Abstract
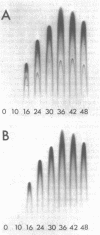
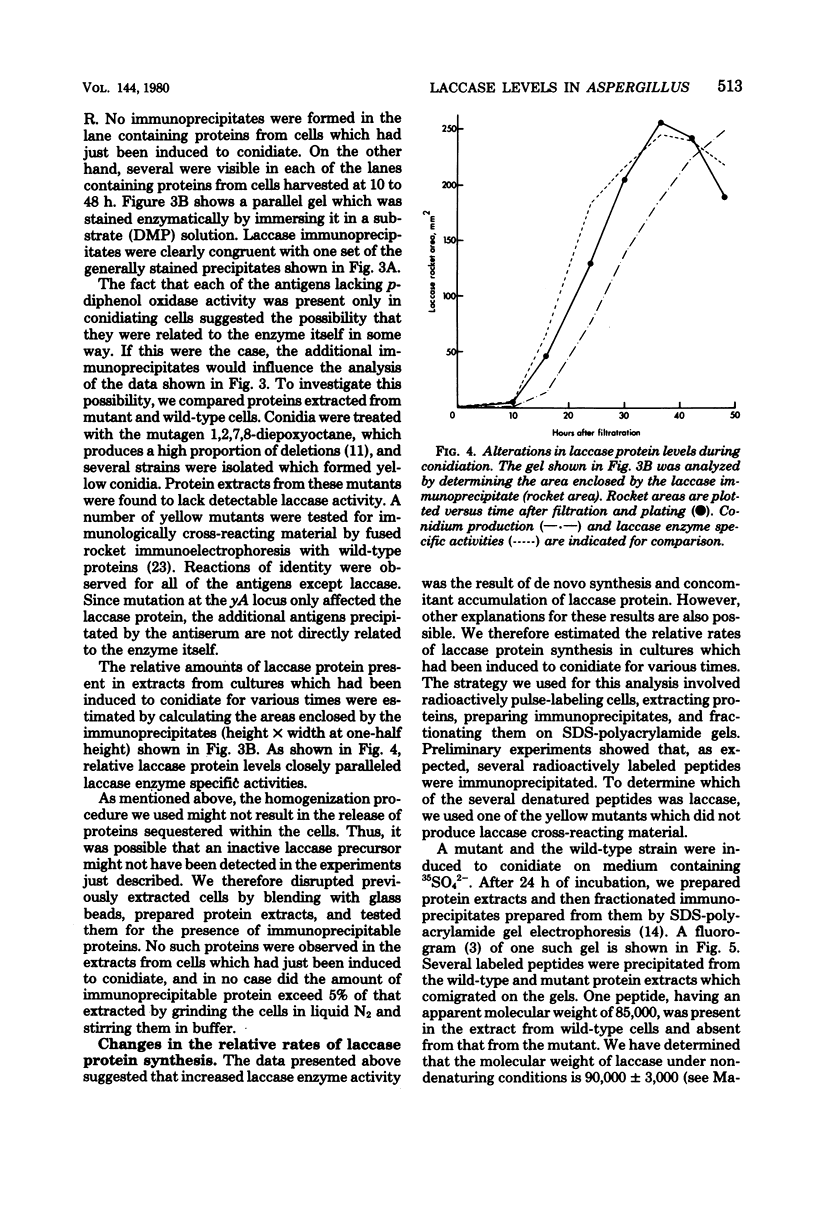
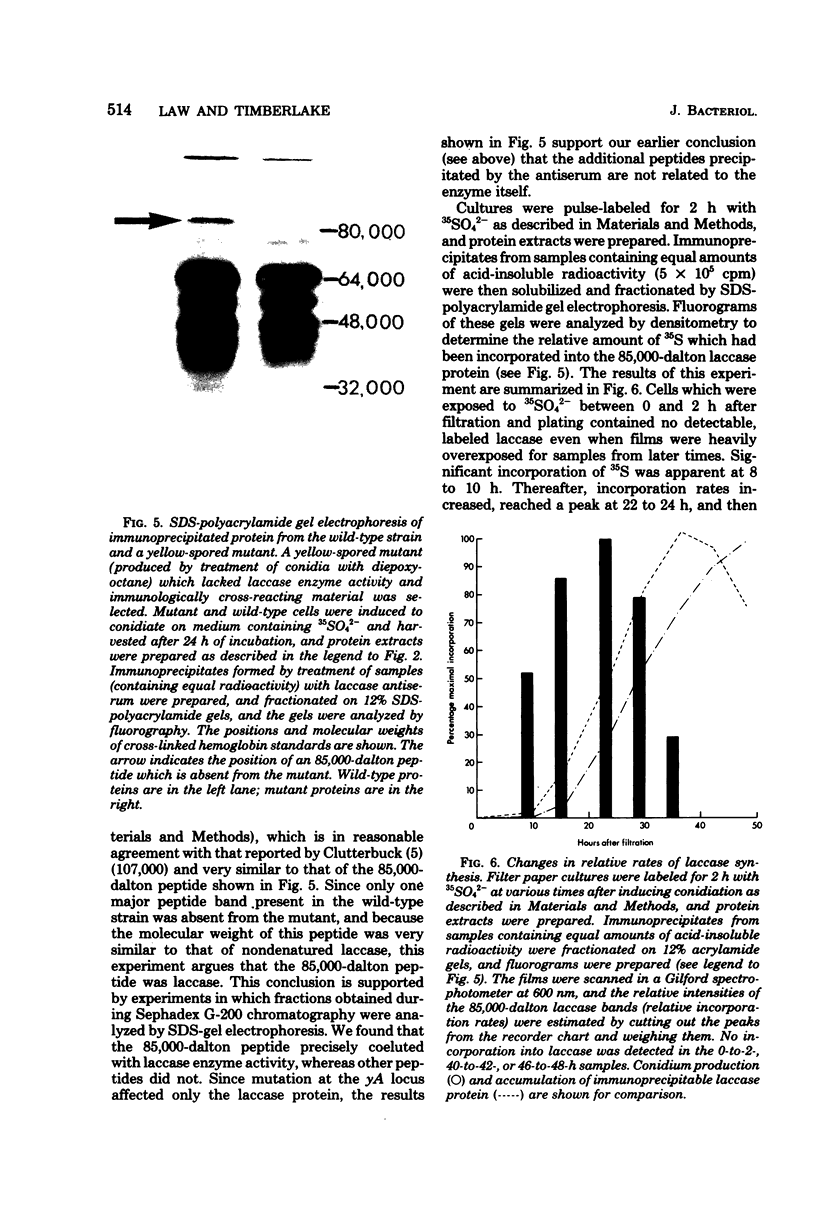
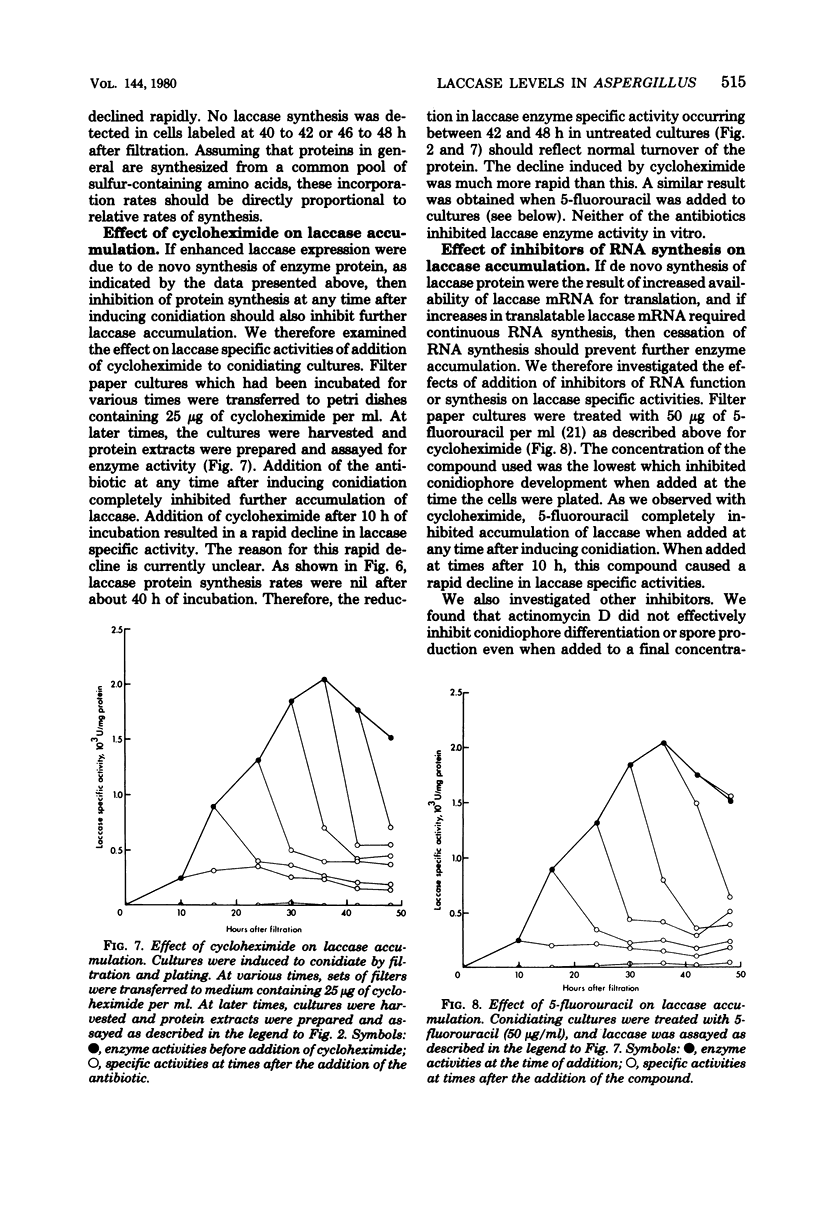
Asexual spores (conidia) of Aspergillus nidulans contain a dark green pigment which is not present in other cell types. Synthesis of this pigment is catalyzed, in part, by a developmentally controlled p-diphenol oxidase, or laccase, encoded at the gamma A genetic locus (A. J. Clutterbuck, J. Gen. Microbiol. 70:423-435, 1972). We have investigated the mechanisms regulating expression of the gamma A gene of A. nidulans. Vegetative hyphae grown in submerged culture lacked detectable laccase enzyme activity and neither contained nor synthesized immunoprecipitable laccase protein. When such cultures were induced to conidiate by harvesting the cells onto filter papers and aerating them, laccase levels began to increase after 10 to 16 h, reached a peak at 20 to 36 h, and then declined slowly. Immunological assays showed that increases in laccase enzyme activity were (i) proceded by a transient rise in the relative rate of laccase protein synthesis and (ii) closely paralleled by increases in the amount of laccase protein. Addition of cycloheximide to cultures at any time after inducing conidiation inhibited further accumulation of laccase enzyme activity. These data are most consistent with increases in laccase levels being due to regulated, de novo synthesis of laccase protein. Addition of inhibitors of ribonucleic acid synthesis to conidiating cultures also inhibited further accumulation of laccase, suggesting that laccase expression is regulated by alterations in the transcriptional activity of the gamma A locus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D. E., Gealt M., Pastushok M. Gene control of developmental competence in Aspergillus nidulans. Dev Biol. 1973 Sep;34(1):9–15. doi: 10.1016/0012-1606(73)90335-7. [DOI] [PubMed] [Google Scholar]
- Axelrod D. E. Kinetics of differentiation of conidiophores and conidia by colonies of Aspergillus nidulans. J Gen Microbiol. 1972 Nov;73(1):181–184. doi: 10.1099/00221287-73-1-181. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Clutterbuck A. J. A mutational analysis of conidial development in Aspergillus nidulans. Genetics. 1969 Oct;63(2):317–327. doi: 10.1093/genetics/63.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutterbuck A. J. Absence of laccase from yellow-spored mutants of Aspergillus nidulans. J Gen Microbiol. 1972 May;70(3):423–435. doi: 10.1099/00221287-70-3-423. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Regulation of gene expression: possible role of repetitive sequences. Science. 1979 Jun 8;204(4397):1052–1059. doi: 10.1126/science.451548. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., Lodish H. F. A small nuclear precursor of messenger RNA in the cellular slime mold Dictyostelium discoideum. J Mol Biol. 1973 Sep 15;79(2):295–314. doi: 10.1016/0022-2836(73)90007-7. [DOI] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Hereford L. M., Rosbash M. Number and distribution of polyadenylated RNA sequences in yeast. Cell. 1977 Mar;10(3):453–462. doi: 10.1016/0092-8674(77)90032-0. [DOI] [PubMed] [Google Scholar]
- Hynes M. J. Fine-structure mapping of the acetamidase structural gene and its controlling region in Aspergillus nidulans. Genetics. 1979 Mar;91(3):381–392. doi: 10.1093/genetics/91.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamalay J. C., Goldberg R. B. Regulation of structural gene expression in tobacco. Cell. 1980 Apr;19(4):935–946. doi: 10.1016/0092-8674(80)90085-9. [DOI] [PubMed] [Google Scholar]
- Kleene K. C., Humphreys T. Similarity of hnRNA sequences in blastula and pluteus stage sea urchin embryos. Cell. 1977 Sep;12(1):143–155. doi: 10.1016/0092-8674(77)90192-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Oliver P. T. Conidiophore and spore development in Aspergillus nidulans. J Gen Microbiol. 1972 Nov;73(1):45–54. doi: 10.1099/00221287-73-1-45. [DOI] [PubMed] [Google Scholar]
- PONTECORVO G., ROPER J. A., HEMMONS L. M., MACDONALD K. D., BUFTON A. W. J. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- Palacios R., Palmiter R. D., Schimke R. T. Identification and isolation of ovalbumin-synthesizing polysomes. I. Specific binding of 125 I-anti-ovalbumin to polysomes. J Biol Chem. 1972 Apr 25;247(8):2316–2321. [PubMed] [Google Scholar]
- Palmiter R. D., Oka T., Schimke R. T. Modulation of ovalbumin synthesis by estradiol-17 beta and actinomycin D as studied in explants of chick oviduct in culture. J Biol Chem. 1971 Feb 10;246(3):724–737. [PubMed] [Google Scholar]
- Payvar F., Schimke R. T. Methylmercury hydroxide enhancement of translation and transcription of ovalbumin and conalbumin mRNA's. J Biol Chem. 1979 Aug 25;254(16):7636–7642. [PubMed] [Google Scholar]
- Timberlake W. E., Shumard D. S., Goldberg R. B. Relationship between nuclear and polysomal RNA populations of Achlya: a simple eucaryotic system. Cell. 1977 Apr;10(4):623–632. doi: 10.1016/0092-8674(77)90095-2. [DOI] [PubMed] [Google Scholar]
- Weeke B. Rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:37–46. doi: 10.1111/j.1365-3083.1973.tb03777.x. [DOI] [PubMed] [Google Scholar]
- Wold B. J., Klein W. H., Hough-Evans B. R., Britten R. J., Davidson E. H. Sea urchin embryo mRNA sequences expressed in the nuclear RNA of adult tissues. Cell. 1978 Aug;14(4):941–950. doi: 10.1016/0092-8674(78)90348-3. [DOI] [PubMed] [Google Scholar]
- Wong L. J., Marzluf G. A. Sequence complexity and abundance classes of nuclear and polysomal polyadenylated RNA in Neurospora crassa. Biochim Biophys Acta. 1980 Mar 28;607(1):122–135. doi: 10.1016/0005-2787(80)90226-9. [DOI] [PubMed] [Google Scholar]