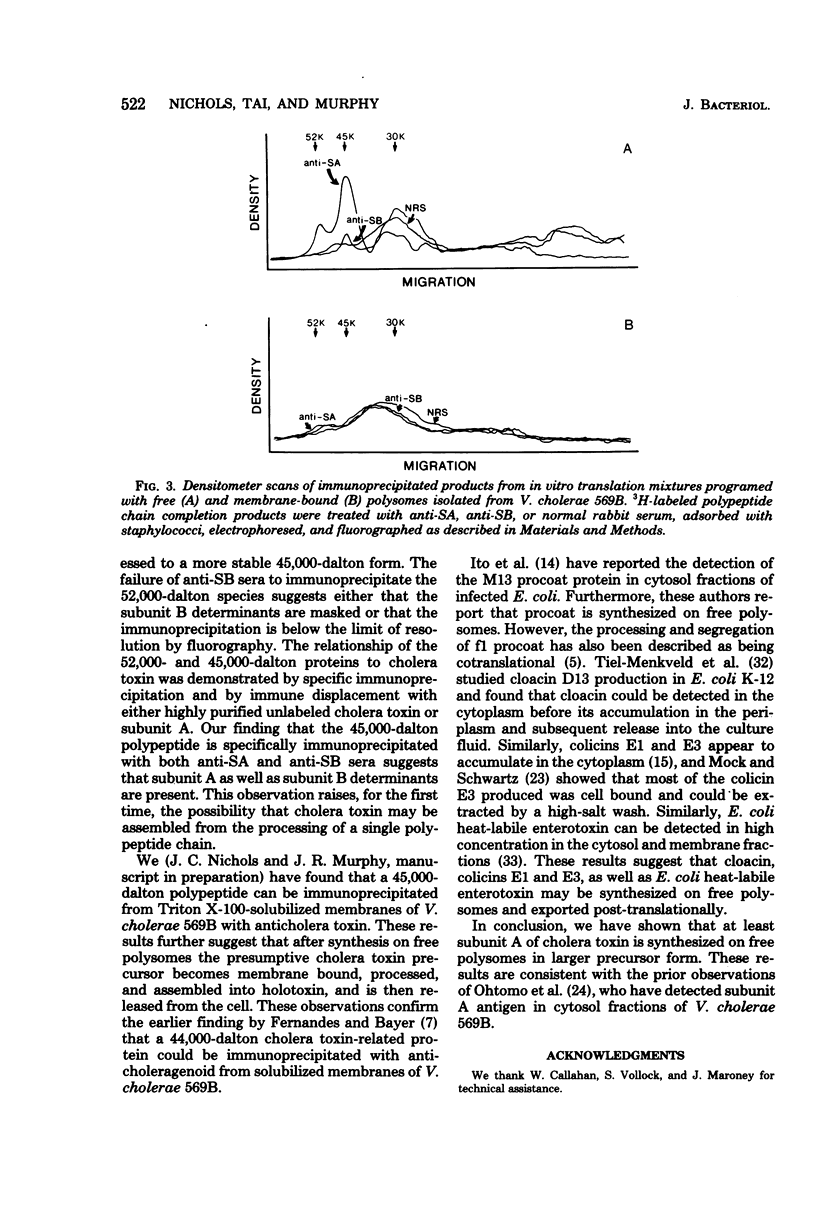
Abstract
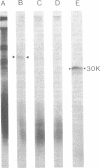
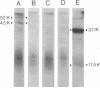
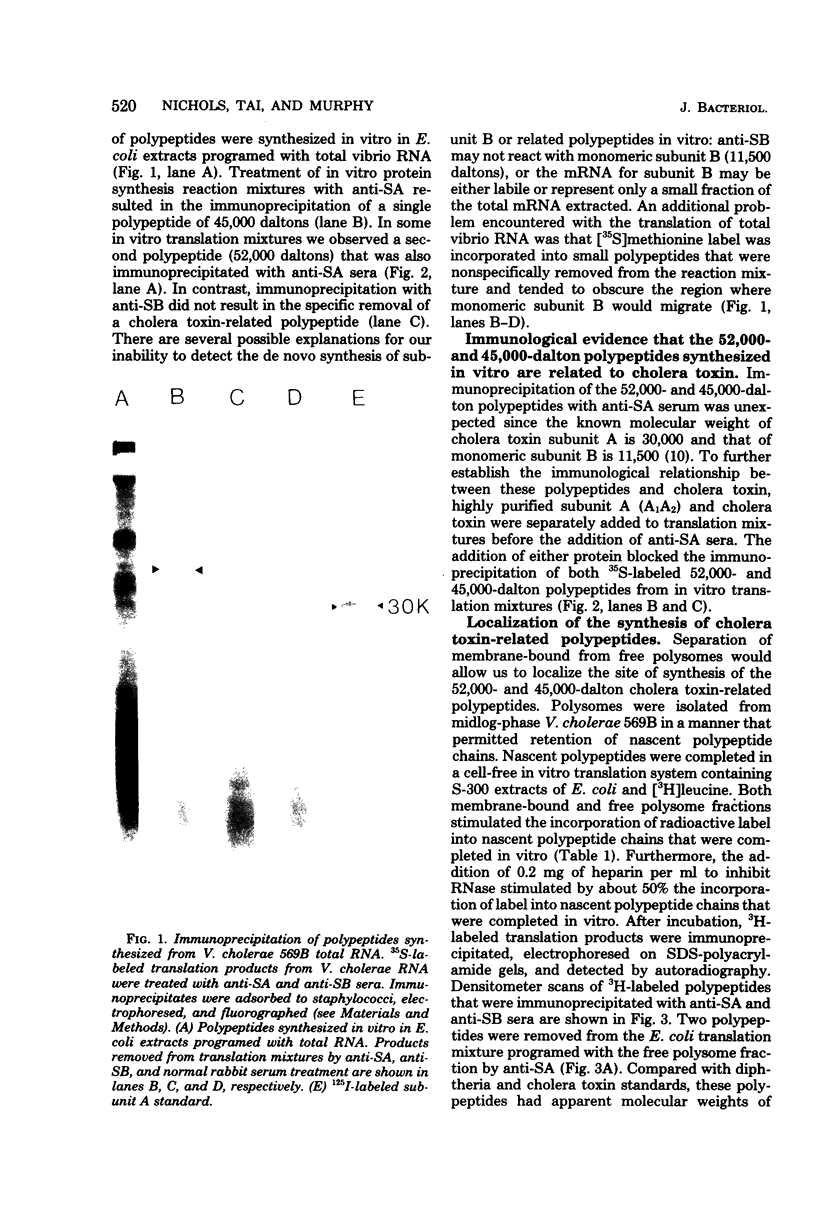
Membrane-bound and free polysomes have been isolated from Vibrio cholerae 569B. Nacent polypeptide chains were completed in a cell-free translation mixture containing Escherichia coli S-300 extracts and [3H]leucine or [35S]methionine. Cholera toxin-related polypeptides synthesized in vitro were immunologically detected after treatment with either anti-subunit A or anti-subunit B serum. Immunoreactive translation products were removed from reaction mixtures with formalinized Cowan's strain of Staphylococcus aureus, electrophoresed on sodium dodecyl sulfate-polyacrylamide gels, and visualized by fluorography. Anti-subunit A serum precipitated two major polypeptide species (molecular weights 52,000 and 45,000) from translation mixtures programed with free polysomes, whereas anti-subunit B serum precipitated only the 45,000-molecular-weight polypeptide. No cholera toxin-related polypeptides were detectable in translation mixtures programed with membrane-bound polysomes. Purified subunit A and cholera toxin competed for anti-subunit A binding sites and blocked the immunoprecipitation of the 35S-labeled 52,000- and 45,000-dalton polypeptides from in vitro translation mixtures. The data presented suggest that cholera toxin is synthesized in the cytoplasm in a precursor form on free polysomes and is secreted post-translationally.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cammack K. A., Wade H. E. The sedimentation behaviour of ribonuclease-active and -inactive ribosomes from bacteria. Biochem J. 1965 Sep;96(3):671–680. doi: 10.1042/bj0960671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. N., Model P., Blobel G. Membrane biogenesis: cotranslational integration of the bacteriophage f1 coat protein into an Escherichia coli membrane fraction. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1251–1255. doi: 10.1073/pnas.76.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. D., Tai P. C. The mechanism of protein secretion across membranes. Nature. 1980 Jan 31;283(5746):433–438. doi: 10.1038/283433a0. [DOI] [PubMed] [Google Scholar]
- FINKELSTEIN R. A., NORRIS H. T., DUTTA N. K. PATHOGENESIS EXPERIMENTAL CHOLERA IN INFANT RABBITS. I. OBSERVATIONS ON THE INTRAINTESTINAL INFECTION AND EXPERIMENTAL CHOLERA PRODUCED WITH CELL-FREE PRODUCTS. J Infect Dis. 1964 Jun;114:203–216. doi: 10.1093/infdis/114.3.203. [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Bayer M. E. Membrane-bound enterotoxin of Vibrio cholerae. J Gen Microbiol. 1977 Dec;103(2):381–387. doi: 10.1099/00221287-103-2-381. [DOI] [PubMed] [Google Scholar]
- GESTELAND R. F., BOEDTKER H. SOME PHYSICAL PROPERTIES OF BACTERIOPHAGE R17 AND ITS RIBONUCLEIC ACID. J Mol Biol. 1964 Apr;8:496–507. doi: 10.1016/s0022-2836(64)80007-3. [DOI] [PubMed] [Google Scholar]
- Gill D. M. Mechanism of action of cholera toxin. Adv Cyclic Nucleotide Res. 1977;8:85–118. [PubMed] [Google Scholar]
- Gill D. M., Rappaport R. S. Origin of the enzymatically active A1 fragment of cholera toxin. J Infect Dis. 1979 Jun;139(6):674–680. doi: 10.1093/infdis/139.6.674. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973 Aug;8(2):208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Mandel G., Wickner W. Soluble precursor of an integral membrane protein: synthesis of procoat protein in Escherichia coli infected with bacteriophage M13. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1199–1203. doi: 10.1073/pnas.76.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakes K. S., Model P. Mechanism of export of colicin E1 and colicin E3. J Bacteriol. 1979 Jun;138(3):770–778. doi: 10.1128/jb.138.3.770-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kronvall G., Frommel D. Definition of staphylococcal protein A reactivity for human immunoglobulin G fragments. Immunochemistry. 1970 Jan;7(1):124–127. doi: 10.1016/0019-2791(70)90036-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levner M. H., Urbano C., Rubin B. A. Lincomycin increases synthetic rate and periplasmic pool size for cholera toxin. J Bacteriol. 1980 Jul;143(1):441–447. doi: 10.1128/jb.143.1.441-447.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnroth I., Holmgren J. Subunit structure of cholera toxin. J Gen Microbiol. 1973 Jun;76(2):417–427. doi: 10.1099/00221287-76-2-417. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Mock M., Schwartz M. Mechanism of colicin E3 production in strains harboring wild-type or mutant plasmids. J Bacteriol. 1978 Nov;136(2):700–707. doi: 10.1128/jb.136.2.700-707.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I. Biologically and chemically pure mRNA coding for a mouse immunoglobulin L-chain prepared with the aid of antibodies and immobilized oligothymidine. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2256–2260. doi: 10.1073/pnas.70.8.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Davis B. D. Extracellular labeling of growing secreted polypeptide chains in Bacillus subtilis with diazoiodosulfanilic acid. Biochemistry. 1979 Jan 9;18(1):198–202. doi: 10.1021/bi00568a030. [DOI] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Murphy J. R., Davis B. D. Precursor in cotranslational secretion of diphtheria toxin. J Bacteriol. 1980 Jan;141(1):184–189. doi: 10.1128/jb.141.1.184-189.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Thompson R. C., Davis B. D. Extracellular labeling of nascent polypeptides traversing the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2830–2834. doi: 10.1073/pnas.74.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P. C., Davis B. D. Isolation of polysomes free of initiation factors. Methods Enzymol. 1979;59:362–371. doi: 10.1016/0076-6879(79)59097-1. [DOI] [PubMed] [Google Scholar]
- Tai P. C., Wallace B. J., Herzog E. L., Davis B. D. Properties of initiation-free polysomes of Escherichia coli. Biochemistry. 1973 Feb;12(4):609–615. doi: 10.1021/bi00728a007. [DOI] [PubMed] [Google Scholar]
- Van Tiel-Menkvled G. J., Rezee A., De Graaf F. K. Production and excretion of cloacin DF13 by Escherichia coli harboring plasmid CloDF13. J Bacteriol. 1979 Nov;140(2):415–423. doi: 10.1128/jb.140.2.415-423.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink J., Gankema H., Jansen W. H., Guinée P. A., Witholt B. Isolation of the membranes of an enterotoxigenic strain of Escherichia coli and distribution of enterotoxin activity in different subcellular fractions. Biochim Biophys Acta. 1978 Dec 4;514(1):128–136. doi: 10.1016/0005-2736(78)90082-2. [DOI] [PubMed] [Google Scholar]