Abstract
Background
To extend investigation beyond global cognitive measures prevalent in the literature, this study examined attention and working memory (WM) abilities of survivors of childhood acute lymphoblastic leukemia (ALL), the separate contributions of attention and WM to IQ and their association with neuroimaging changes.
Methods
Ninety seven children with ALL received risk-directed therapy based on presenting clinical and biological factors. During consolidation therapy, low-risk patients received half the dose of intravenous methotrexate that standard-/high-risk patients received, and fewer doses of triple intrathecal therapy. Patients were classified according to end of consolidation MRIs (normal or leukoencephalopathy) and continuous measures of white matter structure were computed. As part of the protocol study, children completed cognitive assessment two years later (completion of therapy), utilizing Digit Span Forward (DSF) for attention and Digit Span Backward (DSB) for WM.
Results
For the total sample and standard/high risk group, Total Digit Span (TDS), DSF, and DSB were impaired relative to norms (p < .05). In the low risk group, only DSB was impaired (p <.0001). Across groups, a higher percentage of patients performed below the average range (scale score < 7) on DSB (66%) than DSF (14%) or TDS (18%). Regression analysis indicated that DSB predicted Estimated IQ (p <.05), after accounting for DSF. Leukoencephalopathy was predictive of lower TDS (p <.05).
Conclusions
WM appears especially sensitive to treatment-related changes in ALL survivors, detecting difficulties potentially missed by global intelligence measures. These findings ma y facilitate identification of vulnerable neural pathways and development of targeted cognitive interventions.
Keywords: leukemia, working memory, attention, leukoencephalopathy
INTRODUCTION
Acute Lymphoblastic Leukemia (ALL) is the most common malignancy of childhood and adolescence, with this age group comprising approximately two thirds of the 4000 total cases diagnosed annually in the United States. Over the past few decades there have been significant improvements in ALL treatment outcomes, resulting in five year event-free survival rates of nearly 90%.1 Increased cure rate has led to increased attention to the quality of life of these survivors.
Although recent treatment regimens are associated with decreased adverse side effects including cognitive late effects, ALL survivors are still subject to increased cognitive impairments secondary to disease and treatment.2 Historically, cognitive outcomes have been evaluated using global measures assessing abilities such as IQ and academic achievement. These studies typically show that healthy controls perform better than children treated for ALL on these benchmarks.3 Recent research has begun to examine more specific and functional cognitive processes. For example, deficits in attention4, problems with attention and memory5 and decreased processing speed and working memory6 have been demonstrated in ALL survivors.
Curative therapy for ALL includes central nervous system (CNS) directed therapy, generally in the form of intensive systemic and intrathecal chemotherapy. Prophylactic CNS irradiation had been part of the standard treatment historically, but is now generally limited to patients with high-risk ALL and is omitted for all patients in some protocols.1 However, systemic and intrathecal chemotherapy can also cause neurocognitive deficits and cerebral white matter changes. Reddick and colleagues7 determined that smaller white matter volumes observed in ALL survivors are associated with declines in attention, intelligence and academic achievement.
The frontal lobes of the brain are the last to fully develop with respect to cerebral white matter, with myelination extending into the third decade of life.8–10 Given this protracted development, cognitive processes supported by the frontal lobes (e.g., attention and working memory [WM]) may be particularly vulnerable to neurotoxicities associated with CNS-directed therapy. A significant proportion of age-related improvements in IQ can be accounted for by developmental improvements in WM.11 These neuroanatomical developmental trajectories, together with the established declines in the intelligence of ALL survivors, warrant a detailed examination of attention and WM to elucidate potentially vulnerable neural pathways.
To examine these specific cognitive constructs, the current study explores attention and WM abilities of childhood ALL survivors. The study also investigates the unique contribution that attention and WM make to IQ. Finally, the association between these abilities and treatment-related white matter changes is analyzed. Based on the existing literature, we hypothesized that these survivors would show deficits in the areas of intelligence and attention but with the most significant decreases in WM. We also expected that WM would be significantly associated with overall intellectual functioning. Finally, we anticipated a relationship between higher levels of leukoencephalopathy and decreased WM.
METHODS
Patients
All patients in the current study were sequentially enrolled on a frontline institutional ALL treatment protocol which includes serial cognitive assessment.1 Children were assigned to one of three risk groups (low, standard or high) based on comprehensive biological and clinical risk classification, which included blast cell immunophenotype and genotype, presenting clinical features and early treatment response.1 All patients received triple intrathecal therapy with methotrexate, hydrocortisone and cytarabine as CNS-directed therapy (13 to 18 treatments in low-risk group and 16 to 25 treatments in high-risk group), beginning with remission induction therapy. During consolidation therapy, high-dose methotrexate was given intravenously every other week for four doses at 2.5 gm/m2 per dose for low-risk patients and at 5.0 gm/m2 for standard-/high-risk patients. None of the patients received prophylactic cranial irradiation. During continuation treatment, dexamethasone pulses were given at 8 mg/m2 per day for low-risk patients, and at 12 mg/m2 per day for standard-/high-risk patients.
For the purposes of this study, all children between the ages of 6 and 18 years were included in analyses based on the normative ranges for cognitive measures. Children were further excluded from analysis if they were previously diagnosed with a developmental disorder with known cognitive sequelae (Down syndrome, autism; n=6), did not speak English (n=14) or were missing imaging and/or psychological testing data (n=54; these included children with refractory or progressive disease, missed testing appointments, scheduling problems or technical issues interfering with imaging acquisition). The study was approved by the Institutional Review Board. Written informed consent, with assent from the patient, as appropriate, was obtained prior to participation. Study enrollment occurred between 2000 and 2007.
Procedures
Cognitive Assessment
Children were tested two years after the completion of consolidation therapy (Week 120) using measures standardized on large representative normative samples that have demonstrated reliability and validity.12,13 They were administered an age-appropriate Wechsler Intelligence Scale [Wechsler Intelligence Scale for Children – Third Edition (WISC-III) for patients under the age of sixteen and Wechsler Adult Intelligence Scale – Third Edition (WAIS-III) for patients ages sixteen and older]. The current study focused primarily on the Digit Span subtest, which yields age -standardized scores for Total Digit Span, Digit Span Forward and Digit Span Backward. For Digit Span Forward, which is regarded as a measure of attention and immediate recall, the examiner verbally presents specified random sequences of digits that the participant is required to repeat back verbatim. For Digit Span Backward, the participant must reverse the order of the digits before repeating. Given this additional requirement to mentally manipulate information, this task is conventionally considered a measure of verbal WM.14,15 Total Digit Span is the aggregate of Digit Span Forward and Backward.
In addition to the Digit Span subtest, an Estimated Intelligence Quotient (EIQ) was also derived for each participant at study baseline and Week 120. This score is computed from the Information, Similarities and Block Design subtests from either the WISC-III or WAIS-III using a formula provided by Sattler.16 The EIQ was chosen over the Full Scale IQ because Digit Span scores are included in the determination of Full Scale IQ. Thus, EIQ provides a valid and reliable estimate of IQ that can be tested for relationships to attention and WM without the embedded measures of these constructs.
MR Imaging
Conventional MR Imaging was performed on one of two 1.5T whole-body MR systems using the standard circular polarized volume head coil (Avanto, Siemens Medical Systems, Iselin, NJ). Leukoencephalopathy, T2-hyperintensities within the white matter, is best visualized with a T2-weighted sequence, preferably with CSF attenuated. Imaging was acquired and included at least nineteen oblique 4 mm thick axial images with a 1 mm gap. T1-weighted images were acquired with a multi-echo inversion recovery sequence (repetition time between spin excitations [TR] = 8000 ms; echo time [TE] = 20 ms; time between inversion and the excitation pulse [TI] = 300 ms; 11 echoes). T2/PD-weighted images were acquired with a dual spin-echo sequence(TR/TE1/TE2 = 3500/17/102 ms, 7 echoes). Fluid-attenuated inversion recovery (FLAIR) images were acquired with a multi-echo inversion recovery sequence (TR/TE/TI = 9000/119/2470 ms; 11 echoes). These imaging sets covered most of the cerebrum starting at the apex of the brain but did not include the cerebellum.
Patients were classified according to MRI images (normal or leukoencephalopathy) at the completion of consolidation therapy based on retrospective review by a single neuroradiologist (FHL) blinded to results of cognitive assessment. Volumes of regional brain parenchyma on MR images were quantified using an automated hybrid neural network segmentation and classification method.17 Robust reliability and validity have been established for these methods, resulting in a predicted variance of approximately 2% in the repeated measures of normal appearing white matter (NAWM) and gray matter.18 Once each examination was segmented, we assessed the extent of leukoencephalopathy and volume NAWM in left/right and anterior/posterior quadrants. Volumes were assessed both as absolute volumes in cubic centimeters and relative volumes where each tissues volume was normalized to the intracranial volume.
Statistical Analyses
In order to characterize the sample, qualitative analyses of demographic and clinical variables were performed. Demographic and clinical variables were also examined to investigate potential associations with measures of attention and WM.
The extent of cognitive impairment was estimated by comparing the performance of ALL survivors on EIQ and the Digit Span tasks to the normative population (EIQ has a mean of 100 and standard deviation of 15; Digit Span scaled scores have a mean of 10 and standard deviation of 3) using two-tailed independent t-tests. Performance on the Digit Span tasks was also examined to determine the rate of clinical impairment by investigating the percentage of patients who scored at least one standard deviation below the mean for Total Digit Span, Digit Span Forward and Digit Span Backward. Multiple linear regression analysis was used to analyze the contribution of Digit Span Backward to EIQ after accounting for Digit Span Forward.
Finally, to examine possible effects of neuroanatomical findings, the dichotomous neuroradiologist ratings (normal or leukoencephalopathy) were examined for relationship to performance on the Digit Span task using Wilcoxon rank two samples tests. To further investigate these clinical measures, correlations among continuous metrics of white matter structure (absolute and relative NAWM and leukoencephalopathy volumes) and performance on the Digit Span task were conducted. Each of the above analyses was performed across the total patient population as well as with risk-based subgroups (i.e., standard/high and low risk).
RESULTS
Demographic and Clinical Characteristics
Ninety-seven children (55 male and 42 female) were included in this study. Ethnicity of the sample was representative of the overall treatment protocol population. On average, children were 8 years old at diagnosis and 10 years old at assessment, with a baseline EIQ of 100. Treatment risk arm assignment was nearly equal, with 48 patients classified as low risk and 49 patients classified as standard or high risk. Demographic and clinical characteristics are summarized in Table 1.
Table 1.
Patient Demographic and Clinical Characteristics
| n | Percentage | ||
|---|---|---|---|
| Gender | |||
| Male | 55 | 56.7 | |
| Female | 42 | 43.3 | |
| Ethnicity | |||
| Caucasian | 71 | 73.2 | |
| African-American | 20 | 20.6 | |
| Other | 6 | 6.2 | |
| Risk Arm | |||
| Standard/High | 49 | 50.5 | |
| Low | 48 | 49.5 | |
| N | M ± SD | Range | |
| Age at Diagnosis | 97 | 8.22 ± 3.93 | 3.46 – 18.45 |
| Age at Assessment1 | 97 | 10.84 ± 3.93 | 6.02 – 21.00 |
| Baseline Estimated IQ2 | 653 | 100.92 ± 16.54 | 58 – 138 |
Assessment 2 years after completion of consolidation therapy (Week 120)
IQ is estimated using the WISC-III/WAIS-III Similarities, Information and Block Design subtests
Sample size discrepancy is primarily due to patients being too young for assessment at Baseline
M=Mean; SD=Standard Deviation; IQ=Intelligence Quotient; WISC-III=Wechsler Intelligence Scale for Children– Third Edition; WAIS -III=Wechsler Adult Intelligence Scale – Third Edition
No statistical associations were found between demographic variables and attention or WM performance. Of the clinical variables, only risk classification showed an association with attention and WM ability, with standard/high risk patients performing significantly worse (p<.01) on Total Digit Span than low risk patients. This relationship is explored below.
Attention and Working Memory Performance
Patients’ performance on neuropsychological measures in relation to the normative sample is summarized in Table 2. Across the entire sample, EIQ at week 120 did not differ significantly when compared to the normative mean of 100. There was also no statistical departure from the norm for EIQ for standard/high risk or low risk patients. Additionally, week 120 EIQ did not differ significantly from baseline EIQ. This was true of the entire sample and for individual risk groups.
Table 2.
Neuropsychological Variables in Relation to Normative Data
| n | M ± SD | p1 | |
|---|---|---|---|
| Week 120 Estimated IQ | |||
| All Patients | 95 | 100.4 ± 17.34 | 0.8226 |
| Standard/High Risk | 48 | 96.88 ± 18.46 | 0.2469 |
| Low Risk | 47 | 104.00 ± 15.49 | 0.0832 |
| Total Digit Span Std Score | |||
| All Patients | 97 | 8.81 ± 2.89 | 0.0001* |
| Standard/High Risk | 49 | 7.92 ± 2.67 | <.0001* |
| Low Risk | 48 | 9.73 ± 2.85 | 0.5134 |
| Digit Span Forward Std Score | |||
| All Patients | 852 | 9.28 ± 2.68 | 0.0157* |
| Standard/High Risk | 41 | 8.66 ± 2.82 | 0.0040* |
| Low Risk | 44 | 9.86 ± 2.45 | 0.7133 |
| Digit Span Backward Std Score | |||
| All Patients | 852 | 5.79 ± 2.46 | <.0001* |
| Standard/High Risk | 41 | 5.29 ± 2.30 | <.0001* |
| Low Risk | 44 | 6.25 ± 2.54 | <.0001* |
Tested for significant difference from mean of normative sample (Digit Span SS=10±3; Estimated IQ =100±15)
12 patients were greater than 16 years of age and received the WAIS-III which does not provide separate standard scores for Digit Span Forward and Digit Span Backward
p<.05
M=Mean; SD=Standard Deviation; IQ=Intelligence Quotient; Std=Standard
For the sample as a whole, patients showed significant impairment on Total Digit Span, Digit Span Forward and Digit Span Backward (p <.05) in comparison to the normative mean of 10. Standard/high risk patients showed the same pattern, with deficits across all tasks (p<.01). In contrast, the low risk group showed significant impairment only on Digit Span Backward (p<.0001) and maintained statistically normal performance on Total Digit Span and Digit Span Forward. This finding suggests that WM(as measured by Digit Span Backward) may be particularly sensitive as it was impaired in patients with the lowest treatment intensity.
Patients’ performance on the Digit Span tasks was defined as clinically significant if the score fell below one standard deviation from the normative mean (<7) and therefore outside the average range (Figure 1). Across the entire sample, a high percentage of patients fell below this clinical cutoff on Digit Span Backward (65.9%), while expected proportions of children showed this impairment on Total Digit Span (17.5%) and Digit Span Forward (14.1%). The same pattern of clinical significance was seen in the standard/high risk group (Total Digit Span: 24.5%, Digit Span Forward: 19.5%, Digit Span Backward: 70.7%) and the low risk group (Total Digit Span: 10.4%, Digit Span Forward: 9.1%, Digit Span Backward: 61.4%).
Figure 1. Patients with Clinically Significant Digit Span Deficits.
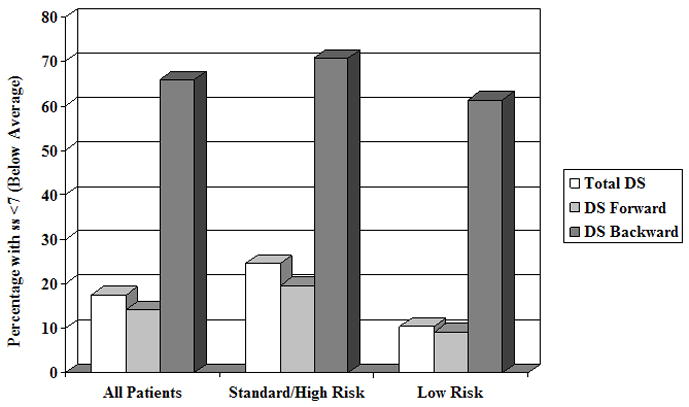
Percentage of patients who score clinically below average on Digit Span Tasks. DS = Digit Span; ss = Standard Score
Regression analysis was used to determine the degree to which Digit Span tasks predict EIQ and investigate whether Digit Span Backward made a unique contribution after Digit Span Forward was taken into account. A scatterplot of these analyses can be found in Figure 2. Results indicated that 21% of the variance of EIQ is explained by Digit Span Forward and Backward. Further analysis showed that Digit Span Backward still contributed significantly (p=.01) after accounting for Digit Span Forward (p=.01). These results suggest that WM is unique aspect of overall intelligence over and above attention.
Figure 2. Contribution of Digit Span Forward and Backward to IQ.
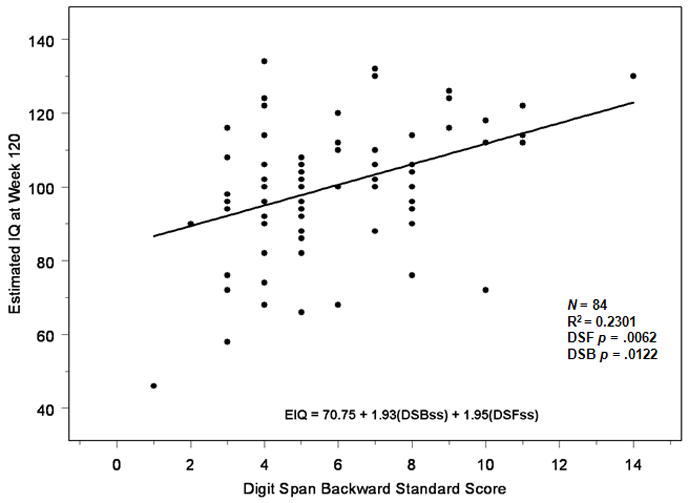
Scatterplot reflecting regression analysis examining the degree to which Digit Span Tasks predict Estimated Intelligence Quotient. DSF=Digit Span Forward; DSB=Digit Span Backward; EIQ=Estimated Intelligence Quotient; ss=Standard Score
Neuroanatomical Changes
Performance on the Digit Span tasks was also related to neuroanatomical findings. Examination of the dichotomous neuroradiologist ratings (normal vs. leukoencephalopathy) of patient MRIs revealed that children with normal ratings had significantly better scores on Total Digit Span (p=.03) across all patients. Results in the low risk group were similar (p=.01) but this pattern was not seen in the standard/high risk group alone. To further investigate this relationship, continuous variables of white matter structure were assessed. Neither the absolute nor the relative NAWM volumes were significantly associated with performance on the Digit Span task. However, greater absolute volumes of leukoencephalopathy in all four quadrants (left/right, anterior/posterior) were significantly associated with lower scores on Total Digit Span (both anterior, p=.01; both posterior, p=.04)across all patients. Results in the low risk group were similar (both anterior, p=.01; both posterior, p=.02) but this pattern was not seen in the standard/high risk group alone.
To ensure that this relationship was not related to patient head size, we also assessed relative volumes. The same associations were shown with greater relative volumes of leukoencephalopathy in all quadrants being significantly associated with lower scores on Total Digit Span across all patients(both anterior, p=.01; both posterior, p=.04) and for the low risk group (both anterior, p=.01; both posterior, p=.02)but was not observed for the standard/high-risk group.
DISCUSSION
Results from this study revealed that, two years after completing therapy, childhood ALL survivors demonstrate impairment in WM, suggesting that WM is particularly sensitive to ALL treatment effects. While the standard/high risk group demonstrated impairment in attention and WM, only deficits in WM were detected in the low risk group. Additionally, these effects on WM proved clinically meaningful with a large percentage of children functioning below the average range. Taken together, we can conclude that WM is sensitive to current treatment regimens despite risk adapted treatment stratification of patients and reduction in the use of neurotoxic agents.
In addition to being particularly sensitive, WM proved to be a unique contributor to overall intellectual functioning. After accounting for attention, WM still played a significant role in predicting IQ. This study gives evidence to WM as a distinct aspect of overall intelligence of ALL survivors. Given the established contributions of WM to developmental improvements in intelligence, WM may therefore be a proximal contributor to previously identified IQ declines in these children.
Treatment-induced white matter changes proved to be associated with a decrease in attention and WM. Using both dichotomous ratings (normal and leukoencephalopathy) and continuous measures of white matter structure, children with leukoencephalopathy scored lower on combined attention and WM tasks. It appears that these neuroanatomical changes are related to decreased abilities in these ALL survivors. Additionally, since imaging was obtained at the end of consolidation therapy and cognitive assessment occurred two years later, there appears to be a predictive nature to this relationship. These findings were statistically significant for the low risk patients and trending in the same direction for patients in the standard/high group. This risk-stratified finding could be explained by the older age of standard/high risk patients. Given more mature white matter, these children may not have suffered as much leukoencephalopathy as younger patients with less myelination.
The study found that WM played a significant role in predicting IQ and that there were significant deficits in WM within our sample. In what looks to be a contradictory finding, however, no IQ deficits were observed in relation to the normative population or baseline intellectual functioning. This finding is also in contrast to prior studies showing global declines following treatment for ALL.7, 5, 19, 20 This is likely due to the use of Estimated IQ instead of Full Scale IQ. While we were able to demonstrate the importance of WM to overall intellectual functioning (as EIQ is highly representative of FSIQ), the selected subtests used in this study (Information, Similarities and Block Design) are not heavily reliant on WM abilities or processing speed. Therefore, EIQ scores may not have been impacted as FSIQ scores containing WM or processing speed subtests, abilities shown to be at risk in this population.6 It should also be remembered that many treatment refinements have taken place since these prior studies. A part of the IQ resiliency may be due to the protection offered by newer therapy regimens. The current study did find global declines in attention (as measured by Total Digit Span) similar to those in the established literature.4
As a result of using the Wechsler tests as measures of intelligence and WM, we were unable to assess the youngest children in our sample (as it is not normed for children under 6 years old) and patients older than 16 were not included in the WM analyses (due to lack of standard scores for Digit Span Forward and Backward on the WAIS-III). This truncated age range may have limited our ability to detect age effects of treatment on attention or WM. This lack of age effects is inconsistent with current literature showing that patients who are younger at treatment have poorer neurocognitive outcomes.21 It should be noted that because of the longitudinal nature of our data, most of the children who were too young for inclusion at baseline were old enough for assessment two years later. The inclusion of multiple WM measures in future studies would allow assessment of a wider age range of patients and provide a more comprehensive picture of WM skills.
In regards to neuroanatomical findings, the literature is very consistent in placing the mediation of executive tasks (including WM) in the frontal and prefrontal cortex.22–24 These brain regions are the last to myelinate and may therefore be particularly vulnerable to insults related to CNS-directed therapies. Accordingly, it was hypothesized that white matter changes associated with decreased WM would be located in the anterior portions of the brain. This hypothesis was not confirmed as identical relationships were found between WM performance and posterior brain regions. One explanation for this finding is that the division of the brain into quadrants for analysis is not based on anatomical or functional regions, so the anterior portions do not represent specific areas within the frontal and prefrontal cortex (e.g., the dorsal lateral prefrontal cortex). Another likely confounding factor is the presence of longitudinal nerve fibers. Even when assessing performance of functional regions within the frontal cortex, long associational fibers connect these regions with more distant cortical and thalamic regions.25, 26 These fibers may span both the anterior and posterior regions of interest and thus make the observed measures abnormal across multiple regions. Subsequent studies should take advantage of diffusion tensor imaging to more specifically investigate the role and location of white matter changes associated with cognitive changes.
Future studies could benefit from expansion and improvement on the current design as we continue to investigate WM abilities in ALL survivors. We believe that the use of a predictive (vs. concurrent) design of the current study gives further weight to the finding that early detected leukoencephalopathy can serve as a warning of later cognitive declines. As demonstrated in Table 3, only a small percentage of patients will develop leukoencephalopathy if these changes are not present at the end of consolidation therapy. While a larger portion of patients show resolution of white matter changes, as reported previously27, 28, the current study is interested in early identification of children at risk for later development of cognitive deficits. Additional longitudinal testing points to assess the persistence or exacerbation of deficits over time would also be beneficial and utilization of a non-CNS cancer control group would serve to further illustrate the effects of CNS-directed treatment. In addition to the use of diffusion tensor imaging, functional MRI techniques may identify changes in resource allocation during task completion.
Table 3.
Change in MRI Classification of White Matter Between End of Consolidation and End of Therapy
| MRI 1 Classification1 | MRI 2 Classification2 | Change Status | Percent | ||
|---|---|---|---|---|---|
| Normal | 67 | Normal | 59 | Unchanged | 95% |
| Abnormal | 3 | Late Change | 5% | ||
| Missing | 5 | ||||
| Abnormal | 26 | Normal | 6 | Resolved | 25% |
| Abnormal | 18 | Persistent | 75% | ||
| Missing | 2 | ||||
| Missing | 4 | Normal | 4 | 100% | |
| Abnormal | 0 | ||||
| Missing | 0 | ||||
| Total | 93 | 90 | |||
MRI 1 images obtained at end of consolidation therapy
MRI 2 images obtained at end of therapy
A major implication of these findings is that treatment related neurocognitive deficits may be more subtle than detectable by global measures of intelligence and achievement. If the medical team relies on the more common global assessments, these more specialized cognitive deficits may be missed. Given what we know about WM’s developmental contribution to overall intelligence11, it may also be that unidentified WM deficits may result in later emerging IQ and academic achievement problems. With these vulnerable pathways in mind, treatment planning for ALL can be more cognizant of early indications of leukoencephalopathy. If the acute neuroimaging changes (i.e., leukoencephalopathy) are associated with later emerging deficits in attention and WM, chemotherapy regimens may be adjusted to provide greater sparing of these sensitive areas. Modern ALL therapy has largely eliminated CNS radiation therapy as a frontline treatment for newly diagnosed patients and therapy continues to evolve. Decreased dosages of corticosteroids and fewer intrathecal therapies across the span of treatment are examples of measures being put in place to help alleviate adverse side effects associated with CNS-directed therapy.
It is important to educate parents about the potential for attention and WM deficits to emerge subsequent to ALL treatment. They should know that “low risk” does not equal “no risk”, since even at the lowest intensity, these CNS-directed agents can still have significant effects on their child’s cognitive functioning. Proactive education of families will allow for closer monitoring and earlier intervention should problems arise.
In addition to the reduction of cognitive late effects through treatment advances, there is encouraging emerging support for interventions that may mitigate the impact of cognitive sequelae that may not be entirely unavoidable. In addition to well established pharmacological interventions,29, 30 there are cognitive remediation programs currently being evaluated that show promise for improving attention and WM.31 Ideally, this will help to synchronize the improved disease-related outcomes with long-term quality of life outcomes for children cured of ALL.
Acknowledgments
This work was supported, in part, by the Cancer Center Support (CORE) Grant (P30 CA21765, R01 CA90246 to WR) from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC). There are no financial disclosures from any of the authors. Portions of this abstract were previously published by the International Neuropsychology Society (February 2009). We thank the patients and their families who volunteered their time to participate.
References
- 1.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009 Jun 25;360(26):2730–41. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moleski M. Neuropsychological, neuroanatomical, and neurophysiological consequences of CNS chemotherapy for acute lymphoblastic leukemia. Arch of Clin Neuropsychol. 2000;15(7):603–30. [PubMed] [Google Scholar]
- 3.Mulhern RK, Butler RW. Neurocognitive sequelae of childhood cancers and their treatment. Pediatr Rehabil. 2004;7(1):1–14. doi: 10.1080/13638490310001655528. discussion 15–6. [DOI] [PubMed] [Google Scholar]
- 4.Rodgers J, Horrocks J, Britton PG, Kernahan J. Attentional ability among survivors of leukaemia. Arch Dis Child. 1999;80(4):318–23. doi: 10.1136/adc.80.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown RW, Madan-Swain A, Pais R, et al. Cognitive status of children treated with central nervous system prophylactic chemotherapy for acute lymphocytic leukemia. Arch of Clin Neuropsychol. 1992;7:481–97. [PubMed] [Google Scholar]
- 6.Schatz J, Kramer JH, Ablin A, Matthay KK. Processing speed, working memory, and IQ: a developmental model of cognitive deficits following cranial radiation therapy. Neuropsychology. 2000;14(2):189–200. doi: 10.1037//0894-4105.14.2.189. [DOI] [PubMed] [Google Scholar]
- 7.Reddick WE, Shan ZY, Glass JO, et al. Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia. Cancer. 2006;106(4):941–9. doi: 10.1002/cncr.21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huttenlocher PR. Synaptic density in human frontal cortex-developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 9.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54(1–3):241–57. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 10.Giedd JN. The anatomy of mentalization: a view from developmental neuroimaging. Bull Menninger Clin. 2003;67(2):132–42. doi: 10.1521/bumc.67.2.132.23445. [DOI] [PubMed] [Google Scholar]
- 11.Fry AS, Hale S. Processing speed, working memory, and fluid intelligence: evidence for a developmental cascade. Psychological Science. 1996;4:237–41. [Google Scholar]
- 12.Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio: Psychological Corporation; 1991. [Google Scholar]
- 13.Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio: Psychological Corporation; 1997. [Google Scholar]
- 14.Lezak MD. Neuropsychological Assessment. 3. New York: Oxford University Press; 1995. [Google Scholar]
- 15.Conklin HM, Curtis CE, Katsanis J, Iacono WG. Verbal working memory impairment in schizophrenia patients and their first-degree relatives: evidence from the digit span task. Am J Psychiatry. 2000;157:275–77. doi: 10.1176/appi.ajp.157.2.275. [DOI] [PubMed] [Google Scholar]
- 16.Sattler JM. Assessment of Children. 3. San Diego, CA: 1992. [Google Scholar]
- 17.Reddick WE, Glass JO, Cook EN, Elkin TD, Deaton R. Automated segmentation and classification of multispectral magnetic resonance images of brain using artificial neural networks. IEEE Transactions on Medical Imaging. 1997;16:911–918. doi: 10.1109/42.650887. [DOI] [PubMed] [Google Scholar]
- 18.Reddick WE, Glass JO, Langston JW, Helton KJ. Quantitative MRI assessment of leukoencephalopathy. Magnetic Resonance in Medicine. 2002;47:912–920. doi: 10.1002/mrm.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giralt J, Ortega JJ, Olive T, Verges R, Forio I, Salvador L. Long-term neuropsychologic sequelae of childhood leukemia: comparison of two CNS prophylactic regimens. Int J Radiat Oncol Biol Phys. 1992;24(1):49–53. doi: 10.1016/0360-3016(92)91020-n. [DOI] [PubMed] [Google Scholar]
- 20.Ochs J, Mulhern R, Fairclough D, et al. Comparison of neuropsychologic functioning and clinical indicators of neurotoxicity in long-term survivors of childhood leukemia given cranial radiation or parenteral methotrexate: a prospective study. J Clin Oncol. 1991 Jan;9(1):145–51. doi: 10.1200/JCO.1991.9.1.145. [DOI] [PubMed] [Google Scholar]
- 21.Reddick WE, White HA, Glass JO, et al. Developmental Model Relating White Matter Volume to Neurocognitive Deficits in Pediatric Brain Tumor Survivors. Cancer. 2004;97:2512–2519. doi: 10.1002/cncr.11355. [DOI] [PubMed] [Google Scholar]
- 22.D’Esposito M, Aquirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 1998 Jul;7(1):1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 23.Smith EE, Jonides J. Working memory: a view from neuroimaging. Cogn Psychol. 1997 Jun;33(1):5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- 24.Petrides M. Functional organization of the human frontal corex for mnemonic processing. Evidence from neuroimaging studies. Ann N Y Acad Sci. 1995 Dec 15;769:85–96. doi: 10.1111/j.1749-6632.1995.tb38133.x. [DOI] [PubMed] [Google Scholar]
- 25.Petrides M, Pandya DN. Association pathways of the prefrontal cortex and functional observations. In: Stuss D, Knight RT, editors. Principles of Frontal Lobe Function. New York, NY: Oxford University Press; 2002. pp. 31–50. [Google Scholar]
- 26.Reddick WE, Glass JO, Johnson DP, Laningham FH, Pui CH. Voxel-based analysis of T2 hyperintensities in white matter during treatment of childhood leukemia. Am J Neuroradiol. 2009 Nov;30(10):1947–54. doi: 10.3174/ajnr.A1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddick WE, Glass JO, Helton KJ, et al. Prevalence of leukoenchepalopathy in children treated for acute lymphoblastic leukemia with high-dose methotrexate. Am J Neuroradiol. 2005 May;26:1263–69. [PMC free article] [PubMed] [Google Scholar]
- 28.Reddick WE, Glass JO, Helton KJ, Langston JW, Li CS, Pui CH. A quantitative MR imaging assessment of leukoencephalopathy in children treated for acute lymphoblastic leukemia without irradiation. Am J Neuroradiol. 2005 Oct;26:2371–2377. [PMC free article] [PubMed] [Google Scholar]
- 29.Conklin HM, Khan RB, Reddick WE, et al. Acute neurocognitive response to methylphenidate among survivors of childhood cancer: a randomized, double-blind, cross-over trial. J Pediatr Psychol. 2007 Oct;32(9):1127–39. doi: 10.1093/jpepsy/jsm045. [DOI] [PubMed] [Google Scholar]
- 30.Thompson SJ, Leigh L, Christensen R, et al. Immediate neurocognitive effects of methylphenidate on learning-impaired survivors of childhood cancer. J Clin Oncol. 2001 Mar 15;19(6):1802–8. doi: 10.1200/JCO.2001.19.6.1802. [DOI] [PubMed] [Google Scholar]
- 31.Butler RW, Copeland DR, Fairclough DL, et al. A multicenter, randomized clinical trial of cognitive remediation program for childhood survivors of a pediatric malignancy. J Consult Clin Psychol. 2008 Jun;76(3):367–78. doi: 10.1037/0022-006X.76.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]


