Abstract
Introduction
Monoclonal antibody (mAb) L8A4 binds specifically to the epidermal growth factor receptor variant III (EGFRvIII) that is present on gliomas but not normal tissues, and is internalized rapidly after receptor binding. Because of the short range of its β-emissions, labeling this mAb with177Lu would be an attractive approach for the treatment of residual tumor margins remaining after surgical debulking of brain tumors.
Materials and Methods
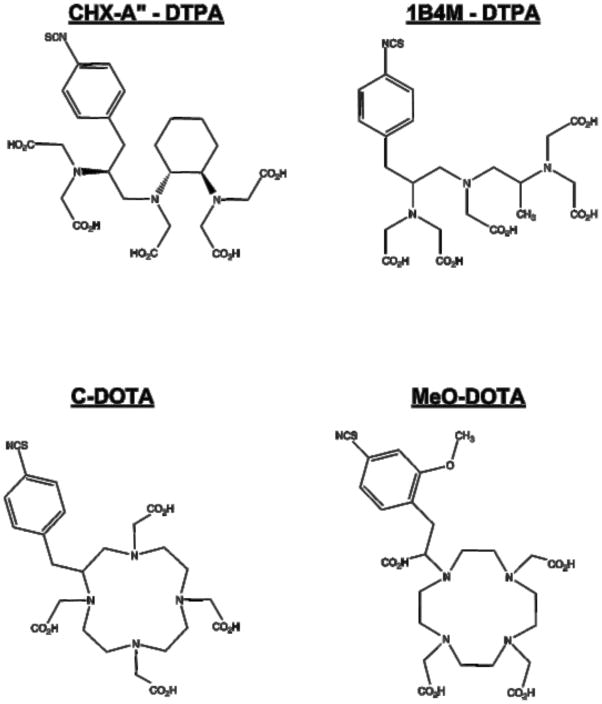
L8A4 mAb was labeled with 177Lu using the acyclic ligands [(R)-2-Amino-3-(4-isothiocyanatophenyl)propyl]-trans-(S,S)-cyclohexane-1,2-diamine- pentaacetic acid (CHX-A″-DTPA) and 2-(4-Isothiocyanatobenzyl)-6-methyldiethylene- triaminepentaacetic acid (1B4M-DTPA), and the macrocyclic ligands S-2-(4- Isothiocyanatobenzyl)-1,4,7,10-tetraazacyclododecane-tetraacetic acid (C-DOTA) and α-(5-isothiocyanato-2-methoxyphenyl)-1,4,7,10-tetraazacyclododecane-1,4,7,10- tetraacetic acid (MeO-DOTA). Paired-label tissue distribution experiments were performed in athymic mice bearing subcutaneous EGFRvIII-expressing U87.)EGFR glioma xenografts over a period of 1 to 8 days to directly compare 177Lu-labeled L8A4 to L8A4 labeled with 125I using N-succinimidyl 4-guanidinomethyl-3-[125I]iodobenzoate ([125I]SGMIB).
Results
Except with C-DOTA, tumor uptake for the 177Lu-labeled mAb was significantly higher than the co-administered radioiodinated preparation; however, this was also the case for spleen, liver, bone and kidneys. Tumor:normal tissue ratios for 177Lu-1B4M-DTPA-L8A4 and to an even greater extent, 177Lu-MeO-DOTA-L8A4, were higher than those for [125I]SGMIB-L8A4 in most other tissues.
Conclusions
Tumor and normal tissue distribution patterns for this anti-EGFRvIII mAb were dependent on the nature of the bifunctional chelate used for 177Lu labeling. Optimal results were obtained with 1B4M-DTPA and MeO-DOTA, suggesting no clear advantage for acyclic vs. macrocyclic ligands for this application.
Keywords: Lu-177, radioimmunotherapy, MeO-DOTA, 1B4M-DTPA
1. Introduction
Radiation and its utility in the destruction of malignant tissues has been recognized almost since its discovery [1]. Selective targeting is the paramount focus of the contemporary therapeutic application of radionuclides. This is achieved by chemically packaging the radionuclide in such a way that it can take advantage of tumor-specific characteristics, hopefully leading to localized radiation deposition within malignant cell populations with minimal exposure of healthy tissues [2, 3]. Much of this effort has involved monoclonal antibodies (mAbs), with 131I being the most frequently utilized radionuclide, due in part to its reasonable cost, the availability of well-established radioiodination protocols and emission of γ-rays that permit imaging to be performed [4- 8]. More recently, radiometalation has emerged as an attractive alternative for mAb labeling, exemplified by the FDA approval of 90Y-labeled ibritumomab tiuxetan (Zevalin→) for the treatment of patients with lymphoma [9].
Although 90Y remains attractive for some applications of targeted radiotherapy, its high energy β-particles are not well matched to the treatment of minimum residual disease, settings thought to be most conducive for this type of therapy. For this reason, 131I has been the radionuclide of choice for treatment of residual tumor cells remaining after surgical resection of brain tumors [10], despite the fact that its 364-keV γ-ray is not ideal for imaging, can increase radiation dose to normal brain, and can prolong patient confinement. Among the radiometals emitting low-energy β-particles, there are at least two attractive alternatives – 67Cu and 177Lu. While both present excellent characteristics for targeted radiotherapy, 177Lu offers a compelling practical advantage – availability on a routine basis. Furthermore, its half-life (6.7 days), Eβ(max) of 497 keV and average range in tissue (1.8 mm) are similar to those of 131I; however, the γ-rays emitted by 177Lu are considerably lower in energy (113, 208 keV) and abundance (7, 11%) compared with those of 131I. The γ-ray emission characteristics of 177Lu are more suitable for imaging and dosimetry determination; in addition, they simplify radiation protection for patients and personnel, and are more amenable for clinical use as an outpatient procedure.
A number of groups including our own have been investigating a variety of bifunctional chelates derived from the acyclic ligand DTPA and the macrocyclic ligand DOTA for labeling mAbs and peptides with 177Lu. As with many other radiometals, the primary prerequisite is the formation of complex with high thermodynamic stability and kinetic inertness under physiological conditions [11, 12] in order to minimize release and translocation of the free radiometal to the bone [13]. A second criterion is minimizing activity levels in organs such as the liver and spleen, which can be higher for mAbs labeled with 177Lu compared with those labeled using 131I [14, 15]. Based on these considerations, in a study comparing the mAb fragment HuCC49 CH2 labeled with 177Lu using three different chelating agents, CHX-A″-DTPA, and C-DOTA were reported to provide good stability and tissue distributions while PA-DOTA did not [16]. Our own work using both the murine and chimeric forms of the anti-tenascin mAb 81C6 demonstrated that the in vivo behavior of complexed 177Lu was more favorable with the MeO-DOTA macrocycle than either the NHS-DOTA macrocycle or the acyclic 1B4M- DTPA ligand [17].
For mAbs that undergo internalization after receptor binding and translocation from the cell surface to lysosomes, there is an additional criterion that must be considered – maximizing trapping of radiolabeled catabolites after proteolysis of the labeled mAb. While there is considerable evidence to suggest that this objective generally can be more readily accomplished with radiometals that with radiohalogens, the best bifunctional chelate for this purpose remains to be ascertained. As a first step, we performed a series of in vitro internalization and cellular processing assays with L8A4, a mAb that reacts with the rapidly internalizing epidermal growth factor receptor variant III, and labeled with177Lu using the acyclic ligands CHX-A″-DTPA, pSCN-Bz-DTPA, 1B4M- DTPA and the macrocyclic ligands C-DOTA and MeO-DOTA [18]. Chelate-dependent differences in the intracellular retention of 177Lu were observed, MeO-DOTA providing maximum internalized counts at early time points and the three DTPA-based ligands offering the highest values at later time points.
In the current study, we have evaluated the tissue distribution of the L8A4 mAb labeled with 177Lu using two acyclic and two macrocyclic ligands previously evaluated in the in vitro studies - CHX-A″-DTPA, C-DOTA, 1B4M-DTPA and MeO-DOTA (Figure 1). A series of four paired label studies were performed in athymic mice with subcutaneous EGFRvIII-expressing xenografts and injected with one of the 177Lu-labeled L8A4-chelate conjugates as well as with L8A4 labeled using N-succinimidyl 4-guanidinomethyl-3- [125I]iodobenzoate [19]. The goal of this study was to identify the best ligand for labeling L8A4 mAb with 177Lu to move forward for clinical evaluation as a targeted radiotherapeutic for glioma.
Figure 1.
Structures of the bifunctional chelators CHX-A″-DTPA, 1B4M-DTPA, C-DOTA and MeO-DOTA used in this study.
2. Materials and methods
All solvents were either ACS-certified or HPLC grade solvents obtained from Sigma- Aldrich and used as received. MeO-DOTA was generously donated by Dr. Keith Frank (IsoTherapeutics Group Angleton, TX) and 1B4M-DTPA was kindly supplied by Dr. Martin Brechbiel (National Cancer Institute, Bethesda, MD). CHX-A″-DTPA and C- DOTA were purchased from Macrocyclics (Dallas, Texas), and used as received. [177Lu]lutetium chloride was obtained from the University of Missouri-Columbia Research Reactor (Columbia, MO, USA) as a 0.05N HCl solution. Iodine-125 was purchased from Perkin-Elmer Life and Analytical Sciences (Boston, MA).
2.1 L8A4 mAb
EGFRvIII is a mutant form of the epidermal growth factor receptor that is characterized by the deletion of a segment of the external domain of wild-type EGFR consisting of amino acids 6 through 273 [20]. EGFRvIII is an attractive target for radioimmunotherapy because it is expressed on several types of human malignancies including glioma, head and neck cancer, breast and ovarian carcinoma, but not on normal tissues including the liver and others that express wild-type EGFR [21]. The development of the murine IgG1 anti-EGFRvIII mAb L8A4 have been described previously [22]. Briefly, mice were immunized with a synthetic peptide that mimicked the novel fusion junction characteristic of EGFRvIII followed several weeks later by inoculation with irradiated EGFRvIII-expressing transfected cells or cell membrane preparations. Purification, characterization, and documentation of the rapid internalization and intracellular degradation of L8A4 after binding to EGFRvIII- expressing cells, were carried out following previously described methods [23].
2.2 Conjugation of Chelates to mAb
Because of the propensity of these chelates to form inert complexes with endogenous metals, all buffers were prepared using procedures designed to minimize metal ion concentrations as described previously [18, 24]. The L8A4 mAb-chelate conjugates were prepared by incubation of 0.5–2.5 mg L8A4 with a 10–20 fold molar excess of the bifunctional chelate in 10–12 mL 1H pH 8.6 conjugation buffer [18] for 24 h at room temperature. The conjugate were then subjected to centrifugal filtration using a Centricon concentrator (Amicon) with a 10 kDa cutoff to remove free chelate. During this process, the conjugation buffer was gradually replaced by 1H NH4OAc (pH 7) buffer. The number of chelates per mAb was determined using a spectrophotometric assay based on the titration of a Pb(II)-Arsenazo(III) complex for MeO-DOTA and C-DOTA [25] and Y(III)-Arsenazo(III) complex for 1B4M-DTPA and CHX-A″-DTPA [26].
2.3 Radiolabeling of L8A4 with 177Lu and 125I
Lutetium-177 chloride (typically, 25 Ci/mg specific activity, 1.2 Ci/mL activity concentration) in 20μL 0.05 M HCl, was diluted with 80μL of 0.15 M NH4OAc (pH 7) buffer, and an aliquot equal to ~2 μCi/μg mAb was transferred to an Eppendorff vial for radiolabeling. The mAb-chelate conjugate (200–300 μg) in 150μL 0.15 M NH4OAc (pH 7) buffer was added to the 177Lu activity, buffered as described above, and incubated for 90 min at 25°C (room temperature). Incubation at higher temperatures and for longer periods did not significantly increase radiolabeling yields. The reaction was quenched with 3 μL of 0.15 M EDTA, and the labeled mAb was purified by passage through a Sephadex G25 PD-10 column (GE Health Care, Piscastawy, NJ).
The L8A4 mAb was labeled with 125I using the SGMIB method, a procedure that has been shown to increase retention of radioiodine in EGFRvIII-positive tumor cells after L8A4 internalization [19]. First, [125I]SGMIB was prepared from the corresponding tin precursor and then about 1 mCi of the radioiodinated active ester was reacted with L8A4 (typically 80 μL, 1.3 mg/mL), previously dialyzed into 0.1 M borate buffer pH 8.5, for 20 min at room temperature [27]. Iodine-125-labeled L8A4 was isolated using a Sephadex G25 PD-10 column.
2.4 Determination of immunoreactive fraction
The immunoreactivity of each radiolabeled L8A4 preparation was determined by a magnetic bead assay using beads coated with the extracellular domain of EGFRvIII or as a measure of nonspecific binding, BSA [28]. Assays were done in paired-label format to directly compare the 125I- and 177Lu-labeled L8A4 preparations administered in each paired-label biodistribution experiment.
2.5 Biodistribution measurements
Animal studies were performed under a protocol approved by the Duke University Institutional Animal Care and Use Committee. BALB/c nu/nu mice were obtained from a closed breeding colony maintained at the Duke University Cancer Center Isolation Facility. Subcutaneous xenografts were established from the U87MG. EGFR human glioma cell line [29] by injection of 50 μL of tumor homogeneate in the flank through a 16 gauge needle. Based on flow cytometry experiments, disaggregated U87MG. EGFR xenografts express a mean of 2.5 H 105 EGFRvIII receptors per cell [30]. Biodistribution measurements were initiated when xenograft volumes were 200–400 mm3.
In the four paired-label tissue distribution studies, mice were injected with either 177Lu-1B4M-DTPA-L8A4 (5 μCi, 1.8 μCi/μg), 177Lu-CHX-A″-DTPA-L8A4 (5 μCi, 2.5 μCi/μg), 177Lu-MeO-DOTA-L8A4 (5 μCi, 0.9 μCi/μg), or 177Lu-C-DOTA-L8A4 (6 μCi, 1.0 μCi/μg) in tandem with [125I]SGMIB-L8A4 (3–5 μCi, 0.7–3.2 μCi/μg). Groups of five animals were killed by isoflurane overdose at 1, 2, 3, 4, 6 and 8 days post-injection. The 3-day time point was not performed in the 177Lu-C-DOTA-L8A4 study. After dissection the tissues of interest were harvested, blot dried, and the samples were weighed and counted for both 177Lu and 125I activity using a dual-channel automated LKB 1282 gamma-counter (Turku, Finland) with decay and crossover correction capability. Tissue radioactivity levels were compared with injected dose (ID) standards, and the results were expressed as percentage of the injected dose per gram tissue (%ID/g). For each animal the 177Lu to 125I uptake ratio in tissues of interest also was determined. Differences in the radioactivity levels of two compounds administered simultaneously were analyzed for statistical significance using a paired t–test while an unpaired t–test was utilized to compare results from different experiments.
3. Results
3.1 Preparation of radiolabeled L8A4
Reaction of L8A4 with a 10–20:1 molar ratio of chelate to protein yielded an average number of chelates per mAb of 0.3 for CHX-A″-DTPA, 1.1 for 1B4M-DTPA 3.1 for C- DOTA and 2.7 for MeO-DOTA. Optimization of 177Lu labeling of these conjugates was not performed in this study; nonetheless, labeling yields between 73–86% were obtained for the four chelates. Immunoreactive fractions determined by the magnetic bead assay also were similar, ranging from 60% for 177Lu-CHX-A″-DTPA to 76% for 177Lu-1B4M- DTPA. The immunoreactive fractions of the [125I]SGMIB-L8A4 preparations used in the biodistribution experiments were in the same range, 60–71%. Prior to in vivo administration, all radiolabeled mAbs and mAb-chelate conjugates were analyzed by size-exclusion HPLC and demonstrated to elute as a single peak with a retention corresponding to that of unmodified L8A4 (data not shown).
3.2 Biodistribution of 177Lu- and 125I-labeled L8A4 in tumor bearing mice
A series of paired label tissue distribution experiments were performed in athymic mice bearing subcutaneous U87MG. EGFR human glioma xenografts. Our objective was to evaluate the potential utility of the two acyclic and two macrocyclic ligands for 177Lu labeling of the anti-EGFRvIII L8A4 mAb, using co-administered [125I]SGMIB-L8A4 as a common point of reference. The percent injected dose per gram (% ID/g) recovered from tumor and normal tissues 1, 4, and 8 days after injection of [125I]SGMIB- L8A4 and L8A4 labeled with 177Lu using 1B4M-DTPA, CHX-A″-DTPA, C-DOTA, and MeO-DOTA is summarized in Tables 1 through 4, respectively (Data for intermediate time points available upon request). With the exception of the study with C-DOTA, the % ID/g for the 177Lu-labeled mAb in tumor was significantly higher (p <0.05) than that for [125I]SGMIB-L8A4 at all time points.
Table 1.
Paired-label biodistribution of L8A4 labeled with 177Lu using 1B4M-DTPA and with 125I using SGMIB in athymic mice with subcutaneous U87MG. EGFR human glioma xenografts
| Nuclide | Tissue | Day 1 | Day 4 | Day 8 |
|---|---|---|---|---|
| 177Lu | Blood | 19.78 ± 2.94 | 10.75 ± 2.88 | 1.35 ± 0.94 |
| Liver | 8.36 ± 0.74 | 7.52 ± 2.18 | 4.16 ± 0.54 | |
| Spleen | 5.64 ± 1.13 | 4.62 ± 1.73 | 2.01 ± 1.01 | |
| Kidneys | 8.06 ± 1.03 | 5.34 ± 1.25 | 2.61 ± 0.32 | |
| Lung | 6.87 ± 1.61 | 6.00 ± 3.12 | 0.93 ± 0.45 | |
| Bone | 3.58 ± 1.44 | 2.44 ± 0.71 | 1.82 ± 0.52 | |
| Muscle | 1.64 ± 0.08 | 0.88 ± 0.22 | 0.30 ± 0.07 | |
| Brain | 0.58 ± 0.13 | 0.40 ± 0.12 | 0.07 ± 0.05 | |
| Thyroida | 0.50 ± 0.13 | 0.27 ± 0.07 | 0.11 ± 0.04 | |
| Tumor | 31.58 ± 7.24 | 29.24 ± 6.03 | 15.27 ± 5.16 | |
| 125I | Blood | 18.78 ± 2.24 | 10.64 ± 2.86 | 1.29 ± 0.87 |
| Liver | 5.11 ± 0.86 | 2.89 ± 0.75 | 0.42 ± 0.32 | |
| Spleen | 4.48 ± 0.72 | 2.26 ± 0.80 | 0.28 ± 0.19 | |
| Kidneys | 4.73 ± 0.68 | 2.74 ± 0.69 | 0.39 ± 0.29 | |
| Lung | 6.81 ± 1.40 | 4.28 ± 0.78 | 0.58 ± 0.41 | |
| Bone | 3.02 ± 0.99 | 1.53 ± 0.49 | 0.33 ± 0.18 | |
| Muscle | 1.66 ± 0.08 | 0.85 ± 0.19 | 0.15 ± 0.10 | |
| Brain | 0.55 ± 0.13 | 0.40 ± 0.10 | 0.05 ± 0.04 | |
| Thyroida | 0.37 ± 0.30 | 0.31 ± 0.07 | 0.02 ± 0.03 | |
| Tumor | 21.64 ± 4.53 | 9.60 ± 1.72 | 2.86 ± 0.98 |
Values presented as percent injected dose per gram tissue (% ID/g), mean ″ standard deviation; five mice per group.
Percent injected dose in the thyroid.
Table 4.
Paired-label biodistribution of L8A4 labeled with 177Lu using MeO-DOTA and with 125I using SGMIB in athymic mice with subcutaneous U87MG. EGFR human glioma xenografts
| Nuclide | Tissue | Day 1 | Day 4 | Day 8 |
|---|---|---|---|---|
| 177Lu | Blood | 15.35 ± 2.41 | 3.73 ± 2.88 | 0.70 ± 0.62 |
| Liver | 7.88± 1.56 | 4.67 ± 1.55 | 2.89 ± 0.71 | |
| Spleen | 4.53 ± 0.52 | 2.72 ± 1.00 | 1.44 ± 0.55 | |
| Kidneys | 7.04 ± 0.69 | 3.53 ± 0.88 | 2.34 ± 0.32 | |
| Lung | 5.01 ± 1.82 | 1.34 ± 0.73 | 0.43 ± 0.27 | |
| Bone | 2.75 ± 0.40 | 0.90 ± 0.39 | 0.82 ± 0.29 | |
| Muscle | 1.65 ± 0.35 | 0.46 ± 0.19 | 0.15 ± 0.08 | |
| Brain | 0.51 ± 0.12 | 0.11 ± 0.07 | 0.04 ± 0.03 | |
| Thyroida | 0.26 ± 0.13 | 0.06 ± 0.05 | 0.02 ± 0.01 | |
| Tumor | 38.77± 8.55 | 26.30 ± 9.68 | 10.37 ± 4.05 | |
| 125I | Blood | 15.62 ± 2.32 | 4.09 ± 3.22 | 0.81 ± 0.71 |
| Liver | 4.07 ± 0.50 | 0.98 ± 0.71 | 0.31 ± 0.26 | |
| Spleen | 2.74 ± 0.49 | 0.76 ± 0.54 | 0.22 ± 0.17 | |
| Kidneys | 3.74 ± 0.57 | 0.88 ± 0.55 | 0.25 ± 0.20 | |
| Lung | 5.16 ± 1.83 | 1.23 ± 0.80 | 0.34 ± 0.29 | |
| Bone | 2.61 ± 0.38 | 0.63 ± 0.38 | 0.26 ± 0.16 | |
| Muscle | 1.67 ± 0.40 | 0.41 ± 0.20 | 0.11 ± 0.08 | |
| Brain | 0.52 ± 0.11 | 0.11 ± 0.07 | 0.03 ± 0.03 | |
| Thyroida | 0.31 ± 0.13 | 0.12 ± 0.06 | 0.08 ± 0.02 | |
| Tumor | 25.19 ± 4.77 | 7.52 ± 3.66 | 2.22 ± 1.44 |
Values presented as percent injected dose per gram tissue (% ID/g), mean ″ standard deviation; five mice per group.
Percent injected dose in the thyroid.
Because 177Lu is a bone-seeking radiometal, it is important to minimize accumulation in this tissue in order to lessen radiotoxicity to proximal bone marrow. With regard to this criterion, we observed that the acyclic chelator CHX-A″-DTPA showed an increase in bone uptake over the course of the experiment starting from 5.95% ± 1.27% ID/g on Day 1 to 7.67% ± 1.71% ID/g on Day 8. Bone uptake values for mAb labeled via C- DOTA and 1B4M-DTPA were significantly lower (p <0.05) than those observed with CHX-A″-DTPA. By far the lowest levels of 177Lu bone-uptake were observed with MeO- DOTA, declining from 2.75% ± 0.40% ID/g on Day 1 to 0.8% ± 0.3% ID/g on Day 8. In other tissues, particularly the liver, spleen and kidneys, the four chelators showed a similar uptake pattern, declining by about a factor of two or three from Day 1 to Day 8.
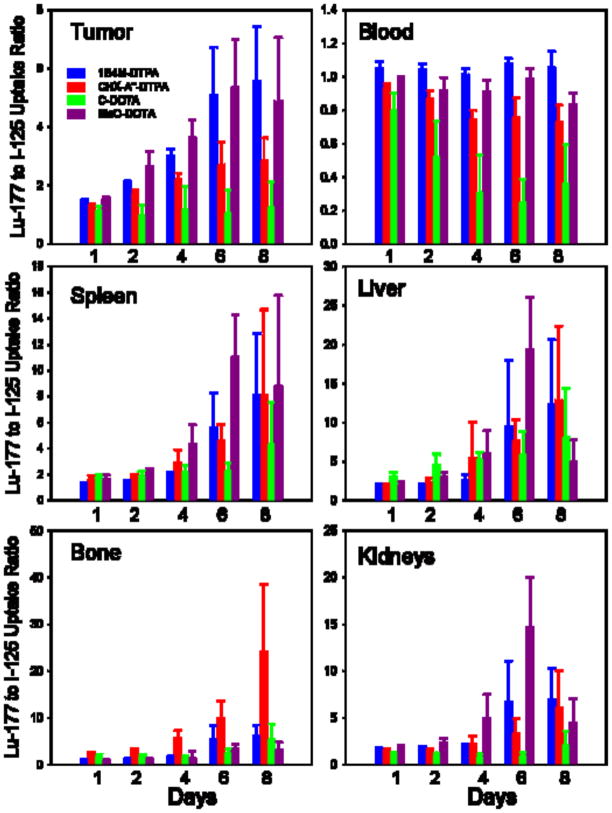
Differences in the in vivo characteristics of the four 177Lu-labeled L8A4-chelate conjugates also were evaluated by calculating their 177Lu/125I tissue uptake ratios. An advantage of using this parameter is that by normalizing each data set to the same co- administered [125I]SGMIB-L8A4, it should be possible to minimize the effects of potential differences among groups of experimental animals such as tumor size. As shown in Figure 2, with the exception of the C-DOTA conjugate, 177Lu/125I uptake ratios in the blood did not vary considerably with time and were close to unity. In tumor, C-DOTA offered no significant delivery advantage compared with SGMIB; however, with the three other bifunctional chelates there was a significant tumor delivery advantage for 177Lu labeling, particularly for 1B4M-DTPA and MeO-DOTA. For example, 6 days after injection, 177Lu/125I tumor uptake ratios were 5.1 ″ 1.7 and 5.4 ″ 1.6 for 1B4M-DTPA and MeO-DOTA, respectively. With the exception of CHX-A″-DTPA, 177Lu/125I uptake ratios in the bone were less than 2.0 from 1–4 days after injection; the lowest value observed at the end of the 8 day experiment was 3.2 ″ 1.7 for MeO-DOTA. In general, 177Lu/125I uptake ratios in the kidney, liver and spleen increased with time, reflecting significantly higher retention of 177Lu compared with 125I in these organs. Except for the results for C-DOTA in the kidney, 177Lu/125I uptake ratios in these tissues reached values greater than 4 by 6 days after injection.
Figure 2.
Ratio of 177Lu to 125I activity in U87. EGFR glioma xenografts and selected normal tissues following four paired label experiments. [125I]SGMIB-L8A4 co- administered with L8A4 mAb labeled with 177Lu using either 1B4M-DTPA, CHX-A″-DTPA, C-DOTA, or MeO-DOTA.
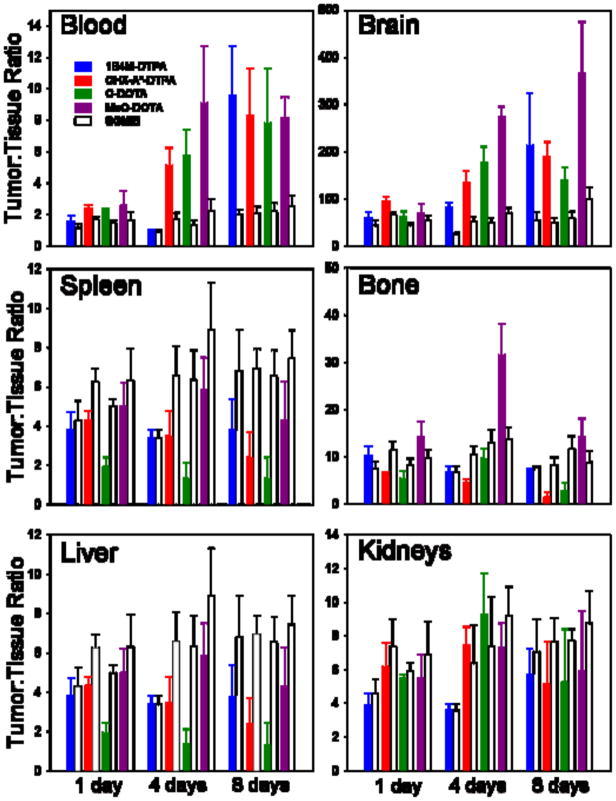
In order to assess the selectivity of tumor targeting for the 177Lu-labeled L8A4- chelate conjugates as well as the co-administered [125I]SGMIB-L8A4 preparations, tumor:normal tissue ratios for the four paired-label experiments were calculated. The results for the blood, brain, bone, spleen, liver and kidneys at 1, 4 and 8 days are summarized in Figure 3. In spleen, liver and kidney, higher Tumor:normal tissue ratios generally were observed with the radioiodinated mAb while the opposite behavior was seen in the blood and the brain. In most tissues and at most time points, use of the MeO-DOTA chelate for labeling L8A4 with 177Lu resulted in the most selective targeting. Table 5 summarizes tumor:normal tissue ratio results at 3 days after injection for 177Lu- MeO-DOTA-L8A4 and co-administered [125I]SGMIB-L8A4 in a more comprehensive set of tissues. Tumor:normal tissue ratios for [125I]SGMIB-L8A4 were marginally higher in liver, spleen and kidneys, however, two- to threefold more selective targeting was seen in all other tissues with 177Lu-MeO-DOTA-L8A4.
Figure 3.
Tumor:normal tissue uptake ratios in athymic mice with subcutaneous U87. EGFR glioma xenografts from four paired label experiments. [125I]SGMIB-L8A4 co-administered with L8A4 mAb labeled with 177Lu using either 1B4M-DTPA, CHX-A″-DTPA, C-DOTA, or MeO-DOTA.
Table 5.
Tumor-to-normal tissue ratios of radioactivity in athymic mice bearing subcutaneous U87. EGFR human glioma xenografts 3 days after injection of L8A4 mAb labeled with 177Lu using MeO-DOTA and 125I using SGMIB
| Tissue | Lu MeO-DOTA | I SGMIB |
|---|---|---|
| Liver | 7.8 ″ 4.1 | 10.4 ″ 2.6 |
| Spleen | 14.6 ″ 4.6 | 14.9 ″ 3.9 |
| Lungs | 25.4 ″ 3.8 | 7.6 ″ 2.5 |
| Heart | 39.4 ″ 8.6 | 10.8 ″ 2.2 |
| Kidney | 9.4 ″ 4.1 | 11.0 ″ 2.4 |
| Stomach | 214 ″ 47 | 72.1 ″ 17.1 |
| Small Intestine | 84.1 ″ 21.6 | 33.0 ″ 8.5 |
| Large Intestine | 96.8 ″ 37.7 | 37.7 ″ 13.7 |
| Blood | 10.8 ″ 3.3 | 2.9 ″ 0.7 |
| Muscle | 69.6 ″ 22.0 | 21.4 ″ 6.9 |
| Bone | 35.9 ″ 9.7 | 15.4 ″ 4.0 |
| Brain | 350 ″ 50 | 88.8 ″ 17.4 |
4. Discussion
The vast majority of targeted radiotherapeutic studies have involved β-emitting radionuclides with 131I and 90Y being the most extensively investigated β-emitters based at least in part on their widespread availability. Now that 177Lu is also routinely available at a reasonable cost, it has emerged as an attractive alternative to 90Y and 131I for targeted radiotherapy. Moreover, recent studies have demonstrated practical advantages for 177Lu for labeling peptides [31] as well as improved therapeutic efficacy with 177Lu as a label for MOv18 mAb compared with 131I and 90Y [32]. The later observation is consistent with the favorable aspects of the 177Lu emissions spectrum – low β-particle energy combined with low γ-ray energies and intensities - which should facilitate maximizing energy deposition in tumor while minimizing toxicity to neighboring normal tissues.
A setting in which 177Lu emission characteristics should be of particular benefit is the treatment of minimum residual disease, where efficient delivery of curative doses of radiation to small tumor foci is of paramount importance. The irradiation of residual glioma cells remaining after the resection of brain tumors is a notable example that has been evaluated in several clinical studies, with the majority of these involving anti- tenascin C mAbs labeled with 131I [10]. The long-term objective of the current study is to develop an improved targeted radiotherapeutic for brain tumor treatment by not only replacing 131I with 177Lu but also utilizing a molecular target with greater tumor specificity. Unlike tenacin-C, which is expressed on liver and spleen, EGFRvIII is not expressed on normal tissues [21], making mAbs such as L8A4 that recognize this mutant receptor attractive candidates for radioimmunotherapy.
A variety of acyclic and macrocyclic bifunctional chelates have been utilized for labeling mAbs with trivalent radiometals; our goal was to determine whether the nature of the ligand had an impact on its suitability for labeling L8A4 mAb with 177Lu. Most investigations regarding bifunctional chelators and radiometals have dealt with 90Y and in general, macrocyclic chelators show greater kinetic inertness than acyclic chelators [12]. The in vivo properties of mAbs and mAb fragments labeled with 177Lu using a variety of chelators also has been evaluated. For example, Milenic et al [16] compared PA-DOTA and C-DOTA with the acyclic chelator CHX-A″-DTPA conjugated for labeling the HuCC49 CH2 fragment with 177Lu. The 177Lu-C-DOTA and 177Lu-CHX-A″-DTPA conjugates exhibited good targeting whereas the 177Lu-PA-DOTA conjugate was not stable in vivo. In a similar type of comparison utilizing NHS-DOTA, MeO-DOTA and 1B4M-DTPA for labeling both the murine and chimeric forms of anti-tenascin mAb 81C6 with 177Lu, we observed that the use of MeO-DOTA was shown to provide the best tumor:normal tissue ratios with both constructs [17].
Unlike the mAbs and MAb fragment evaluated in these chelate comparison studies, the L8A4 mAb is rapidly internalized into EGFRvIII-expressing tumor cells where it undergoes extensive intracellular catabolism [23]. Thus, in addition to 177Lu complex stability and normal tissue clearance of labeled catabolites, maximizing the intracellular trapping of 177Lu after internalization of the mAb-EGFRvIII complex will be an important factor influencing the selection of the best bifunctional chelate for labeling L8A4 with 177Lu. Interpretation of the effect of the last variable should be facilitated by comparison to the results from a previous study [18] in which we evaluated the intracellular trapping of radioactivity by U87. EGFR glioma cells after incubation with177Lu- and 125I-labeled L8A4 prepared via a variety of methods including all those utilized herein.
Because both intracellular trapping in vitro and tumor uptake in vivo are time dependent, and different observation periods were used for the cell culture (1–24 h) and tissue distribution (1–8 Days) experiments, only general trends might be discernable even though the same U87. EGFR glioma cell model was utilized in both cases. In the internalization assays, 177Lu/125I intracellular activity ratios reflected higher uptake for [125I]SGMIB-L8A4 compared with mAb labeled via 1B4M-DTPA, CHX-A″-DTPA and C- DOTA from 1–8 h with the opposite behavior seen at 16 and 24 h [18]. In contrast, 177Lu/125I intracellular activity ratios were greater than 1 at all points. After a 24-h incubation, 177Lu/125I ratios were: C-DOTA, 1.76 ″ 0.24; MeO-DOTA, 2.09 ″ 0.17; 1B4M-DTPA, 2.34 ″ 0.39; CHX-A″-DTPA, 2.86 ″ 0.26. The fact that in the current in vivo study, the 177Lu/125I uptake ratios in U87. EGFR xenografts (Figure 2) were the lowest for the four bifunctional chelates at all time points is consistent with these results. However, the fact that the 177Lu/125I tumor uptake ratios for MeO-DOTA and 1B4M- DTPA are higher than those for CHX-A″-DTPA would not have been predicted based on the in vitro internalization assay results at 24 h. On the other hand, the rank order of the 177Lu/125I tumor uptake ratios are in good agreement with the 177Lu/125I intracellular activity ratios observed at early time points, at which time MeO-DOTA and 1B4M-DTPA have higher values than CHX-A″-DTPA. Thus, although internalization assays may be of value for predicting the relative merit of labeling methods for internalizing mAbs; this must be done with caution.
Although our intended clinical application involves direct injection of labeled mAbs into surgically created tumor resection cavities, leakage of radioactivity from the cavity does occur with time [10], making it important to select a labeling method that maximizes tumor:normal tissue ratios. In our previous studies with 177Lu-labeled anti- tenascin mAb 81C6, which reacts with a non-internalizing molecular target, the selectivity of tumor uptake was dependent both on the properties of the chelate and the form of the mAb. With the more stable chimeric construct, both 1B4M-DTPA and MeO- DOTA were effective in providing acceptable levels of 177Lu in bone, liver, spleen and kidneys; however, with murine 81C6, labeling via MeO-DOTA resulted in significantly lower 177Lu levels in these tissues. The results of the current study with the rapidly internalizing L8A4 mAb also demonstrate chelate-dependent differences in normal organ uptake expressed in terms of % ID/g, 177Lu/125I uptake ratio and tumor:normal tissue ratio.
The magnitude of tumor uptake and tumor:normal tissue ratios probably are the most relevant parameters for evaluating the relative merit of these radiolabeled L8A4 conjugates for targeted radiotherapy. Although tumor:tissue ratios for [125I]SGMIB-L8A4 in spleen, liver and kidneys are more favorable than those observed with any of the 177Lu-labeled mAb conjugates, this must be balanced by the fact that for 1B4M-DTPA and MeO-DOTA, the magnitude of tumor uptake is up to 4 to 5 times higher than seen with the radioiodinated mAb. Given that the anticipated non-intravenous route of delivery will delay exposure to normal tissue and that 177Lu has better emission properties than 131I, the potential benefits of labeling L8A4 mAb with 177Lu via these two chelates appear to outweigh the disadvantages. Furthermore, tumor:normal tissue ratios for 177Lu-1B4M-DTPA-L8A4 and to an even greater extent, 177Lu-MeO-DOTA- L8A4, were higher than those for [125I]SGMIB-L8A4 in most other tissues. Finally, uptake and retention of 177Lu in blood and bone was lower for 177Lu-MeO-DOTA-L8A4 than 177Lu-1B4M-DTPA-L8A4, which should help decrease bone marrow toxicity. Based on these observations, we have selected MeO-DOTA as the bifunctional chelate for labeling L8A4 mAb with 177Lu for our planned clinical radioimmunotherapy trial in patients with malignant brain tumors.
Table 2.
Paired-label biodistribution of L8A4 labeled with 177Lu using CHX-A″-DTPA and with 125I using SGMIB in athymic mice with subcutaneous U87MG. EGFR human glioma xenografts
| Nuclide | Tissue | Day 1 | Day 4 | Day 8 |
|---|---|---|---|---|
| 177Lu | Blood | 15.18 ± 3.30 | 6.10 ± 2.47 | 0.99 ± 0.71 |
| Liver | 8.53 ± 2.39 | 9.36 ± 3.59 | 4.45 ± 0.87 | |
| Spleen | 5.98 ± 1.86 | 3.97 ± 0.53 | 1.84 ± 0.70 | |
| Kidneys | 6.07 ± 1.56 | 3.96 ± 1.24 | 2.00 ± 0.37 | |
| Lung | 5.39 ± 1.28 | 2.44 ± 0.94 | 0.56 ± 0.23 | |
| Bone | 5.95 ± 1.27 | 6.55 ± 1.77 | 7.56 ± 1.88 | |
| Muscle | 1.30 ± 0.44 | 0.64 ± 0.21 | 0.19 ± 0.05 | |
| Brain | 0.46 ± 0.12 | 0.21 ± 0.08 | 0.05 ± 0.03 | |
| Thyroida | 0.44 ± 0.13 | 0.24 ± 0.07 | 0.14 ± 0.07 | |
| Tumor | 36.28 ± 6.60 | 29.00 ± 7.07 | 8.67 ± 3.42 | |
| 125I | Blood | 16.24 ± 3.73 | 8.18 ± 3.25 | 1.50 ± 1.32 |
| Liver | 4.43 ± 0.77 | 2.14 ± 0.86 | 0.46 ± 0.37 | |
| Spleen | 3.77 ± 1.43 | 1.51 ± 0.52 | 0.35 ± 0.30 | |
| Kidneys | 3.87 ± 1.03 | 2.16 ± 1.18 | 0.42 ± 0.34 | |
| Lung | 5.70 ± 1.64 | 2.90 ± 1.30 | 0.50 ± 0.40 | |
| Bone | 2.51 ± 0.86 | 1.24 ± 0.51 | 0.36 ± 0.27 | |
| Muscle | 1.37 ± 0.58 | 1.29 ± 0.49 | 0.16 ± 0.13 | |
| Brain | 0.50 ± 0.13 | 0.28 ± 0.10 | 0.09 ± 0.09 | |
| Thyroida | 0.50 ± 0.13 | 0.27 ± 0.09 | 0.09 ± 0.02 | |
| Tumor | 27.56 ± 4.82 | 13.09 ± 2.97 | 3.39 ± 2.19 |
Values presented as percent injected dose per gram tissue (% ID/g), mean ″ standard deviation; five mice per group.
Percent injected dose in the thyroid.
Table 3.
Paired-label biodistribution of L8A4 labeled with 177Lu using C-DOTA and with 125I using SGMIB in athymic mice with subcutaneous U87MG. EGFR human glioma xenografts
| Nuclide | Tissue | Day 1 | Day 4 | Day 8 |
|---|---|---|---|---|
| 177Lu | Blood | 10.95 ± 1.53 | 2.47 ± 1.80 | 1.33 ± 1.60 |
| Liver | 13.00 ± 4.79 | 9.34 ± 1.71 | 5.39 ± 2.54 | |
| Spleen | 5.43 ± 1.50 | 3.61 ± 1.02 | 2.47 ± 1.84 | |
| Kidneys | 4.26 ± 0.70 | 1.28 ± 0.60 | 1.22 ± 0.67 | |
| Lung | 4.21 ± 1.28 | 0.99 ± 0.70 | 0.72 ± 0.67 | |
| Bone | 4.49 ± 0.84 | 1.21 ± 0.53 | 2.39 ± 1.55 | |
| Muscle | 1.32 ± 0.26 | 0.30 ± 0.14 | 0.25 ± 0.16 | |
| Brain | 0.37 ± 0.05 | 0.22 ± 0.37 | 0.05 ± 0.04 | |
| Thyroida | 0.17 ± 0.09 | 0.04 ± 0.03 | 0.06 ± 0.05 | |
| Tumor | 23.52 ± 3.14 | 12.04 ± 6.29 | 7.47 ± 6.89 | |
| 125I | Blood | 13.93 ± 3.17 | 8.16 ± 1.08 | 3.82 ± 3.70 |
| Liver | 4.15 ± 0.85 | 1.78 ± 0.25 | 1.14 ± 1.07 | |
| Spleen | 2.89 ± 0.59 | 1.62 ± 0.18 | 0.81 ± 0.78 | |
| Kidneys | 3.47 ± 0.63 | 1.61 ± 0.36 | 1.00 ± 0.93 | |
| Lung | 5.46 ± 1.86 | 2.49 ± 0.53 | 1.60 ± 1.50 | |
| Bone | 2.47 ± 0.21 | 0.87 ± 0.17 | 0.57 ± 0.41 | |
| Muscle | 1.64 ± 0.32 | 0.59 ± 0.09 | 0.40 ± 0.32 | |
| Brain | 0.48 ± 0.08 | 0.41 ± 0.46 | 0.13 ± 0.10 | |
| Thyroida | 0.29 ± 0.11 | 0.22 ± 0.10 | 0.18 ± 0.12 | |
| Tumor | 20.76 ± 5.02 | 11.24 ± 3.09 | 7.37 ± 6.27 |
Values presented as percent injected dose per gram tissue (% ID/g), mean ″ standard deviation; five mice per group.
Percent injected dose in the thyroid.
Acknowledgments
The authors would like to thank Dr. Martin Brechbiel, National Cancer Institute, Bethesda, MD, USA, and Dr. Keith Frank, IsoTherapeutics Group, Arlington, TX, USA, for generously supplying the 1B4M-DTPA and MeO-DOTA, respectively. This work was supported by Grants NS20023 and CA42324 from the National Institutes of Health, and a grant from the Pediatric Brain Tumor Foundation of the United States.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cleaves MA. Radium Therapy. Med Rec. 1903;64:601. [Google Scholar]
- 2.McDevitt MR, Ma D, Lai LT, Simon J, Borchardt P, Frank RK, Wu K, Pellegrini V, et al. Tumor Therapy with Targeted Atomic Nanogenerators. Science. 2001;294:1537–1540. doi: 10.1126/science.1064126. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava S, Dadachova E. Recent advances in radionuclide therapy. Semin Nucl Med. 2001;31:330–341. doi: 10.1053/snuc.2001.27043. [DOI] [PubMed] [Google Scholar]
- 4.Knox SJ, Meredith RF. Clinical radioimmunotherapy. Semin Rad Oncol. 2000;10:73–93. doi: 10.1016/s1053-4296(00)80045-4. [DOI] [PubMed] [Google Scholar]
- 5.DeNardo G, DeNardo S, O’Donnell R, Kroger L, Kukis D, Meares C, et al. Are Radiometal-Labeled Antibodies Better Than Iodine-131–Labeled Antibodies: Comparative Pharmacokinetics and Dosimetry of Copper-67–, Iodine-131–, and Yttrium-90–Labeled Lym-1 Antibody in Patients with Non-Hodgkin’s Lymphoma. Clin Lymphoma. 2000;1:118–126. doi: 10.3816/clm.2000.n.010. [DOI] [PubMed] [Google Scholar]
- 6.DeNardo G, DeNardo S, O’Donnell R, Kroger L, Shen S, DeNardo DA, et al. Radiation Dosimetry, Toxicity and Tumor Regression in Patients with Lymphoma. J Nucl Med. 1999;40:302–310. [PubMed] [Google Scholar]
- 7.Wilder RB, DeNardo G, DeNardo S. Radioimmunotherapy: Recent Results and Future Directions. J Clin Oncol. 1996;14:1383–1400. doi: 10.1200/JCO.1996.14.4.1383. [DOI] [PubMed] [Google Scholar]
- 8.Kairemo KJA. Radioimmunotherapy of Solid Cancers. Acta Oncol. 1996;35:343–355. doi: 10.3109/02841869609101651. [DOI] [PubMed] [Google Scholar]
- 9.Witzig TE, White CA, Gordon LI, Wiseman GA, Emmanouilides C, Murray JL, et al. Safety of yttrium-90 ibritumomab tiuxetan radioimmunotherapy for relapsed low-grade, follicular, or transformed non-hodgkin’s lymphoma. J Clin Oncol. 2003;21:1263–1270. doi: 10.1200/JCO.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 10.Reardon DA, Zalutsky MR, Bigner DD. Antitenascin-C monoclonal antibody radioimmunotherapy for malignant glioma patients. Expert Rev Anti-Cancer Therapy. 2007;7:675–687. doi: 10.1586/14737140.7.5.675. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Edwards DS. Fundamentals of receptor-based diagnostic metalloradiopharmaceuticals. Top Curr Chem. 2002;222:259–278. [Google Scholar]
- 12.Liu S, Edwards DS. Bifunctional chelators for target-specific therapeutic lanthanide radiopharmaceuticals. Bioconjug Chem. 2001;12:7–34. doi: 10.1021/bc000070v. [DOI] [PubMed] [Google Scholar]
- 13.Durbin PW. Metabolic characteristics within a chemical family. Health Phys. 1960;2:225–238. doi: 10.1097/00004032-195907000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Postema EJ, Frielink C, Oyen WJ, Raemaekers JM, Goldenberg DM, Corstens FH, et al. Biodistribution of 131I-, 186Re-, 177Lu-, and 88Y-labeled hLL2 (Epratuzumab) in nude mice with CD22-positive lymphoma. Cancer Biother Radiopharm. 2003;18:525–33. doi: 10.1089/108497803322287592. [DOI] [PubMed] [Google Scholar]
- 15.Brouwers AH, van Eerd JEM, Frielink C, Oosterwijk E, Oyen WJG, Corstens FHM, et al. Optimization of radioimmunotherapy of renal cell carcinoma: Labeling of monoclonal antibody cG250 with 131I, 90Y, 177Lu, or 186Re. J Nucl Med. 2003;45:327–337. [PubMed] [Google Scholar]
- 16.Milenic DE, Garmestani K, Chappell LL, Dadochova E, Yordanov A, Ma D, et al. In vivo comparison of monoclonal antibodies with 177Lu for radioimmuno- therapeutic applications. Nucl. Med. Biol. 2002;29:431–442. doi: 10.1016/s0969-8051(02)00294-9. [DOI] [PubMed] [Google Scholar]
- 17.Yordanov AT, Hens M, Pegram C, Bigner DD, Zalutsky MR. Anti-Tenascin Antibody 81C6 Armed with 177Lu: In Vivo Comparison of Macrocyclic and Acyclic Ligands. Nucl Med Biol. 2006;34:173–183. doi: 10.1016/j.nucmedbio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Hens M, Vaidyanathan G, Welsh P, Pegram C, Bigner DD, Zalutsky MR. Labeling internalizing anti-epidermal growth factor receptor variant III monoclonal antibody with177Lu: in vitro comparison of acyclic and macrocyclic ligands. Nucl Med Biol. 2009;36:117–128. doi: 10.1016/j.nucmedbio.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaidyanathan G, Affleck D, Li J, Welsh P, Zalutsky MR. A polar substituent- containing acylation agent for the radioiodination of internalizing monoclonal antibodies: N-Succinimidyl 4-guanidinomethyl-3-[131I]iodobenzoate ([131I]SGMIB) Bioconjugate Chem. 2001;12:428–438. doi: 10.1021/bc0001490. [DOI] [PubMed] [Google Scholar]
- 20.Humphrey PA, Wong AJ, Vogelstein B, Zalutsky MR, Fuller GN, Archer GE, et al. Anti-synthetic peptide antibody reacting at the fusion junction of deletion- mutant epidermal growth factor receptors in human glioblastoma. Proc Natl Acad Sci. 1990;87:4207–4211. doi: 10.1073/pnas.87.11.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wikstrand CJ, Reist CJ, Archer GE, Zalutsky MR, Bigner DD. The class III variant of the epidermal growth factor receptor (EGFRvIII): characterization and utilization as an immunotherapeutic target. J NeuroVirol. 1998;4:148–158. doi: 10.3109/13550289809114515. [DOI] [PubMed] [Google Scholar]
- 22.Wikstrand CJ, Hale LP, Batra SK, Hill L, Humphrey PA, Kurpad SK, et al. Monoclonal antibodies against EGFRvllI are tumor specific and react with breast and lung carcinomas and malignant gliomas. Cancer Res. 1995;55:3140–3148. [PubMed] [Google Scholar]
- 23.Reist CJ, Archer GE, Kurpad SN, Wikstrand CJ, Vaidyanathan G, Willingham MC, et al. Tumor-specific Anti-Epidermal Growth Factor Receptor Variant III Monoclonal Antibodies: Use of the Tyramine-Cellobiose Radioiodination Method Enhances Cellular Retention and Uptake in Tumor Xenografts. Cancer Res. 1995;55:4375–4382. [PubMed] [Google Scholar]
- 24.Stetter H, Frank W. Complex formation with tetraazacycloalkane-N,N′,N″,N‴tetraacetic acids as a function of ring size. Angewandte Chemie. 1976;88:760. [Google Scholar]
- 25.Dadachova E, Chapell LL, Brechbiel MW. Spectrophotometric method for the determination of bifunctional macrocyclic ligands in macrocyclic ligand-protein conjugates. Nucl Med Biol. 1999;26:977–982. doi: 10.1016/s0969-8051(99)00054-2. [DOI] [PubMed] [Google Scholar]
- 26.Pippin CG, Parker T, McMurry TJ, Brechbiel MW. Spectrophotometric method for the determination of a bifunctional DTPA ligand in DTPA–monoclonal antibody conjugates. Bioconjugate Chemistry. 1992;3:342–344. doi: 10.1021/bc00016a014. [DOI] [PubMed] [Google Scholar]
- 27.Vaidyanathan G, Zalutsky MR. Synthesis of N-succinimidyl 4-guanidinomethyl-3-[*I]iodobenzoate: a radioiodination agent for labeling internalizing proteins and peptides. Nature Protocols. 2007;2:282–286. doi: 10.1038/nprot.2007.20. [DOI] [PubMed] [Google Scholar]
- 28.Foulon CF, Reist CJ, Bigner DD, Zalutsky MR. Radioiodination via D-amino acid peptide enhances cellular retention and tumor xenograft targeting of an internalizing anti-epidermal growth factor receptor variant III monoclonal antibody. Cancer Res. 2000;60:4453–4460. [PubMed] [Google Scholar]
- 29.Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorgenicity. ProC Natl Acad Sci USA. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reist CJ, Archer GE, Wikstrand CJ, Bigner DD, Zalutsky MR. Improved targeting of an anti-epidermal growth factor receotor variant III monoclonal antibody after labeling using N-succinimidyl 5-iodo-3-pyridinecarboxylate. Cancer Res. 1997;57:1510–1515. [PubMed] [Google Scholar]
- 31.Koumarianou E, Mikolajczak R, Pawlak D, Zikos X, Bouziotis P, Garnuszek P, et al. Comparative study on DOTA-derivatized bombesin analog labeled with 90Y and 177Lu: in vitro and in vivo evaluation. Nucl Med Biol. 2009;36:591–603. doi: 10.1016/j.nucmedbio.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Zacchetti A, Coliva A, Luison E, Seregni E, Bombardieri E, Giussani A, et al. 177Lu- labeled MOv18 as compared to 131I- or 90Y-labeled MOv18 has the better therapeutic effect in eradication of alpha folate receptor-expressing tumor xenografts. Nucl Med Biol. 2009;36:759–770. doi: 10.1016/j.nucmedbio.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Edwards DS. Bifunctional chelators for target-specific lanthanide radiopharmaceuticals. Bioconj Chem. 2001;12:7–34. doi: 10.1021/bc000070v. [DOI] [PubMed] [Google Scholar]