects of MK-801 on memory. A final issue that deserves attention is identifying the types of learning and memory affected by H3 antagonists. Using an automated delayed alternation task, Yoon et al., (2008) discovered that the medial prefrontal cortex regulates working memory but not reference memory, and that the hippocampus is required for both. In a radial arm maze task, at the doses used in this study, MK-801 impairs working and reference memory (Bardgett et al., 2008); moreover, preliminary work from our lab indicates that ciproxifan can reduce some aspects of this impairment. The use of the automated delayed alternation task or a radial arm maze task, along with other non-spatial or non-appetitive memory tasks, would allow one to determine the types of memory influenced by H3 receptors.
It is puzzling that ciproxifan alleviated the debilitating effect of MK-801 on delayed spatial alternation, yet augmented the effect of MK-801 on locomotor activity. It is tempting to minimize the alleviating effect of ciproxifan in the delayed spatial alternation task given that it was only observed with one dose of ciproxifan against one dose of MK-801 at one delay. This finding nonetheless deserves serious consideration since it is consistent with the previous research showing that ciproxifan improves memory in several other animal models, the near significant interaction between ciproxifan and MK-801 seen at the 10-second delay in the delayed alternation task, and our own preliminary data on ciproxifan’s positive effects against MK-801-induced deficits in the radial arm maze. One way to account for the disparate effects of ciproxifan on delayed spatial alternation versus motor activity in the MK-801 model may be to consider the idea that ciproxifan, like many H3 antagonists, acts as wake-promoting, mild stimulant drug (see Bonaventure et al., 2007 for review). This feature may allow it to exert positive effects on delayed spatial alternation similar to the cognitive-enhancing effects of moderate doses of stimulants such as amphetamine, nicotine, or caffeine. From this perspective, the stimulant effect of ciproxifan may provide a modicum of attentional focus in the midst of the cognitive disinhibition produced by MK-801. As for the results of the locomotor experiments, it remains possible that a modest stimulant effect of ciproxifan coupled to the disinhibitory motoric action of MK-801 enhanced the locomotor response to a moderate dose of the latter drug despite the fact that ciproxifan alone did little to locomotor activity. However, a slight stimulant effect of ciproxifan would also be expected to limit the ataxia produced by a higher dose of MK-801, whereas the results illustrate an opposite trend. Another idea, raised previously, is that ciproxifan interacts with NMDA-mediated mechanisms in significantly different ways as a function of brain region. The behavioral results would suggest that ciproxifan alleviates NMDA receptor hypofunction in prefrontal or hippocampal regions, exacerbates receptor hypofunction in brain regions subserving locomotor activity, and does not alter receptor hypofunction in brain regions critical to sensorimotor gating. These rather cursory ideas obviously merit much caution, and more substantial explanations will certainly arise as additional data are compiled on the interaction between H3 and NMDA receptors.
The present results raise interesting questions about the nature of basic H3 and NMDA receptor interactions in the brain and the potential use of H3 antagonists as cognitive-enhancing drugs. Given that the interaction of ciproxifan and MK-801 depended on the behavior examined, and, in the case of locomotor activity, the dose of MK-801, it is suggested that the interactions between H3 and NMDA receptors vary as a function of brain region. Further work is needed to examine these interactions in cortical, limbic, and striatal areas at cellular, electrophysiological, and even behavioral levels. The data also support the idea that some H3 antagonists may possess cognitive enhancing qualities, at least in the context of NMDA receptor hypofunction. The limitation to this claim is that H3 antagonists may exacerbate other behavioral outcomes associated with such pathophysiology. At the very least, the data do not support the idea of H3 antagonists as stand-alone antipsychotic drugs, although it still remains possible that H3 antagonists may augment the cognitive-enhancing effects of antipsychotic drugs (Pillot et al., 2002b; Bardgett et al., 2006a; 2006b, Grayson et al., 2007) by modifying neurotransmitter release upstream of post-synaptic receptor sites occupied by such drugs. Additional research is needed to determine if H3 antagonists can improve memory without perturbing the impact of pathophysiology on other behaviors associated with mental disorders or cognitive dysfunction.
Acknowledgements
This research was supported by National Center for Research Resources Grant 2P20 RR16481 and National Institute of Mental Health Grant R15 MH076788. We would like to acknowledge the NIMH Chemical Synthesis and Drug Supply Program for providing the ciproxifan used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bacciottini L, Passani MB, Giovannelli L, Cangioli I, Mannaioni PF, Schunack W, Blandina P. Endogenous histamine in the medial septum-diagonal band complex increases the release of acetylcholine from the hippocampus: a dual-probe microdialysis study in the freely moving rat. European Journal of Neuroscience. 2002;15:1669–1680. doi: 10.1046/j.1460-9568.2002.02005.x. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Geyer MA. Multiple limbic regions mediate the disruption of prepulse inhibition produced in rats by the noncompetitive NMDA antagonist dizocilpine. Journal of Neuroscience. 1998;18:8394–8401. doi: 10.1523/JNEUROSCI.18-20-08394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett ME. Behavioral models of atypical antipsychotic drug action. In: Csernansky JG, Laurello J, editors. Atypical antipsychotics: From bench to bedside. Marcel Dekker; New York: 2004. pp. 61–93. [Google Scholar]
- Bardgett ME, Baum KT, O’Connell SM, Lee NM, Hon JC. Effects of risperidone on locomotor activity and spatial memory in rats with hippocampal damage. Neuropharmacology. 2006a;51:1156–1162. doi: 10.1016/j.neuropharm.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Boeckman R, Krochmal D, Fernando H, Ahrens R, Csernansky JG. NMDA receptor blockade and hippocampal neuronal loss impair fear conditioning and position habit reversal in C57Bl/6 mice. Brain Research Bulletin. 2003;60:131–142. doi: 10.1016/s0361-9230(03)00023-6. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Jacobs PS, Jackson JL, Csernansky JG. Kainic acid lesions enhance locomotor responses to novelty, saline, amphetamine, and MK-801. Behavioural Brain Research. 1997;84:47–55. doi: 10.1016/s0166-4328(96)00132-5. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Griffith MS, Foltz RF, Hopkins JA, Massie CM, O’Connell SM. The effects of clozapine on delayed spatial alternation deficits in rats with hippocampal damage. Neurobiology of Learning & Memory. 2006b;85:86–94. doi: 10.1016/j.nlm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Points M, Ramsey-Faulkner C, Topmiller J, Roflow J, McDaniel T, Lamontagne T, Griffith MS. The effects of clonidine on discrete-trial delayed spatial alternation in two rat models of memory loss. Neuropsychopharmacology. 2008;33:1980–1991. doi: 10.1038/sj.npp.1301580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett ME, Points M, Roflow J, Blankenship M, Griffith MS. Effects of the H3 receptor antagonist, thioperamide, on behavioral alterations induced by systemic MK-801 administration. Psychopharmacology. 2009;205:589–597. doi: 10.1007/s00213-009-1566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron SP, Wright D, Wenger GR. Effects of drugs of abuse and scopolamine on memory in rats: delayed spatial alternation and matching to position. Psychopharmacology. 1998;137:7–14. doi: 10.1007/s002130050587. [DOI] [PubMed] [Google Scholar]
- Bernaerts P, Lamberty Y, Tirelli E. Histamine H3 antagonist thioperamide dose-dependently enhances memory consolidation and reverses amnesia induced by dizocilpine or scopolamine in a one-trial inhibitory avoidance task in mice. Behavioural Brain Research. 2004;154:211–219. doi: 10.1016/j.bbr.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Berry SD, Seager MA. Hippocampal theta oscillations and classical conditioning. Neurobiology of Learning and Memory. 2001;76:298–313. doi: 10.1006/nlme.2001.4025. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Letavic M, Dugovic C, Wilson S, Aluisio L, Pudiak C, Lord B, Mazur C, Kamme F, Nishino S, Carruthers N, Lovenberg T. Histamine H3 receptor antagonists: from target identification to drug leads. Biochemical Pharmacology. 2007;73:1084–1096. doi: 10.1016/j.bcp.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Brabant C, Alleva L, Grisar T, Quertemont E, Lakaye B, Ohtsu H, Lin JS, Jatlow P, Picciotto MR, Tirelli E. Effects of the H3 receptor inverse agonist thioperamide on cocaine-induced locomotion in mice: role of the histaminergic system and potential pharmacokinetic interactions. Psychopharmacology. 2009;202:673–687. doi: 10.1007/s00213-008-1345-y. [DOI] [PubMed] [Google Scholar]
- Brosnan-Watters G, Wozniak DF, Nardi A, Olney JW. Acute behavioral effects of MK-801 in the mouse. Pharmacology Biochemistry and Behavior. 1996;53:701–711. doi: 10.1016/0091-3057(95)02073-x. [DOI] [PubMed] [Google Scholar]
- Browman KE, Komater VA, Curzon P, Rueter LE, Hancock AA, Decker MW, Fox GB. Enhancement of prepulse inhibition of startle in mice by the H3 receptor antagonists thioperamide and ciproxifan. Behavioural Brain Research. 2004;153:69–76. doi: 10.1016/j.bbr.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Heusner CL, Palmiter RD. Dopamine is not required for the hyperlocomotor response to NMDA receptor antagonists. Neuropsychopharmacology. 2005;30:1324–1333. doi: 10.1038/sj.npp.1300678. [DOI] [PubMed] [Google Scholar]
- Clapham J, Kilpatrick GJ. Histamine H3 receptors modulate the release of [3H]-acetylcholine from slices of rat entorhinal cortex: Evidence for the possible existence of H3 receptor subtypes. British Journal of Pharmacology. 1992;107:919–923. doi: 10.1111/j.1476-5381.1992.tb13386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham J, Kilpatrick GJ. Thioperamide, the selective histamine H3 receptor antagonist, attenuates stimulant-induced locomotor activity in the mouse. European Journal of Pharmacology. 1994;259:107–114. doi: 10.1016/0014-2999(94)90498-7. [DOI] [PubMed] [Google Scholar]
- De Leonibus E, Mele A, Oliverio A, Pert A. Distinct pattern of c-fos mRNA expression after systemic and intra-accumbens amphetamine and MK-801. Neuroscience. 2002;115:67–78. doi: 10.1016/s0306-4522(02)00415-3. [DOI] [PubMed] [Google Scholar]
- Diez-Ariza M, Redondo C, García-Alloza M, Lasheras B, Del Río J, Ramírez MJ. Flumazenil and tacrine increase the effectiveness of ondansetron on scopolamine-induced impairment of spatial learning in rats. Psychopharmacology. 2003;169:35–41. doi: 10.1007/s00213-003-1467-1. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D, Dickson CT. Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience. 2009;161:359–369. doi: 10.1016/j.neuroscience.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Esbenshade TA, Krueger KM, Miller TR, Kang CH, Denny LI, Witte DG, Yao BB, Fox GB, Faghih R, Bennani YL, Williams M, Hancock AA. Two novel and selective nonimidazole histamine H3 receptor antagonists A-304121 and A-317920: I. In vitro pharmacological effects. Journal of Pharmacological and Experimental Therapeutics. 2003;305:887–896. doi: 10.1124/jpet.102.047183. [DOI] [PubMed] [Google Scholar]
- Etou K, Kuroki T, Kawahara T, Yonezawa Y, Tashiro N, Uchimura H. Ceruletide inhibits phencyclidine-induced dopamine and serotonin release in rat prefrontal cortex. Pharmacology Biochemistry and Behavior. 1998;61:427–434. doi: 10.1016/s0091-3057(98)00128-2. [DOI] [PubMed] [Google Scholar]
- Faucard R, Armand V, Héron A, Cochois V, Schwartz JC, Arrang JM. N-methyl-D-aspartate receptor antagonists enhance histamine neuron activity in rodent brain. Journal of Neurochemistry. 2006;98:1487–1496. doi: 10.1111/j.1471-4159.2006.04002.x. [DOI] [PubMed] [Google Scholar]
- Ferrada C, Ferré S, Casadó V, Cortés A, Justinova Z, Barnes C, Canela EI, Goldberg SR, Leurs R, Lluis C, Franco R. Interactions between histamine H3 and dopamine D2 receptors and the implications for striatal function. Neuropharmacology. 2008;55:190–197. doi: 10.1016/j.neuropharm.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox GB, Pan JB, Radek RJ, Lewis AM, Bitner RS, Esbenshade TA, Faghih R, Bennani YL, Williams M, Yao BB, Decker MW, Hancock AA. Two novel and selective nonimidazole H3 receptor antagonists A-304121 and A-317920: II. In vivo behavioral and neurophysiological characterization. Journal of Pharmacology and Experimental Therapeutics. 2003;305:897–908. doi: 10.1124/jpet.102.047241. [DOI] [PubMed] [Google Scholar]
- Fox GB, Esbenshade TA, Pan JB, Radek RJ, Krueger KM, Yao BB, Browman KE, Buckley M, Ballard ME, Komater VA, Miner H, Zhang M, Faghih R, Rueter LE, Bitner RS, Drescher KU, Wetter J, Marsh K, Lemaire M, Porsolt RD, Bennani YL, Sullivan JP, Cowart MD, Decker MW, Hancock AA. Pharmacological properties of ABT-239 [4-2-{2-[2R-2-Methylpyrrolidinyl]ethyl}-benzofuran-5-ylbenzonitrile]: II. Neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H3 receptor antagonist. Journal of Pharmacology and Experimental Therapeutics. 2005;313:176–190. doi: 10.1124/jpet.104.078402. [DOI] [PubMed] [Google Scholar]
- Galici R, Boggs JD, Aluisio L, Fraser IC, Bonaventure P, Lord B, Lovenberg TW. JNJ-10181457, a selective non-imidazole histamine H3 receptor antagonist, normalizes acetylcholine neurotransmission and has efficacy in translational rat models of cognition. Neuropharmacology. 2009;56:1131–1137. doi: 10.1016/j.neuropharm.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Grayson B, Idris NF, Neill JC. Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behavioural Brain Research. 2007;184:31–38. doi: 10.1016/j.bbr.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Hajós M, Siok CJ, Hoffmann WE, Li S, Kocsis B. Modulation of hippocampal theta oscillation by histamine H3 receptors. Journal of Pharmacology and Experimental Therapeutics. 2008;324:391–398. doi: 10.1124/jpet.107.130070. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Yamada K, Hasegawa T, Nabeshima T. Role of dopaminergic neuronal system in dizocilpine-induced acetylcholine release in the rat brain. Journal of Neural Transmission. 1996;103:651–660. doi: 10.1007/BF01271225. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. Journal of Neuroscience. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YW, Hu WW, Chen Z, Zhang LS, Shen HQ, Timmerman H, Leurs R, Yanai K. Effect of the histamine H3-antagonist clobenpropit on spatial memory deficits induced by MK-801 as evaluated by radial maze in Sprague-Dawley rats. Behavioural Brain Research. 2004;151:287–293. doi: 10.1016/j.bbr.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proceedings of the National Academy of Sciences. 2004;101:8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs PS, Taylor BM, Bardgett ME. Maturation of locomotor and Fos responses to NMDA antagonists, PCP and MK-801. Developmental Brain Research. 2000;122:91–95. doi: 10.1016/s0165-3806(00)00059-6. [DOI] [PubMed] [Google Scholar]
- Jones CK, Eberle EL, Shaw DB, McKinzie DL, Shannon HE. Pharmacologic interactions between the muscarinic cholinergic and dopaminergic systems in the modulation of prepulse inhibition in rats. Journal of Pharmacological & Experimental Therapeutics. 2005;312:1055–1063. doi: 10.1124/jpet.104.075887. [DOI] [PubMed] [Google Scholar]
- Kubota T, Hirota K, Yoshida H, Takahashi S, Ohkawa H, Anzawa N, Kushikata T, Matsuki A. Inhibitory effect of clonidine on ketamine-induced norepinephrine release from the medial prefrontal cortex in rats. British Journal of Anaesthesia. 1999;83:945–947. doi: 10.1093/bja/83.6.945. [DOI] [PubMed] [Google Scholar]
- Le S, Gruner JA, Mathiasen JR, Marino MJ, Schaffhauser H. Correlation between ex vivo receptor occupancy and wake-promoting activity of selective H3 receptor antagonists. Journal of Pharmacology and Experimental Therapeutics. 2008;325:902–909. doi: 10.1124/jpet.107.135343. [DOI] [PubMed] [Google Scholar]
- Ligneau X, Landais L, Perrin D, Piriou J, Uguen M, Denis E, Robert P, Parmentier R, Anaclet C, Lin JS, Burban A, Arrang JM, Schwartz JC. Brain histamine and schizophrenia: potential therapeutic applications of H3-receptor inverse agonists studied with BF2.649. Biochemical Pharmacology. 2007a;73:1215–1224. doi: 10.1016/j.bcp.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Ligneau X, Lin J, Vanni-Mercier G, Jouvet M, Muir JL, Ganellin CR, Stark H, Elz S, Schunack W, Schwartz J. Neurochemical and behavioral effects of ciproxifan, a potent histamine H3-receptor antagonist. Journal of Pharmacology and Experimental Therapeutics. 1998;287:658–666. [PubMed] [Google Scholar]
- Ligneau X, Perrin D, Landais L, Camelin JC, Calmels TP, Berrebi-Bertrand I, Lecomte JM, Parmentier R, Anaclet C, Lin JS, Bertaina-Anglade V, la Rochelle CD, d’Aniello F, Rouleau A, Gbahou F, Arrang JM, Ganellin CR, Stark H, Schunack W, Schwartz JC. BF2.649 [1-{3-[3-4-Chlorophenylpropoxy]propyl}piperidine, hydrochloride], a nonimidazole inverse agonist/antagonist at the human histamine H3 receptor: Preclinical pharmacology. Journal of Pharmacology and Experimental Therapeutics. 2007b;320:365–375. doi: 10.1124/jpet.106.111039. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Aultman JM, Verma A, Weinberger DR, Moghaddam B. Neonatal damage of the ventral hippocampus impairs working memory in the rat. Neuropsychopharmacology. 2002;27:47–54. doi: 10.1016/S0893-133X(02)00282-8. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Schaffhauser H, Campbell UC, Baccei CS, Correa LD, Rowe B, Rodriguez DE, Anderson JJ, Varney MA, Pinkerton AB, Vernier JM, Bristow LJ. Group II mGlu receptor activation suppresses norepinephrine release in the ventral hippocampus and locomotor responses to acute ketamine challenge. Neuropsychopharmacology. 2003;28:1622–1632. doi: 10.1038/sj.npp.1300238. [DOI] [PubMed] [Google Scholar]
- Lubenov EV, Siapas AG. Hippocampal theta oscillations are travelling waves. Nature. 2009;459:534–549. doi: 10.1038/nature08010. [DOI] [PubMed] [Google Scholar]
- Marcus MM, Jardemark KE, Wadenberg ML, Langlois X, Hertel P, Svensson TH. Combined alpha2 and D2/3 receptor blockade enhances cortical glutamatergic transmission and reverses cognitive impairment in the rat. International Journal of Neuropsychopharmacology. 2005;8:315–327. doi: 10.1017/S1461145705005328. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Kocsis B, Hajós M. Elicited hippocampal theta rhythm: a screen for anxiolytic and procognitive drugs through changes in hippocampal function? Behavioural Pharmacology. 2007;18:329–346. doi: 10.1097/FBP.0b013e3282ee82e3. [DOI] [PubMed] [Google Scholar]
- Medhurst AD, Atkins AR, Beresford IJ, Brackenborough K, Briggs MA, Calver AR, Cilia J, Cluderay JE, Crook B, Davis JB, Davis RK, Davis RP, Dawson LA, Foley AG, Gartlon J, Gonzalez MI, Heslop T, Hirst WD, Jennings C, Jones DN, Lacroix LP, Martyn A, Ociepka S, Ray A, Regan CM, Roberts JC, Schogger J, Southam E, Stean TO, Trail BK, Upton N, Wadsworth G, Wald JA, White T, Witherington J, Woolley ML, Worby A, Wilson DM. GSK189254, a novel H3 receptor antagonist that binds to histamine H3 receptors in Alzheimer’s disease brain and improves cognitive performance in preclinical models. Journal of Pharmacology and Experimental Therapeutics. 2007;321:1032–1045. doi: 10.1124/jpet.107.120311. [DOI] [PubMed] [Google Scholar]
- Mogensen J, Boyd MH, Nielsen MD, Kristensen RS, Malá H. Erythropoietin improves spatial delayed alternation in a T-maze in rats subjected to ablation of the prefrontal cortex. Brain Research Bulletin. 2008;77:1–7. doi: 10.1016/j.brainresbull.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Moor E, Schirm E, Jacsó J, Westerink BH. Involvement of medial septal glutamate and GABAA receptors in behaviour-induced acetylcholine release in the hippocampus: a dual probe microdialysis study. Brain Research. 1998;789:1–8. doi: 10.1016/s0006-8993(97)01445-5. [DOI] [PubMed] [Google Scholar]
- Morisset S, Pilon C, Tardivel-Lacombe J, Weinstein D, Rostene W, Betancur C, Sokoloff P, Schwartz JC, Arrang JM. Acute and chronic effects of methamphetamine on tele-methylhistamine levels in mouse brain: selective involvement of the D(2) and not D(3) receptor. Journal of Pharmacological & Experimental Therapeutics. 2002;300:621–628. doi: 10.1124/jpet.300.2.621. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Burk JA, Bruno JP, Sarter M. Effects of acute and repeated systemic administration of ketamine on prefrontal acetylcholine release and sustained attention performance in rats. Psychopharmacology. 2002;161:168–179. doi: 10.1007/s00213-002-1004-7. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Archives of General Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, Schwartz JC, Arrang JM. A detailed mapping of the histamine H(3) receptor and its gene transcripts in rat brain. Neuroscience. 2002a;114:173–193. doi: 10.1016/s0306-4522(02)00135-5. [DOI] [PubMed] [Google Scholar]
- Pillot C, Ortiz J, Héron A, Ridray S, Schwartz JC, Arrang JM. Ciproxifan, a histamine H3-receptor antagonist/inverse agonist, potentiates neurochemical and behavioral effects of haloperidol in the rat. Journal of Neuroscience. 2002b;22:7272–7280. doi: 10.1523/JNEUROSCI.22-16-07272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi C, Zou H, Zhang R, Zhao G, Jin M, Yu L. Age-related differential sensitivity to MK-801-induced locomotion and stereotypy in C57BL/6 mice. European Journal of Pharmacology. 2008;580:161–168. doi: 10.1016/j.ejphar.2007.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz B, Fendt M, Pedersen V, Koch M. Sensitization of prepulse inhibition deficits by repeated administration of dizocilpine. Psychopharmacology. 2001;156:177–181. doi: 10.1007/s002130100776. [DOI] [PubMed] [Google Scholar]
- Southam E, Cilia J, Gartlon JE, Woolley ML, Lacroix LP, Jennings CA, Cluderay JE, Reavill C, Rourke C, Wilson DM, Dawson LA, Medhurst AD, Jones DN. Preclinical investigations into the antipsychotic potential of the novel histamine H3 receptor antagonist GSK207040. Psychopharmacology. 2009;201:483–494. doi: 10.1007/s00213-008-1310-9. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Schoepp DD. The group II metabotropic glutamate receptor agonist (−)-2-oxa-4-aminobicyclo[3.1.0.]hexane-4,6-dicarboxylate ( LY379268) and clozapine reverse phencyclidine-induced behaviors in monoamine-depleted rats. Journal of Pharmacological & Experimental Therapeutics. 2002;303:919–927. doi: 10.1124/jpet.102.038422. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Martinez ZA, Hanlon FM, Platten A, Farid M, Auerbach P, Braff DL, Geyer MA. Toward understanding the biology of a complex phenotype: rat strain and substrain differences in the sensorimotor gating-disruptive effects of dopamine agonists. Journal of Neuroscience. 2000;20:4325–4336. doi: 10.1523/JNEUROSCI.20-11-04325.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Crain S, Goins J, Onozuka K, Auerbach PP. Sensitivity to drug effects on prepulse inhibition in inbred and outbred rat strains. Pharmacology, Biochemistry, & Behavior. 2004;77:291–302. doi: 10.1016/j.pbb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. Journal of Neuroscience. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DJ, Herbert MR, Stanton ME. NMDA receptor involvement in spatial delayed alternation in developing rats. Behavioral Neuroscience. 2009;123:44–53. doi: 10.1037/a0013633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedzony K, Maćkowiak M, Zajaczkowski W, Fijal K, Chocyk A, Czyrak A. WAY 100135, an antagonist of 5-HT1A serotonin receptors, attenuates psychotomimetic effects of MK-801. Neuropsychopharmacology. 2000;23:547–559. doi: 10.1016/S0893-133X(00)00150-0. [DOI] [PubMed] [Google Scholar]
- Wozniak DF, Olney JW, Kettinger L, 3rd, Price M, Miller JP. Behavioral effects of MK-801 in the rat. Psychopharmacology. 1990;101:47–56. doi: 10.1007/BF02253717. [DOI] [PubMed] [Google Scholar]
- Yoon T, Okada J, Jung MW, Kim JJ. Prefrontal cortex and hippocampus subserve different components of working memory in rats. Learning & Memory. 2008;15:97–105. doi: 10.1101/lm.850808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Ballard ME, Pan L, Roberts S, Faghih R, Cowart M, Esbenshade TA, Fox GB, Decker MW, Hancock AA, Rueter LE. Lack of cataleptogenic potentiation with non-imidazole H3 receptor antagonists reveals potential drug-drug interactions between imidazole-based H3 receptor antagonists and antipsychotic drugs. Brain Research. 2005;1045:142–149. doi: 10.1016/j.brainres.2005.03.018. [DOI] [PubMed] [Google Scholar]


