Abstract
Purpose
To investigate correlation between ocular Demodex infestation and serum.
Design
A prospective study to correlate clinical findings with laboratory data.
Participants
We consecutively enrolled 59 patients: 34 men and 25 women with a mean age of 60.4±17.6 years (range, 17–93).
Methods
Demodex counting was performed based on lash sampling. Serum immunoreactivity to two 62-kDa and 83-kDa proteins derived from B oleronius was determined by Western blot analysis. Facial rosacea, lid margin, and ocular surface inflammation were documented by photography and graded in a masked fashion.
Main Outcome Measures
Statistical significance based on correlative analyses of clinical and laboratory data.
Results
These 59 patients were age matched, but not gender matched, regarding serum immunoreactivity, ocular Demodex infestation, or facial rosacea. There was a significant correlation between serum immunoreactivity and facial rosacea (P = 0.009), lid margin inflammation (P = 0.040), and ocular Demodex infestation (P = 0.048), but not inferior bulbar conjunctival inflammation (P = 0.573). The Demodex count was significantly higher in patients with positive facial rosacea (6.6±9.0 vs. 1.9±2.2; P = 0.014). There was a significant correlation of facial rosacea with lid margin inflammation (P = 0.016), but not with inferior bulbar conjunctival inflammation (P = 0.728). Ocular Demodex infestation was less prevalent in patients with aqueous tear-deficiency dry eye than those without (7/38 vs. 12/21; P = 0.002).
Conclusions
The strong correlation provides a better understanding of comorbidity between Demodex mites and their symbiotic B oleronius in facial rosacea and blepharitis. Treatments directed to both warrant future investigation.
Demodex, a microscopic, obligate, and elongated mite, is the most common ectoparasite in humans. In the skin, D folliculorum is found in hair follicles, whereas D brevis lives in sebaceous glands. They often coexist in the same skin area such as the face, cheeks, forehead, nose, and external ear tract, where active sebum excretion favors their habitation and breeding (for reviews see Baima and Sticherling1 and Forton et al2). In the eye, D folliculorum can be found in the lash follicle, whereas D brevis burrows deep into lash sebaceous glands and meibomian glands.
The pathogenic potential of these 2 mites remains arguable because a low number of Demodex can be found in the skin and lashes of asymptomatic individuals. To probe into the pathogenic role Demodex has in the external eye, we have improved the accuracy of the method of lash sampling and mite detection3,4 and reported that the clinical sign of cylindrical dandruff (CD) in the lash root is pathognomic for ocular Demodex infestation.3 Subsequently, using a method of monitoring in vitro killing of adult Demodex mites, we have discovered that lid scrubs with tea tree oil (TTO), but not baby shampoo, can successfully eradicate ocular mite infestations.5 Because previously refractory ocular surface inflammation resolved after a significant reduction of mite counts, we concluded that ocular Demodex infestation in some patients might lead to ocular surface inflammation in lashes (trichiasis and madarosis), lid (blepharitis), meibomian glands (lipid tear deficiency and tear film disturbance), conjunctiva (conjunctivitis), and various corneal lesions.6,7 Intriguingly, 4 of the 6 patients with Demodex blepharitis that manifested corneal lesions also suffered from facial rosacea and their ocular surface inflammation was notably reduced by lid scrubs with TTO, but not by conventional treatments such as lid hygiene with baby shampoo, topical steroids and antibiotics, and systemic doxycycline.7 Nevertheless, it remains unclear whether the aforementioned clinical improvement is derived solely from eradication of mite infestation or from other known antibacterial,8–10 antifungal,11–13 and anti-inflammatory14,15 actions of TTO. Even if we assume that the aforementioned clinical improvement is causatively linked to the therapeutic action of TTO for killing mites, we still cannot rule out whether there is concomitant microbial involvement in mite infestation.
Papulopustular rosacea is a chronic inflammatory dermatosis of the convexities of the central face characterized by the presence of multiple small, dome-shaped erythematous papules and papulopustules arising on a background of fixed inflammatory erythema.16 Although the diagnostic criteria, classification, and grading of rosacea have recently been outlined,17,18 its etiology and pathogenesis are poorly understood.19 The D folliculorum mites are frequently seen in the follicles of skin of patients with rosacea.20 Using a skin surface biopsy technique, which extracts mites from epidermal follicular canals, several investigators have shown a significantly increased density of D folliculorum mites in the facial skin of patients with rosacea when compared with control subjects,21,22 but the relevance of this finding to the pathogenesis of the condition is disputed.
To reconcile the apparently disparate findings of increased numbers of D folliculorum mites, perifollicular inflammation, and response of papulopustular rosacea to selective antibiotic therapy in some patients, Kavanagh et al23 recently isolated Bacillus oleronius inside of Demodex mites from 1 patient with papulopustular rosacea. They discovered that this bacterium produced antigens capable of stimulating proliferation of peripheral blood mononuclear cells in 16 of 22 (73%) patients with rosacea but only 5 of 17 (29%) control subjects (P=0.0105). Furthermore, they pooled serum from 6 patients with papulopustular rosacea and identified serum immunoreactivity to 2 proinflammatory 62-kDa and 83-kDa proteins produced by this bacterium.23
As a first step toward addressing the aforementioned questions, we carried out a prospective study of 59 patients and correlated their serum immunoreactivity to these 2 bacillus proteins with ocular Demodex infestation as well as with their clinical presentation of facial rosacea, ocular surface inflammation, aqueous tear-deficiency dry eye, and conjunctivochalasis. Our results show a strong correlation among positive serum immunoreactivity and ocular Demodex infestation in facial rosacea and lid margin inflammation. The significance of comorbidity of both microbial infection and mite infestation in ocular surface inflammation is further discussed.
Patients and Methods
Patients
This study followed the tenets of the Helsinki Declaration of Human Studies and has been approved by the ethics committee of the Ocular Surface Research and Education Foundation. A total of 59 patients who presented with a number of ocular surface diseases (Table 1; available online at http://aaojournal.org) were consecutively enrolled in the Ocular Surface Center (Miami, FL) from September 2008 to April 2009. For all patients, in addition to routine complete eye examinations, photographs were taken to document findings in the face and lid margins and bulbar conjunctiva of both eyes. The fluorescein clearance test was performed to determine if there was aqueous tear-deficiency dry eye as previously published.24,25 Fluorescein staining and maneuvers such as frequent blinking and digital pressure against the lid to the globe were used to rule out conjunctivochalasis as another common cause of dry eye.26,27 Afterward, lashes were epilated for microscopic detection and counting of Demodex mites and the blood was drawn for detection of serum immunoreactivity to microbial proteins.
Table 1.
Key Patient Clinical Information Subdivided by Serum Immunoreactivity
| Case No | Age | Gender | Ocular Demodex Counts* | Facial Rosacea | Lid Margin Inflammation | Inferior Conjunctiva Inflammation | Dry Eye | Cch | Other Ocular Surface Diseases |
|---|---|---|---|---|---|---|---|---|---|
| Group I. Positive serum immunoreactivity | |||||||||
| 1 | 24 | M | 1/1 | Y | 1 | 1 | N | N | — |
| 2 | 35 | M | 3 | Y | 2 | 2 | N | N | — |
| 3 | 42 | M | 4 | Y | 0 | 1 | N | N | Chemical burn |
| 4 | 49 | F | 1 | N | 0 | 0 | N | N | — |
| 5 | 51 | M | 3 | N | 0 | 1 | N | N | Scleral melt |
| 6 | 52 | M | 2 | Y | 2 | 1 | Y | N | Pterygium |
| 7 | 60 | F | 0 | N | 0 | 0 | Y | Y | — |
| 8 | 61 | F | 0 | N | 0 | 0 | Y | N | — |
| 9 | 65 | M | 1 | Y | 0 | 1 | Y | N | — |
| 10 | 67 | M | 2 | Y | 2 | 0 | N | N | Pterygium |
| 11 | 67 | M | 5 | N | 2 | 1 | N | Y | Floppy eye lids |
| 12 | 69 | M | 10 | Y | 2 | 0 | Y | N | Floppy eye lids |
| 13 | 75 | M | 44 | Y | 2 | 2 | N | N | — |
| 14 | 75 | M | 0 | Y | 1 | 2 | Y | Y | — |
| 15 | 77 | M | 4 | Y | 0 | 0 | N | N | — |
| 16 | 77 | M | 2 | N | 0 | 0 | N | N | — |
| 17 | 78 | M | 11 | Y | 1 | 1 | N | Y | Floppy eye lids |
| 18 | 80 | M | 8/1 | Y | 1 | 0 | N | Y | — |
| 19 | 82 | M | 0 | N | 1 | 0 | N | N | — |
| 20 | 85 | M | 12 | Y | 0 | 1 | N | N | — |
| 21 | 90 | F | 11 | Y | 1 | 0 | N | Y | Floppy eye lids |
| Group II. Negative serum immunoreactivity | |||||||||
| 1 | 17 | M | 4 | Y | 0 | 0 | N | N | — |
| 2 | 26 | F | 4 | N | 1 | 1 | N | N | Pterygium |
| 3 | 27 | M | 0 | N | 0 | 1 | Y | N | Floppy eye lids, allergic conjunctivitis |
| 4 | 28 | F | 3 | N | 0 | 1 | N | N | Pterygium |
| 5 | 31 | M | 0 | N | 0 | 1 | N | N | — |
| 6 | 35 | M | 0 | Y | 0 | 1 | N | N | Floppy eye lids, pterygium |
| 7 | 41 | F | 7 | N | 0 | 1 | Y | N | — |
| 8 | 43 | M | 12 | Y | 2 | 1 | N | N | Pterygium |
| 9 | 47 | M | 2 | N | 0 | 2 | Y | N | Floppy eye lids |
| 10 | 48 | M | 2 | Y | 0 | 0 | N | N | — |
| 11 | 49 | F | 0 | N | 0 | 0 | N | Y | Allergic conjunctivitis |
| 12 | 49 | F | 0 | N | 1 | 0 | N | N | Superior limbic keratoconjunctivitis |
| 13 | 53 | F | 0 | Y | 1 | 1 | N | N | — |
| 14 | 56 | M | 0 | N | 0 | 0 | Y | N | Pterygium |
| 15 | 56 | M | 0 | N | 1 | 2 | Y | N | Chemical burn |
| 16 | 58 | M | 12/9 | Y | 1 | 0 | Y | N | Chemical burn |
| 17 | 59 | F | 1 | N | 1 | 0 | N | N | — |
| 18 | 60 | F | 1 | N | 0 | 0 | N | Y | — |
| 19 | 62 | F | 14 | Y | 0 | 0 | Y | N | — |
| 20 | 62 | F | 1 | N | 0 | 0 | Y | N | — |
| 21 | 65 | F | 0 | N | 0 | 1 | N | N | Membranous cicatricial pemphigoid |
| 22 | 65 | F | 0 | Y | 0 | 0 | N | N | — |
| 23 | 66 | F | 4 | N | 1 | 1 | N | Y | — |
| 24 | 66 | F | 0 | N | 1 | 1 | N | N | — |
| 25 | 67 | M | 5 | Y | 1 | 1 | Y | Y | Floppy eye lids |
| 26 | 67 | F | 4 | N | 0 | 0 | Y | N | Pterygium |
| 27 | 67 | M | 0 | N | 0 | 0 | N | Y | — |
| 28 | 68 | M | 5 | N | 0 | 2 | N | N | — |
| 29 | 68 | M | 10 | Y | 0 | 0 | N | Y | — |
| 30 | 71 | M | 7 | N | 2 | 1 | Y | N | Conjunctival papilloma |
| 31 | 72 | M | 3/1 | N | 1 | 0 | N | Y | — |
| 32 | 72 | F | 5 | N | 0 | 0 | Y | Y | Pterygium |
| 33 | 72 | F | 0 | N | 0 | 0 | N | N | — |
| 34 | 75 | F | 0 | Y | 1 | 0 | N | N | Floppy eye lids |
| 35 | 76 | F | 0 | Y | 1 | 0 | N | N | — |
| 36 | 77 | F | 0 | N | 0 | 0 | Y | Y | — |
| 37 | 87 | F | 0 | N | 0 | 1 | N | N | — |
| 38 | 93 | M | 3 | N | 0 | 0 | N | Y | — |
Cch = conjunctivochalasis; F = female; M = male; N = no; Y = yes.
A total Demodex count is reported. For those with D brevis, its count is reported after/if detected.
Clinical Grading
Clinical grading of facial rosacea, lid margin inflammation, and inferior bulbar conjunctival inflammation was performed by 2 independent masked readers (HS and VKR). Both mite counting and clinical grading were performed by examiners who had no knowledge about each patient's clinical information and serum immunoreactivity. Standard photographs for grading these three lesions were prepared by JL and SCGT. Facial rosacea was graded according to erythema, flushing, telangiectasia, dome-shaped erythematous papules, pustules in a nasal or central-facial distribution, and thickened skin with prominent pores (Fig 1). Lid margin inflammation was graded according to the presence and extent of blood vessels and redness, and scored as 0 for none or trace, 1 for mild, and 2 for severe (Fig 2, left). Because inflammation of the bulbar conjunctiva was not uniformly distributed in all 4 quadrants, the inferior bulbar conjunctiva was chosen and scored in a similar fashion as 0, 1, and 2 (Fig 2, right). After reviewing these standard photographs, the 2 masked readers were asked to grade photographs of all patients that were labeled only by an enrolled case serial number. Photographs of all 4 lids were reviewed at once and the score of the most severe grade among all 4 lids was chosen for comparison. Any inconsistent grading between the 2 masked readers was arbitrated by SCGT, who had no knowledge about the result of each patient's serum immunoreactivity at the time.
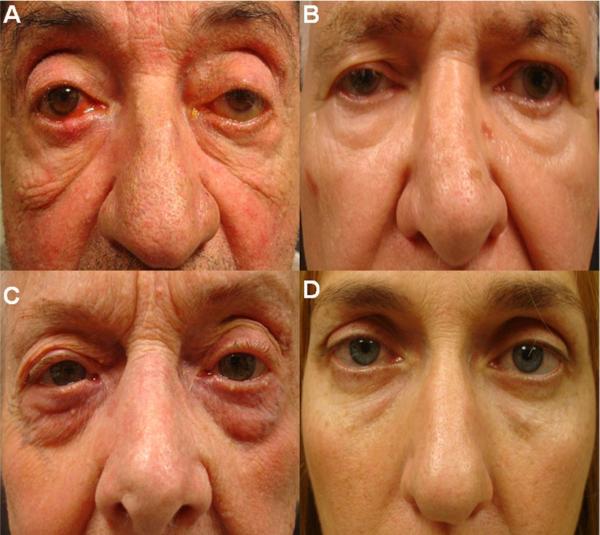
Figure 1.
Standard face photographs of rosacea (A, C) and normal subjects (B, D) of men (A, B) and women (C, D).
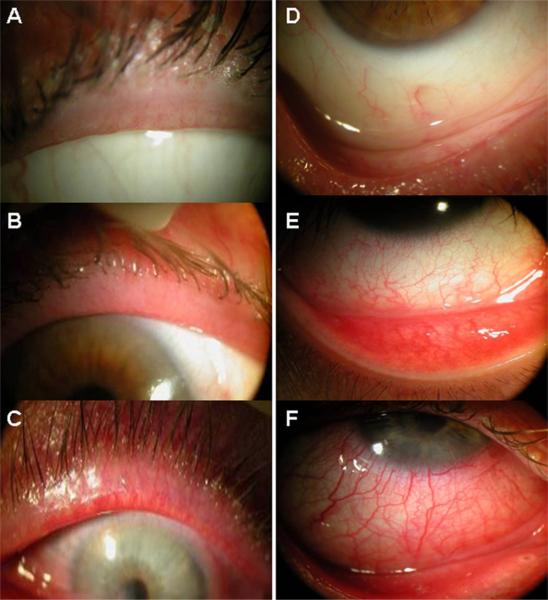
Figure 2.
Standard external photographs of eyelids (left column) and inferior bulbar conjunctiva (right column). Inflammation of the lid margin and the inferior bulbar conjunctiva is graded as 0 for no (A) to trace (D), as 1 for mild (B, E), and 2 for severe (C, F).
Lash Sampling and Demodex Counting
Demodex mites were counted by microscopic examination of epilated lashes as previously reported3 with recent modification.4 Briefly, 2 lashes with CD per lid were removed by fine forceps under slit-lamp examination. Under a light microscope, 1 drop of saline was applied by a pipette to the edge of the coverslip for lashes without retained CD, and 1 drop of fluorescein solution was added for those with retained CD to allow embedded Demodex to migrate out.4 The total number of mites was used for comparison. In addition, D brevis was recorded separately.
Extraction of Bacterial Antigens
The Nutrient Broth (Oxoid) in the volume of 250 ml, pH 7, was inoculated with a loopful of B oleronius and incubated for 48 hours at 30°C and 200 rpm. Late stationary phase cells were sedimented by centrifugation at 4000×g for 20 minutes (Beckman GS-6). The supernatant was discarded and cells were washed twice with phosphate-buffered saline (pH 7.2). Cells were then resuspended in 0.2% Triton-X 100 in Lamberts Breaks buffer (pH 7) with added protease inhibitors (10 μg/ml each of leupeptin, pepstatin A, aprotonin, and tosyl lysyl chlormethyl ketone). The suspension was inverted for 1 hour at 4°C before sonication at 20% power for three 10-second blasts using a soniprobe sonicator (HD 2200; Bandelin Sonopuls, Berlin, Germany). The supernatant was centrifuged at 6000×g at 4°C for 2 minutes to separate the protein preparation. Protein concentration was assessed by Bradford assay and if necessary was precipitated with 3 times the volume of ice cold acetone.
Western Blot Analysis of Each Patient's Serum
The Western blot was based on 12.5% (wt/vol) sodium dodecyl sulphate-polyacrylamide gel electrophoresis performed in a discontinuous buffer system, in which each well was loaded with 20-μL samples, which consisted of 20 μgof B oleronius protein extract. For each patient, a total of 5 ml of peripheral blood was drawn and centrifuged at 3500×g for 10 minutes. The serum was transferred to a sterile, 7-ml bottle under a lamellar flow hood, labeled with a case serial number and the sampling date, and stored in a −80°C freezer. After collection, a number of serum samples were shipped from Miami to Ireland, where each serum sample was diluted 1/150 (V/V) with the antibody-diluting buffer (50 mmol/L Tris-HCl, 150 mmol/l NaCl containing 0.05% Tween-20, 1% bovine serum albumin, and 3% nonfat dried milk) and used as the primary antibody. The secondary antibody was anti-human immunoglobulin G-horseradish peroxidase-linked whole antibody (Sigma Aldrich Chemical Co. Ltd, Poole, UK), which was diluted 1/1000 with the antibody-diluting buffer. The immunoreactive protein bands were visualized by incubating for 10 minutes the membranes in 10 mg of diaminobenzidine tetrahydrochloride in 15 ml of 100 mmol/l Tris-HCl (pH 7) containing 10 μl of hydrogen peroxide before washing in distilled water and drying. Standard positive and negative controls of immunoreactive bands to either 62-kDa and 83-kDa proteins are demonstrated in Figure 3. Densitometry was performed using a Typhoon computer package with Quant-L imaging system to quantify the intensity of these 2 protein bands, and a value < 10 000–12 000 for the 62-kDa band or <10 000 for the 83-kDa protein band was considered positive.
Figure 3.
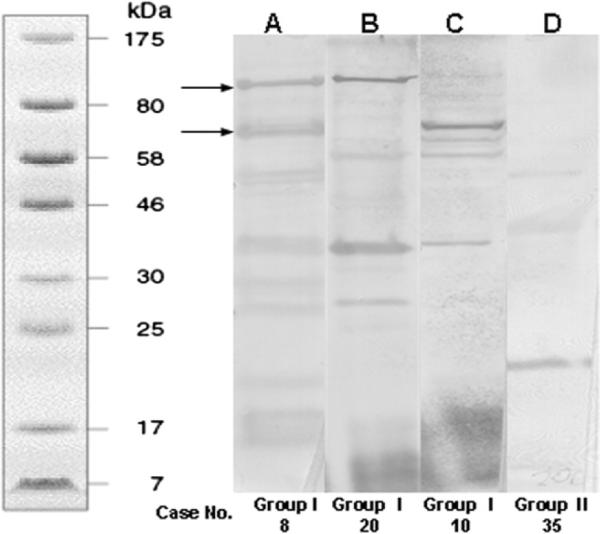
Western blot analyses. Representative examples of (A) positive serum immunoreactivity to both 83-kDa and 62-kDa protein bands (marked by arrows; from group I case no. 8). B, Positive to the 83-kDa protein band (from group I, case no. 20). C, Positive to the 62-kDa protein band (from group I case no. 10). D, Negative to both protein bands (from group II, case no. 35). The left lane shows the molecular weight standard.
Statistical Analysis
Numerical data were reported as the mean values ± SD, and nonnumerical data were recorded as the presence (yes) or absence (no). We used the independent samples t-test to determine age matching and to compare the Demodex counts between 2 groups. We used the Pearson chi-square analysis to determine gender matching and the correlation of any variable between 2 groups. We used Pearson bivariate correlation to determine whether the Demodex counts were influenced by age in different groups. All these statistical analyses were performed using SPSS software version 11.5 (SPSS, Inc., Chicago, IL), and reported as 2-tailed probabilities, with P<0.05 considered significant.
Results
The 59 patients consisted of 34 men and 25 women with a mean age of 60.4±17.6 years (range, 17–93). Their age distribution was not different when they were subdivided into 2 groups according to the presence or absence of serum immunoreactivity, ocular Demodex infestation, facial rosacea, or aqueous tear-deficiency dry eye (P = 0.151, 0.805, 0.868, and 0.706, respectively; Table 2). When the Demodex counts were analyzed, we did not note any significant correlation in the age distribution regardless of whether it was analyzed as a whole group (P=0.153), with or without serum immunoreactivity (P=0.159 and 0.847, respectively), or with or without facial rosacea (P=0.163 and 0.988, respectively). Although there was a trend toward older ages having higher Demodex counts in patients with ocular Demodex infestation (P=0.099), such a difference did not reach significance.
Table 2.
Summary of Statistical Analyses
| No. | Analysis | Statistical Methods | P Value |
|---|---|---|---|
| I. Age-matched with or without | |||
| 1 | Serum immunoreactivity | Independent-samples t-test | 0.151 |
| 2 | Ocular Demodex infestation | Independent-samples t-test | 0.805 |
| 3 | Facial rosacea | Independent-samples t-test | 0.868 |
| 4 | Dry eye | Independent-samples t-test | 0.706 |
| II. Age correlation between Demodex counts in | |||
| 5 | All patients | Pearson bivariate correlation | 0.153 |
| 6 | Positive serum immunoreactivity | Pearson bivariate correlation | 0.159 |
| 7 | Negative serum immunoreactivity | Pearson bivariate correlation | 0.847 |
| 8 | Ocular Demodex infestation | Pearson bivariate correlation | 0.099 |
| 9 | Negative facial rosacea | Pearson bivariate correlation | 0.163 |
| 10 | Negative facial rosacea | Pearson bivariate correlation | 0.988 |
| III. Gender-matched with or without | |||
| 11 | Serum immunoreactivity | Pearson chi-square analysis | 0.012 |
| 12 | Ocular Demodex infestation | Pearson chi-square analysis | 0.024 |
| 13 | Facial rosacea | Pearson chi-square analysis | 0.008 |
| 14 | Dry eye | Pearson chi-square analysis | 0.977 |
| IV. Serum immunoreactivity correlation with | |||
| 15 | Ocular Demodex infestation | Pearson chi-square analysis | 0.048 |
| 16 | Facial rosacea | Pearson chi-square analysis | 0.009 |
| 17 | Lid margin inflammation | Pearson chi-square analysis | 0.040 |
| 18 | Inferior bulbar conjunctival inflammation | Pearson chi-square analysis | 0.573 |
| V. Ocular Demodex infestation/counts correlation with | |||
| 19 | Facial rosacea | Pearson chi-square analysis | 0.075 |
| 20 | Lid margin inflammation | Pearson chi-square analysis | 0.073 |
| 21 | Inferior bulbar conjunctival inflammation | Pearson chi-square analysis | 0.599 |
| 22 | Counts with serum immunoreactivity | Independent-samples t-test | 0.014 |
| 23 | Counts with facial rosacea | Independent-samples t-test | 0.014 |
| VI. Other correlations | |||
| 24 | Dry Eye vs serum immunoreactivity | Pearson chi-square analysis | 0.657 |
| 25 | Dry Eye vs ocular Demodex infestation | Pearson chi-square analysis | 0.002 |
| 26 | Dry Eye vs facial rosacea | Pearson chi-square analysis | 0.361 |
| 27 | Facial rosacea vs lid margin inflammation | Pearson chi-square analysis | 0.016 |
| 28 | Facial rosacea vs inferior bulbar conjunctival inflammation | Pearson chi-square analysis | 0.728 |
| 29 | Lid margin inflammation vs inferior bulbar conjunctival inflammation | Pearson chi-square analysis | 0.162 |
| 30 | Cch vs serum immunoreactivity | Pearson chi-square analysis | 0.852 |
| 31 | Cch vs ocular Demodex infestation | Pearson chi-square analysis | 0.425 |
| 32 | Cch vs facial rosacea | Pearson chi-square analysis | 0.535 |
Cch = conjunctivochalasis.
Regarding the gender, there was no difference in these 59 patients with or without aqueous tear deficiency (P=0.977). However, there was a significant difference between 2 groups with or without serum immunoreactivity, ocular Demodex infestation, or facial rosacea (P=0.012, 0.024, and 0.008, respectively). Specifically, more men were found in the group with positive serum immunoreactivity or facial rosacea, but more women were in the group without ocular Demodex infestation. Such a gender difference suggested that higher ocular Demodex infestation was correlated with higher serum immunoreactivity and facial rosacea in male patients.
Clinical Grading
After reviewing the standard photographs illustrated in Figures 1 and 2, the 2 masked readers had no difficulty in grading coded photographs in all 59 patients without knowing patients' clinical information and serum immunoreactivity. The agreement rate between them reached 47/59 (80.0%), 44/59 (74.6%), and 46/59 (78.0%) in grading facial rosacea, lid margin inflammation, and inferior bulbar inflammation, respectively. For those patients presented with discrepant grading, the final score was arbitrated by the third reader.
Correlative Analyses
Table 1 (available online at http://aaojournal.org) lists the final grading of all relevant clinical information of 59 patients, who were subdivided into 2 groups according to positive (n = 21) or negative (n = 38) serum immunoreactivity. Further comparison of these 2 groups showed that positive serum immunoreactivity was significantly correlated with ocular Demodex infestation (P=0.048), facial rosacea (P=0.009), and lid margin inflammation (P = 0.040), but not with inferior bulbar conjunctival inflammation (P=0.573).
When these 59 patients were subdivided into 2 groups with (n = 38) or without (n = 21) ocular Demodex infestation, our analyses showed a significant correlation between the presence of ocular Demodex infestation and serum immunoreactivity (P=0.048). However, ocular Demodex infestation only had a borderline but not significant correlation with facial rosacea (P=0.075) and lid margin inflammation (P=0.073), but had no correlation with inferior bulbar conjunctival inflammation (P=0.599). The Demodex count in patients with facial rosacea (6.6±9.0) was significantly higher than that in patients without (1.9±2.2; P=0.014). However, the Demodex count was not different between the 2 groups with or without positive serum immunoreactivity (P=0.178). Consistent with what has previously been reported,3,6,7 D folliculorum was the main species found in epilated lashes, and was detected in 37 of 38 patients with positive ocular Demodex infestation. In contrast, D brevis was found in 4 of 38 patients (Table 1, available online at http://aaojournal.org; group I, case nos. 1 and 18 and group II, case nos. 16 and 31). Although the detection of D brevis was not correlated with serum immunoreactivity, 3 of 4 such patients also had facial rosacea.
Other analyses showed a significant correlation of facial rosacea with lid margin inflammation (P=0.016), but not with inferior bulbar conjunctival inflammation (P=0.728). As a result, there was also no correlation between lid margin inflammation and inferior bulbar conjunctival inflammation (P=0.162). Aqueous tear-deficiency dry eye, defined by the fluorescein clearance test, was detected in 19 of 59 (32%) patients. Interestingly, ocular Demodex infestation was more commonly detected in patients without aqueous tear-deficiency dry eye than in patients with (57.1% [12/21] vs 18.4% [7/38]; P=0.002). There is no difference in age (P=0.706) or gender (P=0.977) in patients with or without aqueous tear-deficiency. In contrast, the prevalence of aqueous tear-deficiency dry eye was not different between the 2 groups of patients with or without serum immunoreactivity (P=0.657) or with or without facial rosacea (P=0.361). Conjunctivochalasis, which generates similar dry eye complaints and can clinically be differentiated from aqueous tear-deficiency dry eye,27 was found in 16 of 59 (27%) patients. The occurrence of conjunctivochalasis was also not different between 2 groups with or without serum immunoreactivity (P=0.852), ocular Demodex infestation (P = 0.425), or facial rosacea (P=0.535). These 59 patients also had other types of ocular surface diseases including floppy eyelids (n = 9), pterygium (n = 9), chemical burns (n = 3), allergic conjunctivitis (n = 2), scleral melt (n = 1), superior limbic keratoconjunctivitis (n = 1), conjunctival papilloma (n = 1), and scleral melt (n = 1; Table 1, available online at http://aaojournal.org). Their occurrences did not correlate with any of these analyses.
Representative Case Report
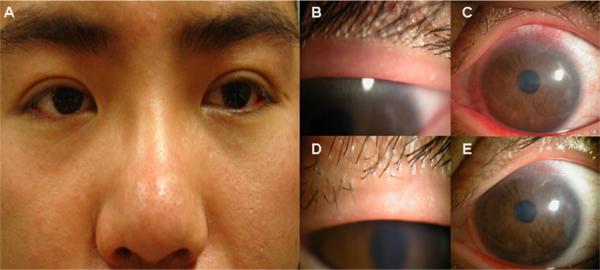
A 24-year-old man (group I, case no. 1) had complained of redness and blurred vision, more so in the left eye than the right, since December 2006. Previously, he had worn monthly contact lenses for 2 years without any problems. From February 2007, the left cornea developed lesions prompting the suspicion of bacterial and viral infection. These problems persisted despite discontinuation of contact lens wear, topical and systemic steroids, antibiotics, and antiviral medication. Because of the progressive worsening of his vision, superficial keratectomy and amniotic membrane transplantation were performed in a foreign country without success. External examination showed mild rosacea in the face (Fig 4A). His best corrected visual acuity was 20/20 in the right eye and 20/30 in the left eye. Lashes were largely clean without CD. The Demodex count was zero per 8 lashes randomly sampled from both eyes. Lid margin inflammation (graded as 1) and meibomian gland dysfunction evidenced by oil plugging of the orifice were noted more in the left eye (Fig 4B). The left cornea showed prominent, superficial neovascularization in the superior aspect and superficial haze in the central region (Fig 4C). Furthermore, perilimbal (Fig 4C) and conjunctival inflammation (not shown), of which the latter was graded as 1 in the inferior bulbar area, was also noted more in the left eye. Because his condition did not improve despite mechanical cannulization of the meibomian gland orifice and hot compresses, sampling of 16 lashes was repeated 5 months later and revealed 1 D brevis. The Western blot analysis of his serum performed then showed strong positive immunoreactivity to both 62-kDa and 83-kDa protein bands. A further inquiry of his history revealed that he slept with a dog at night, and exhibited the strongest 4+ reaction to dust mites among all allergens examined by the skin test. Because of the refractory nature, he started lid scrub with 50% TTO solution every other day and lid massage with 5% TTO ointment twice a day. Three months later, his visual acuity improved from 20/30 to 20/25 in the left eye; lid margin inflammation (Fig 4D) as well as perilimbal conjunctival inflammation and superficial corneal haze were notably reduced (Fig 4E).
Figure 4.
Representative case (group I, no. 1). A 24 year-old man presented with mild facial rosacea (A), grade 1 lid margin inflammation with meibomian gland dysfunction (B), and superior corneal neovascularization, haze, and perilimbal injection (C). Three months after lid scrub with tea tree oil, there was notable reduction of lid margin inflammation (D), perilimbal injection, and corneal haze (E).
Discussion
Lacey et al23 first linked skin mite infestation and microbial infection to explain why cutaneous inflammation occurs in facial rosacea. Specifically, their experimental evidence supports the notion that cutaneous inflammation might be exacerbated by strong host immune responses to proteins produced by B oleronius living inside Demodex mites. Herein, our collaborative study further established the comorbidity between Demodex infestation and Bacillus infection in facial rosacea. This finding allows a better understanding of how ocular surface inflammation might occur in some patients inflicted with ocular Demodex infestation.
Their previous study demonstrated 1 host immune response is positive immunoreactivity to Bacillus 62-kDa and 83-kDa proteins that can be demonstrated by Western blot analysis using pooled patient sera.23 In the present correlative study, we looked into such immunoreactivity in each individual patient serum. Furthermore, we measured Demodex infestation by sampling eyelashes using our improved counting method.3,4 For the first time, our comprehensive analyses disclosed a significant correlation among serum immunoreactivity to Bacillus antigens, ocular Demodex infestation, and facial rosacea. In a total of 59 patients prospectively and consecutively enrolled, positive serum immunoreactivity had a significant correlation with facial rosacea (P=0.009) and ocular Demodex infestation (P=0.048). Patients with facial rosacea had a significantly higher Demodex count than those without (P=0.014). Our correlative analyses were age matched, but not gender matched. The lack of gender matching could be explained in part by the finding that such correlations among positive serum immunoreactivity, facial rosacea, and ocular Demodex infestation were greater in male patients.
The strong correlation between serum immunoreactivity and ocular Demodex infestation was linked not only to facial rosacea, but also ocular surface inflammation. Specifically, we noted that lid margin inflammation had a significant correlation with facial rosacea (P=0.016) and positive serum immunoreactivity (P=0.040) and a borderline correlation with ocular Demodex infestation (P=0.075). This correlative result bodes well with a known fact that patients with facial rosacea frequently manifested blepharitis. However, we did not observe similar significant correlations with inferior bulbar conjunctival inflammation (Table 2). Of course, the extent of conjunctival inflammation in our patients might have been influenced by several factors such as the severity of facial rosacea and the association with other ocular surface diseases. Another important consideration was that the host inflammatory response in the conjunctiva might be much less as it is further away from the site of mite infestation, namely, hair/lash follicles and glands. Along this vein of reasoning, it was worth noting that the rate of detecting D brevis in 4 of 59 patients was notably lower than the 3 of 6 patients reported to have developed conjunctivitis and keratitis.7 Because D brevis primarily resides in meibomian glands, its detection would suggest the inflammation might more easily reach the conjunctiva and the cornea. This scenario was illustrated in the representative case report (Fig 4). In this representative case, we also learned that D brevis might require more lashes to be detected. Interestingly, ocular Demodex infestation was significantly less prevalent in patients with aqueous tear-deficiency dry eye (P=0.002), but not in patients with conjunctivochalasis (P=0.425). Future studies are needed to determine whether the tear-deficient state and treatment might interfere with ocular Demodex infestation. Collectively, such strong correlation reported herein suggests that a point-of-care diagnostic tool might be developed to detect patient's serum immunoreactivity.
If the strong correlation among serum immunoreactivity, ocular Demodex infestation, rosacea, and blepharitis were causative, we speculate a new pathogenic paradigm in linking both Demodex infestation and microbial infection by B oleronius in ocular surface inflammation. The comorbidity of both Demodex mites and B oleronius rests in the symbiosis of the latter in the mite, a scenario first reported in the hindgut of a termite.28 The final host inflammatory response may be affected by a combination of factors such as whether such symbiosis exists in ocular mite infestation, whether there is a sufficient load of released 83-kDa and 62-kDa bacterial antigens, to which host elicits a proinflammatory response, and whether the host has an increasing susceptibility to elicit such a response. For example, as shown in the representative case, the host immune-inflammatory response might be modified and exaggerated because of being allergic to dust mites. In this study, we cannot rule out the role of other microbes that may have been colonized in the lid because of mites as the vector. Because ocular surface inflammation is notably reduced by lid scrub with TTO, but not by conventional treatments including lid hygiene with baby shampoo, topical steroids, and antibiotics or systemic doxycycline in some rosacea patients that present with corneal lesions,7 the mite's role in stirring up host inflammatory response cannot be ruled out. In short, the comorbidity based on a symbiotic relationship of B oleronius in Demodex mites also justifies the consideration of a therapeutic strategy directed to killing the symbiotic bacterium via oral antibiotics such as tetracycline and to killing and preventing mating/reinfestation of Demodex mites, for example, lid scrub with TTO and general hygiene at the same time. Future investigation into this comorbidity between mites and microbes may shed new light not only on the understanding of the pathogenesis of this centuries-old common ailment of the skin and eye, but also other similar unresolved human diseases.
References
- 1.Baima B, Sticherling M. Demodicidosis revisited. Acta Derm Venereol. 2002;82:3–6. doi: 10.1080/000155502753600795. [DOI] [PubMed] [Google Scholar]
- 2.Forton F, Germaux MA, Brasseur T, et al. Demodicosis and rosacea: epidemiology and significance in daily dermatologic practice. J Am Acad Dermatol. 2005;52:74–87. doi: 10.1016/j.jaad.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 3.Gao YY, Di Pascuale MA, Li W, et al. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005;46:3089–94. doi: 10.1167/iovs.05-0275. [DOI] [PubMed] [Google Scholar]
- 4.Kheirkhah A, Blanco G, Casas V, Tseng SC. Fluorescein dye improves microscopic evaluation and counting of Demodex in blepharitis with cylindrical dandruff. Cornea. 2007;26:697–700. doi: 10.1097/ICO.0b013e31805b7eaf. [DOI] [PubMed] [Google Scholar]
- 5.Gao YY, Di Pascuale MA, Li W, et al. In vitro and in vivo killing of ocular Demodex by tea tree oil. Br J Ophthalmol. 2005;89:1468–73. doi: 10.1136/bjo.2005.072363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao YY, Di Pascuale MA, Elizondo A, Tseng SC. Clinical treatment of ocular demodecosis by lid scrub with tea tree oil. Cornea. 2007;26:136–43. doi: 10.1097/01.ico.0000244870.62384.79. [DOI] [PubMed] [Google Scholar]
- 7.Kheirkhah A, Casas V, Li W, et al. Corneal manifestations of ocular Demodex infestation. Am J Ophthalmol. 2007;143:743–9. doi: 10.1016/j.ajo.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 8.Messager S, Hammer KA, Carson CF, Riley TV. Assessment of the antibacterial activity of tea tree oil using the European EN 1276 and EN 12054 standard suspension tests. J Hosp Infect. 2005;59:113–25. doi: 10.1016/j.jhin.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Edwards-Jones V, Buck R, Shawcross SG, et al. The effect of essential oils on methicillin-resistant Staphylococcus aureus using a dressing model. Burns. 2004;30:772–7. doi: 10.1016/j.burns.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Halcon L, Milkus K. Staphylococcus aureus and wounds: a review of tea tree oil as a promising antimicrobial. Am J Infect Control. 2004;32:402–8. doi: 10.1016/S0196655304003657. [DOI] [PubMed] [Google Scholar]
- 11.Oliva B, Piccirilli E, Ceddia T, et al. Antimycotic activity of Melaleuca alternifolia essential oil and its major components. Lett Appl Microbiol. 2003;37:185–7. doi: 10.1046/j.1472-765x.2003.01375.x. [DOI] [PubMed] [Google Scholar]
- 12.Gupta AK, Nicol K, Batra R. Role of antifungal agents in the treatment of seborrheic dermatitis. Am J Clin Dermatol. 2004;5:417–22. doi: 10.2165/00128071-200405060-00006. [DOI] [PubMed] [Google Scholar]
- 13.Hammer KA, Carson CF, Riley TV. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J Antimicrob Chemother. 2004;53:1081–5. doi: 10.1093/jac/dkh243. [DOI] [PubMed] [Google Scholar]
- 14.Caldefie-Chezet F, Guerry M, Chalchat JC, et al. Anti-inflammatory effects of Melaleuca alternifolia essential oil on human polymorphonuclear neutrophils and monocytes. Free Radic Res. 2004;38:805–11. doi: 10.1080/1071576042000220247. [DOI] [PubMed] [Google Scholar]
- 15.Carson CF, Riley TV. Safety, efficacy and provenance of tea tree (Melaleuca alternifolia) oil. Contact Dermatitis. 2001;45:65–7. doi: 10.1034/j.1600-0536.2001.045002065.x. [DOI] [PubMed] [Google Scholar]
- 16.Powell FC. Clinical practice. Rosacea. N Engl J Med. 2005;352:793–803. doi: 10.1056/NEJMcp042829. [DOI] [PubMed] [Google Scholar]
- 17.Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584–7. doi: 10.1067/mjd.2002.120625. [DOI] [PubMed] [Google Scholar]
- 18.Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907–12. doi: 10.1016/j.jaad.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 19.Powell FC. What's going on in rosacea? J Eur Acad Dermatol Venereol. 2000;14:351–2. doi: 10.1046/j.1468-3083.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- 20.Aroni K, Tsagroni E, Lazaris AC, et al. Rosacea: a clinicopathological approach. Dermatology. 2004;209:177–82. doi: 10.1159/000079886. [DOI] [PubMed] [Google Scholar]
- 21.Bonnar E, Eustace P, Powell FC. The Demodex mite population in rosacea. J Am Acad Dermatol. 1993;28:443–8. doi: 10.1016/0190-9622(93)70065-2. [DOI] [PubMed] [Google Scholar]
- 22.Forton F, Seys B. Density of Demodex folliculorum in rosacea: a case-control study using standardized skin-surface biopsy. Br J Dermatol. 1993;128:650–9. doi: 10.1111/j.1365-2133.1993.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 23.Lacey N, Delaney S, Kavanagh K, Powell FC. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007;157:474–81. doi: 10.1111/j.1365-2133.2007.08028.x. [DOI] [PubMed] [Google Scholar]
- 24.Prabhasawat P, Tseng SC. Frequent association of delayed tear clearance in ocular irritation. Br J Ophthalmol. 1998;82:666–75. doi: 10.1136/bjo.82.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afonso AA, Monroy D, Stern ME, et al. Correlation of tear fluorescein clearance and Schirmer test scores with ocular irritation symptoms. Ophthalmology. 1999;106:803–10. doi: 10.1016/S0161-6420(99)90170-7. [DOI] [PubMed] [Google Scholar]
- 26.Meller D, Tseng SC. Conjunctivochalasis: literature review and possible pathophysiology. Surv Ophthalmol. 1998;43:225–32. doi: 10.1016/s0039-6257(98)00037-x. [DOI] [PubMed] [Google Scholar]
- 27.Di Pascuale MA, Espana EM, Kawakita T, Tseng SC. Clinical characteristics of conjunctivochalasis with or without aqueous tear deficiency. Br J Ophthalmol. 2004;88:388–92. doi: 10.1136/bjo.2003.025460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhnigk T, Borst EM, Breunig A, et al. Bacillus oleronius sp.nov., a member of the hindgut flora of the termite Reticulitermes santonensis (Feytaud) Can J Microbiol. 1995;41:699–706. doi: 10.1139/m95-096. [DOI] [PubMed] [Google Scholar]