Abstract
The high sensitivity of scotopic vision depends on the efficient retinal processing of single photon responses generated by individual rod photoreceptors. At the first synapse in the mammalian retina, rod outputs are pooled by a rod “ON” bipolar cell, which uses a G-protein signaling cascade to enhance the fidelity of the single photon response under conditions where few rods absorb light. Here we show in mouse rod bipolar cells that both splice variants of the Go α subunit, Gαo1 and Gαo2, mediate light responses under the control of mGluR6 receptors, and their coordinated action is critical for maximizing sensitivity. We found that the light response of rod bipolar cells was primarily mediated by Gαo1, but the loss of Gαo2 caused a reduction in the light sensitivity. This reduced sensitivity was not attributable to the reduction in the total number of Go α subunits, or the altered balance of expression levels between the two splice variants. These results indicate that Gαo1 and Gαo2 both mediate a depolarizing light response in rod bipolar cells without occluding each other’s actions, suggesting they might act independently on a common effector. Thus, Gαo2 plays a role in improving the sensitivity of rod bipolar cells through its action with Gαo1. The coordinated action of two splice variants of a single Gα may represent a novel mechanism for the fine control of G-protein activity.
INTRODUCTION
At the first synapse of the visual system, the output of the photoreceptor cells is segregated into ON and OFF pathways, which respond to increments and decrements of light intensity, respectively. ON bipolar cells use a G protein–coupled receptor-signaling pathway to signal light-evoked reductions in glutamate release from the rod photoreceptor spherule. However, unlike the phototransduction cascade, many of the components of the bipolar signaling cascade have yet to be identified. What is known is that a metabotropic glutamate receptor, mGluR6 (Nakajima et al., 1993; Nomura et al., 1994; Masu et al., 1995), senses glutamate release from photoreceptors and conveys this activity through a heterotrimeric G protein, Gαo (Nawy, 1999; Dhingra et al., 2000), to close nonselective cation channels, recently identified to be TRPM1 (Bellone et al., 2008; Koike et al., 2009; Morgans et al., 2009; Shen et al., 2009). However the target of the G protein and the gating particle controlling the TRPM1 current remain unidentified.
Despite the lack of identity of key signaling components in the mGluR6 pathway, work on mammalian rod ON bipolar cells has led to several insights about the pathway’s functional properties. For instance, rod bipolar cells generate responses to light that are briefer than the response of rods (Field and Rieke, 2002; see also Sampath et al., 2005). In addition, a nonlinear threshold for signal transmission between rods and rod bipolar cells (van Rossum and Smith, 1998; Field and Rieke, 2002; Berntson et al., 2004a) produced by saturation of the mGluR6 signaling cascade (Sampath and Rieke, 2004) improves the signal-to-noise ratio of the single photon response by preserving responses in rods absorbing photons while eliminating noise from the majority of rods that do not. These properties are ultimately dependent on the speed and sensitivity of G-protein signaling in the rod bipolar dendrites.
Here we investigated the role played by the Gαo splice variants in setting the properties of the light response in mouse rod bipolar cells. The expression of Gαo in the mouse retina is mainly restricted to ON bipolar cells, with little or no expression in the photoreceptors (Vardi et al., 1993; Vardi, 1998; Dhingra et al., 2000; Dhingra et al., 2002). Two splice variants of the Go α subunit (Gαo1 and Gαo2) are found in mouse ON bipolar cells (Dhingra et al., 2002). However, the expression of Gαo2 is much lower than Gαo1, and electroretinography from knockout mice for each splice variant suggests that rod bipolar responses appeared to require Gαo1, but not Gαo2 (Dhingra et al., 2002). We find surprisingly that both Gαo2 and Gαo1 contribute to dark-adapted responses of rod bipolar cells. Rod bipolar cells in mice lacking Gαo2 exhibited reduced light sensitivity. The reduction in sensitivity was not attributable to the reduction in the retinal expression level of Gαo protein, as ∼50% reduction in total Gαo expression for Gαo+/− mice did not alter light sensitivity. Furthermore light sensitivity was not affected by the altered balance of retinal expression levels between two splice variants in Gαo1+/− mice. These data indicate that the saturation within the mGluR6 signaling cascade that separates the rod single photon response from rod noise is not set by Gαo concentration, and that Gαo2 works in a coordinated manner with Gαo1 to improve the light sensitivity of rod bipolar cells.
MATERIALS AND METHODS
Animals and preparation
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Southern California (Protocol 10890) and followed guidelines set by the National Institutes of Health on the care and use of animals. Several lines of mice were crossed and used in these experiments, including mice lacking Gαo (Jiang et al., 1998), lacking either Gαo splice variants Gαo1 or Gαo2 (Dhingra et al., 2002), or lacking the gap junction subunit connexin 36 (Deans et al., 2002). Wild-type (WT), Cx36−/−, Gαo+/−, Gαo1+/−, and Gαo2−/− mice were used between 6 wk and 3 mo of age. Gαo−/−, Gαo1−/−, and Gαo1−/− Cx36−/− mice rarely survived more than 4 wk and were used at the age of 3–4 wk when their retina reached maturity as assessed by morphology and electroretinography (see Dhingra et al., 2000). Given the mixed 129Sv/C57BL-6J background of these mice (Jiang et al., 1998), comparisons in cellular responses were always made between littermates. The preparation of retinal slices was performed under infrared illumination as described previously (Sampath et al., 2005; Okawa et al., 2010). In brief, mice were dark adapted overnight and sacrificed, and the lens and cornea were removed. Retinas were isolated and kept in Ames’ media equilibrated with 5% CO2/95% O2 at 32°C. A small piece of retina was embedded in agar, and slices were cut with a vibrating microtome, transferred into a recording chamber, and superfused with Ames’ media heated to 35–37°C for recordings.
Electrophysiology and light stimulation
Light-evoked currents in rod bipolar cells and AII amacrine cells were recorded by whole-cell voltage clamp (Vm = −60 mV). The intracellular solution for bipolar cells consisted of (in mM): 125 potassium-aspartate, 10 KCl, 10 HEPES, 5 NMG-HEDTA, 0.5 CaCl2, 1 ATP-Mg, 0.2 GTP-Mg; pH was adjusted to 7.2 with NMG-OH. The intracellular solution for AII amacrine cells consisted of (in mM): 110 cesium-methanesulfonate, 20 TEA-Cl, 10 HEPES, 10 EGTA, 2 QX-314, 1 ATP-Mg, 0.2 GTP-Mg; pH was adjusted to 7.2 with Cs-OH. Both rod bipolar cells and AII amacrine cells types were identified both by the location of cell somas within the inner nuclear layer and their distinct response properties. However when the cell types were difficult to distinguish by these criteria, such as for cells in Gαo−/− and Gαo1−/− mice, they were confirmed by visualizing the axonal stratification within the inner plexiform layer with 100–200 µM Alexa 750 (Invitrogen) added to the internal solution. Full-field 10-ms flashes were delivered from a blue LED (λmax ∼ 470 nm, FWHM ∼ 30 nm) and focused onto the retinal slice with 20X 0.75NA objective (Nikon). Light-evoked currents were low-pass filtered at 300 Hz with an 8-pole Bessel filter and digitized at 1 kHz. The series resistance in these recordings was 10−25 MΩ and was uncompensated. Light intensity was calibrated daily and converted to an effective photon flux at the peak of spectral sensitivity for mouse rhodopsin (λmax ∼ 501 nm) by convolving the power-scaled LED output spectrum with the normalized spectral sensitivity curve for mouse rhodopsin. The number of activated rhodopsins per rod for a given flash was calculated by multiplying this effective photon flux with the estimated collecting area of mouse rods in retinal slices, which we calculated in the experimental setup to be 0.18 µm2 (Cao et al., 2008; Okawa et al., 2010).
Western blotting
Isolated retinas were homogenized in lysis buffer containing protease inhibitor (Roche), 50 mM Tris-HCl (pH 8), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS. The homogenate was treated with 100 U/ml DNase for 30 min at room temperature. The protein concentration was checked using a BCA Protein Quantification Assay (Thermo Fisher Scientific). The extracted protein was run on a 10% NuPAGE Bis-Tris gel (Invitrogen) and transferred to a nitrocellulose membrane using a Transphor Electrophoresis Unit (Hoefer). The membrane was blocked in 10% milk in Tris-buffered saline with Tween-20 (TBST) for 1 h at room temperature and incubated in a Gαo rabbit polyclonal antibody (Santa Cruz Biotechnology, Inc.) in TBST (1:200), or in a Gαo2 mouse monoclonal antibody (clone#101.4, provided by R. Jahn [Max-Planck-Institute for Biophysical Chemistry, Göttingen, Germany] and G. Ahnert-Hilger [Medical University of Berlin, Berlin, Germany]; see Winter et al., 2005) in TBST (1:5,000) at 4°C overnight. The membrane was washed with TBST and incubated with IRDye 800 CW anti-rabbit antibody or anti-mouse antibody (LI-COR) in TBST (1:20,000) for 1 h at room temperature and then washed with TBST. The positive bands were detected and expression quantified using an Odyssey Infrared Image System (LI-COR), with the expression of β-actin used as a loading control for total protein.
Online supplemental material
The supplemental material (Fig. S1) is available online at http://www.jgp.org/cgi/content/full/jgp.201010477/DC1. Fig. S1 A displays the average response to the dimmest flash tested in WT and Gαo2−/− rod bipolar cells. Fig. S1 B documents the relationship between the maximal response to light and the flash strength that evokes a half-maximal response across all WT rod bipolar cells in this study.
RESULTS
Residual responses in Gαo1−/− rod bipolar cells are mediated by Gαo2
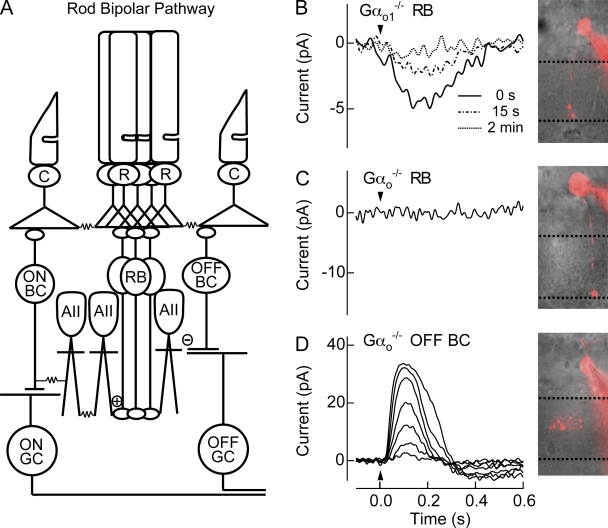
Experimental evidence suggests strongly that Gαo is responsible for transduction channel closure (Nawy, 1999; Dhingra et al., 2000, 2002; Koike et al., 2009), with a splice variant of Gαo, Gαo1, mediating the ON bipolar response (Dhingra et al., 2002). We recorded from rod bipolar cells (Fig. 1 A) in Gαo1−/− mice in an attempt to characterize the influence of Gαo1 on transduction channel gating. Fig. 1 B shows the average response to the first flash for nine rod bipolar cells from the Gαo1−/− retina after achieving the whole-cell voltage-clamp recording (one such cell is visualized). Surprisingly, we found that ON responses persisted in the absence of Gαo1. In Gαo1−/− retinas that showed light responses, rod bipolar cell responses were typically small in amplitude (5.3 + 0.8 pA; n = 9) and decayed quickly after establishing the whole-cell configuration (Fig. 1 B). For comparison, the maximal amplitude of WT rod bipolar responses routinely exceeds several hundred picoamperes (see Table I). Thus, the electroretinography appears to have failed to detect this small remaining ON response (see Dhingra et al., 2002).
Figure 1.
Rod bipolar responses are partially mediated by Gαo2. (A) Schematic of the mammalian rod bipolar pathway. Rod photoreceptors (R) synapse onto rod bipolar cells (RB), which in turn synapse onto AIIACs (AII). Signals from AIIACs, which are coupled to one another by Cx36 gap junctions (Deans et al., 2002), send light-driven signals to ON cone bipolar cells (ON BC) through gap junctions composed of Cx36 on the AII side, and make glycinergic (−) synapses with OFF cone bipolar cells (OFF BC). Each bipolar cells synapses with its respective ganglion cell (GC). Cone photoreceptors (C) are also depicted. (B) A representative Gαo1−/− rod bipolar cell visualized with Alexa 750 and the average flash response of 9 Gαo1−/− rod bipolar cells immediately after whole-cell break in (0 s), and 15 s and 2 min later. The flash strength was 15 Rh*/rod, a strength that saturates WT rod bipolar cells. (C) A representative Gαo−/− rod bipolar cell visualized with Alexa 750 did not generate light responses to flashes producing 32 Rh*/rod. In every rod bipolar cell tested from Gαo−/− mice, rod bipolar light responses were never observed. (D) To confirm viability within the retinal slice, a Gαo−/− Off-bipolar cell located near rod bipolar cell was visualized with Alexa 750, and displayed normal response families, indicating that the lack of rod bipolar responses was not due to the conditions of the retinal slice. Flash strengths were 0.5, 1.0, 2.0, 4.0, 8.0, 16, and 32 Rh*/rod.
Table I.
Response properties of rod bipolar cells and AIIACs in different mouse lines
| Rod bipolar | ||||||
| Gαo+/+ | Gαo+/− | Gαo−/− | Gαo1+/+ | Gαo1+/− | Gαo1−/− | |
| Imax (pA) | 420 ± 38 (14)b | 250 ± 22(15)bd | −0.3 ± 0.3 (10)d | 490 ± 36 (16)b | 350 ± 28(17)bd | 5.3 ± 0.8 (9)d |
| Idark (pA) | −33 ± 4.2 (14) | −28 ± 3.3 (15) | −27 ± 2.2 (10) | −25 ± 3.0 (16) | −27 ± 2.4 (17) | −33 ± 5.0 (9) |
| σ2 (pA2) | 14 ± 3.1 (13)a | 6.4 ± 1.6 (15)ac | 2.2 ± 0.3 (10)c | 9.6 ± 1.3 (14) | 12 ± 2.0 (16)c | 4.7 ± 0.6 (9)c |
| I1/2 (Rh*/rod) | 2.5 ± 0.13 (14) | 2.5 ± 0.17 (15) | 2.8 ± 0.12 (16) | 2.5 ± 0.15 (17) | ||
| n | 1.5 ± 0.04 (14) | 1.6 ± 0.06 (15) | 1.4 ± 0.05 (16) | 1.5 ± 0.07 (17) | ||
| τint (ms) | 120 ± 10 (9.1) | 100 ± 8 (10.0) | 110 ± 6 (11.7) | 120 ± 6 (10.3) | ||
| Tpeak (ms) | 120 ± 4 (9.1)a | 130 ± 5 (10.0)a | 120 ± 3 (11.7) | 120 ± 4 (10.3) | ||
| % Isat | 16 ± 1.2 (9.1) | 15 ± 2.0 (10.0) | 16 ± 1.4 (11.7) | 16 ± 1.7 (10.3) | ||
| Rod bipolar | AIIAC | |||||
| Gαo2+/+ | Gαo2−/− | Gαo1+/+ Cx36−/− | Gαo1−/− Cx36−/− | |||
| Imax (pA) | 430 ± 44 (15) | 370 ± 37 (16) | 210 ± 44 (10)a | 100 ± 22 (9)a | ||
| Idark (pA) | −30 ± 4.3 (16) | −29 ± 2.7 (16) | ||||
| σ2 (pA2) | 12 ± 2.0 (14) | 13 ± 1.8 (16) | ||||
| I1/2 (Rh*/rod) | 2.2 ± 0.15 (15)a | 2.6 ± 0.19 (16)a | 0.17 ± 0.01(10)b | 2.6 ± 0.13 (9)b | ||
| n | 1.5 ± 0.02 (15) | 1.6 ± 0.05 (16) | 1.6 ± 0.09 (10) | 1.5 ± 0.09 (9) | ||
| τint (ms) | 120 ± 7 (9.2) | 130 ± 11 (8.0) | ||||
| Tpeak (ms) | 120 ± 4 (9.2) | 130 ± 8 (8.0) | ||||
| % Isat | 19 ± 1.8 (9.2)a | 14 ± 1.6 (8.0)a | ||||
All the values are given as mean ± SEM (n). The effective number of cells was used to calculate the SEM of τint, Tpeak, and % Isat (see Sampath et al., 2005). Imax is the maximal response amplitude. Idark and σ2 are the mean and the variance of holding current measured in the first 5 s after the establishment of whole-cell configuration. I1/2 is the half-maximal flash strength. n is the exponent in the Hill Equation fit to flash strength vs. normalized response amplitude curves. τint and Tpeak are the integration time and the time-to-peak of dim flash responses. % Isat is the fractional amplitude of a dim flash response to an average flash strength of 1 Rh*/rod.
P < 0.05, significant difference between littermates.
P < 0.01, significant difference between littermates.
P < 0.05, significant difference between Gαo−/− or Gαo1−/− rod bipolar cells compared to their heterozygote.
P < 0.01, significant difference between Gαo−/− or Gαo1−/− rod bipolar cells compared to their heterozygote.
Previous work indicated that ON bipolar cells also express at a lower level the splice variant Gαo2 in addition to Gαo1 (Dhingra et al., 2002). To determine if Gαo2 generated the small residual response in Gαo1−/− mice, we recorded from rod bipolar cells in the full Gαo knockout (Gαo−/−). Voltage-clamp recordings (Vm = −60 mV) from rod bipolar cells in Gαo−/− mice are shown in Fig. 1 C, and indicate that the ON response was completely lost from all ON bipolar cells tested (n = 23), including rod bipolar cells (10 of 23). Neighboring OFF bipolar cells in the same retinal slices demonstrated normal responses (n = 6; Fig. 1 D). Thus, Gαo2 appears to mediate the remaining response in Gαo1−/− rod bipolar cells. Interestingly, the initial holding current in voltage-clamp recordings from Gαo−/− rod bipolar cells was not statistically different from that in WT cells (Table I), indicating that transduction channels remained closed despite the loss of Gαo.
Characterization of Gαo2-mediated rod bipolar responses in AII amacrine cells
The Gαo2-mediated ON response in Gαo1−/− rod bipolar cells was small and decayed too quickly to be characterized. To assess the sensitivity of the Gαo2-mediated response in rod bipolar cells we instead recorded their output in the postsynaptic AII amacrine cells (AIIAC; see Fig. 1 A). Because AIIACs are more sensitive than rod bipolar cells and operate at light levels where few of the rod bipolar cell inputs are active (Pang et al., 2004; Dunn et al., 2006), their light responses will reflect subtle changes in the rod bipolar response. In addition, AIIACs are not subject to washout because their response is mediated by ionotropic glutamate receptors (Boos et al., 1993; Hartveit and Veruki, 1997). To isolate the direct output of rod bipolar cells, we eliminated input to the recorded AIIACs from neighboring AIIACs and ON cone bipolar cells by crossing Gαo1 mice with Cx36−/− mice (Deans et al., 2002; see Fig. 1 A).
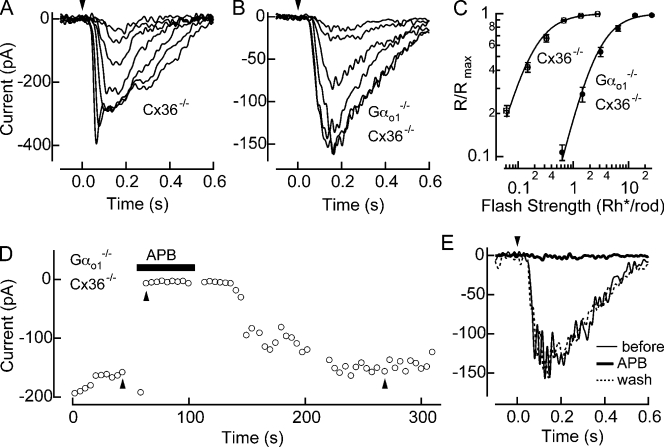
Fig. 2 (A and B) shows voltage-clamped (Vm = −60 mV) response families to flashes of increasing strength from Gαo1+/+ Cx36−/− and Gαo1−/− Cx36−/− AIIACs. The maximum response amplitude among all the Gαo1−/− Cx36−/− AIIACs tested was ∼200 pA (n = 9), indicating that even small rod bipolar responses mediated by Gαo2 can produce more substantial changes in downstream signals. In Fig. 2 C, the normalized response amplitude is plotted versus the flash strength and reveals that response families in Gαo1−/− Cx36−/− AIIACs are shifted to ∼10-fold brighter flash strengths compared with Gαo1+/+ Cx36−/− AIIACs. Furthermore, the maximal response amplitude of Gαo1−/− Cx36−/− AIIACs was, on average, approximately twofold smaller than Gαo1+/+ Cx36−/− AIIACs (Table I). Provided that AIIACs provide an accurate measure of rod bipolar sensitivity, this suggests that the rod bipolar response mediated by Gαo2 alone is ∼20-fold less sensitive than the response mediated by both Gαo1 and Gαo2. Interestingly we find that dark-adapted light responses to the strongest flashes in the Gαo1−/− Cx36−/− AIIACs lacked the initial nose seen under normal circumstances (Nelson, 1982), suggesting that rod bipolar responses mediated by Gαo2 alone are not able to fully drive glutamate release from the rod bipolar synaptic terminal.
Figure 2.
Gαo2-mediated light responses measured in Gαo1−/− Cx36−/− AIIACs. (A and B) Flash response families were recorded in a Cx36−/− (i.e., Gαo1+/+ Cx36−/− littermate) AIIAC (A) and a Gαo1−/− Cx36−/− AIIAC (B). Flash strengths in the Cx36−/− AIIAC were 0.04, 0.1, 0.22, 0.46, 0.94, 1.9, and 3.8 Rh*/rod, and in the Gαo1+/+ Cx36−/− AIIAC were 0.63, 1.5, 3.1, 6.5, 13, and 27 Rh*/rod. (C) Normalized response amplitudes from individual families were averaged across cells for Cx36−/− AIIACs (n = 10) and Gαo1−/− Cx36−/− AIIACs (n = 9), and plotted as a function of the flash strength. Half-maximal flash strengths estimated from the Hill curve fits were 0.17 ± 0.01 and 2.56 ± 0.13 Rh*/rod (mean ± SEM) for Cx36−/− and Gαo1−/− Cx36−/− AIIACs, respectively. (D) Changes in the amplitude of the maximal flash response as a function of time before, during, and after application of APB are plotted. (E) Maximal flash responses (27 Rh*/rod) in a Gαo1−/− Cx36−/− AIIAC before, during, and after the bath application of 8 µM APB, as marked by upward arrows in D.
Gαo2-mediated responses were also controlled by mGluR6. Fig. 2 D plots the maximal inward response amplitude during the application of the mGluR6 agonist, l-2-aminophosphonobutyric acid (APB), for Gαo1−/− Cx36−/− AIIACs. APB (8 μM) completely suppressed the response in Gαo1−/− Cx36−/− AIIACs, an effect that was reversible after washout (Fig. 2 E). Thus, both the Gαo1 and the Gαo2 mediate a depolarizing light response in rod bipolar cells through the activity of mGluR6.
Reduced amplitude and sensitivity of light responses in Gαo2−/− rod bipolar cells
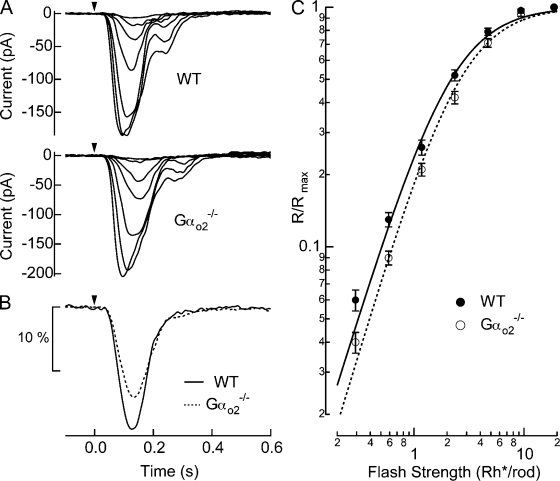
We assessed the functional role played by Gαo2 on the dark-adapted response of rod bipolar cells in Gαo2−/− mice (Fig. 3 A). Response families in Gαo2−/− rod bipolar cells appeared similar to WT, with statistically indistinguishable maximal amplitudes (Table I). The time-to-peak and integration time (defined as the integral of the dim flash response divided by its peak amplitude) of the dim flash response was also statistically indistinguishable from WT rod bipolar cells (Fig. 3 B; see Table I). However, the loss of Gαo2 caused a reduction in the amplitude of the Gαo2−/− dim flash responses (Fig. 3 B; see also Fig. S1 A), which led to an overall reduction of light sensitivity of rod bipolar cells, as seen by the shift to higher flash strengths in the plot of normalized response amplitude versus flash strength (Fig. 3 C). The half-maximal flash strength provides a robust measure of the sensitivity of rod bipolar cells that is independent of the maximal response amplitude (Fig. S1 B). Thus the presence of Gαo2 increases the sensitivity of the average response to a dim flash in rod bipolar cells of WT mice.
Figure 3.
Gαo2−/− rod bipolar cells exhibited reduced light sensitivity. (A) Responses to a family of flashes producing 0.29, 0.59, 1.2, 2.3, 4.7, 9.4, and 19 Rh*/rod were recorded in a WT (i.e., Gαo2+/+ littermate) and a Gαo2−/− rod bipolar cell. (B) Normalized rod bipolar response to dim flashes producing 1 Rh*/rod was estimated by averaging normalized responses to dim flashes casing 5–25% of maximal responses and dividing those by the average flash strength, which was 0.60 Rh*/rod for WT and 0.72 Rh*/rod for Gαo2−/− rod bipolar cells. The WT response is the average of 332 dim flash responses across 15 cells from 8 mice, and Gαo2−/− response is the average of 321 dim flash responses across 16 cells from 6 mice. (C) Normalized response amplitudes from individual families were averaged across cells for WT (n = 15) and Gαo2−/− rod bipolar cells (n = 16), and plotted as a function of flash strength. Half-maximal flash strengths estimated from the Hill curve fits were 2.2 ± 0.15 vs. 2.6 ± 0.19 Rh*/rod, and the Hill exponents were 1.51 ± 0.02 vs. 1.55 ± 0.05 for WT vs. Gαo2−/− rod bipolar cells, respectively (mean ± SEM). While differences in the Hill exponent were not statistically significant (P = 0.13), the shift in half-maximal flash strengths was significant (P = 0.047).
To determine how the decreased amplitude of the dim flash response influenced its detection, we characterized how the absence of Gαo2 impacted the dark noise. We calculated the total variance (0–300 Hz) of the noise in darkness for Gαo2−/− and WT rod bipolar cells in the 5 s immediately after establishing the whole-cell recording for the cells shown in Fig. 3. The total variance of the dark noise in WT rod bipolar cells was 11.5 ± 2.0 pA2 (n = 14) and in Gαo2−/− rod bipolar cells was 12.7 ± 1.8 pA2 (n = 16) (mean ± SEM; P = 0.67), values that are indistinguishable statistically. The loss of Gαo2 appears then to cause a reduction in the amplitude of the light response with the magnitude of the dark noise remaining unchanged, resulting in an overall reduced signal-to-noise ratio in Gαo2−/− rod bipolar cells.
Reducing the total expression of Gαo does not alter rod bipolar responses
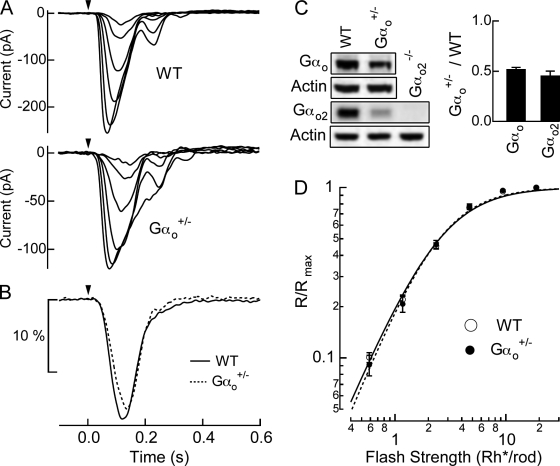
Reduced sensitivity in Gαo2−/− rod bipolar cells may be simply due to the decrease in the total amount of Gαo protein rather than any specific role played by Gαo2. To test whether the concentration of Gαo influenced response sensitivity, we recorded rod bipolar responses from heterozygous mice for Gαo (Gαo+/−). As shown in Fig. 4 C, Western blot analysis for the whole retina using an antibody raised against Gαo confirmed that Gαo+/− retinas had reduced Gαo expression by ∼50% compared with WT. Since Gαo expression in the mouse retina is primarily in ON bipolar cells (Vardi et al., 1993; Vardi, 1998; Dhingra et al., 2000, 2002), and rod bipolar cells are approximately one third of all bipolar cells (Dhingra et al., 2008; Wassle et al., 2009), we expect the Gαo expression in rod bipolar cells is also approximately halved. Despite the loss of half of Gαo1 and Gαo2 (Fig. 4 C), the overall response kinetics and the sensitivity of Gαo+/− rod bipolar cells remained similar to those of the littermate WT rod bipolar cells (Fig. 4, A and D; Table I). While the average time-to-peak was delayed slightly in Gαo+/− rod bipolar cells (from 118 to 133 ms; see Table I), the integration time of the dim flash response was statistically indistinguishable from WT rod bipolar cells (Fig. 4 B; Table I). Thus, the reduced sensitivity in Gαo2−/− rod bipolar cells appears instead to result from a specific effect of Gαo2, and not from a reduction in the overall Gαo level. Furthermore, Fig. 4 D shows the Hill exponent between WT and Gαo+/− rod bipolar cells are statistically identical, indicating that saturation within the mGluR6 cascade is not set by the Gαo concentration (see Discussion).
Figure 4.
Reduced Gαo expression in Gαo+/− mice does not alter rod bipolar responses (A) Responses to a family of flashes producing 0.59, 1.2, 2.4, 4.7, 9.4, and 19 Rh*/rod were recorded in a WT (Gαo+/+ littermate), and a Gαo+/− rod bipolar cell. (B) Normalized rod bipolar response to dim flashes producing 1 Rh*/rod was estimated by averaging normalized responses to dim flashes casing 5–25% of maximal responses and dividing it by the average dim flash strength, which was 0.73 Rh*/rod for WT and 0.79 Rh*/rod for Gαo+/−. The WT response is the average of 437 dim flash responses across 14 cells from 3 mice, and the Gαo+/− response is the average of 271 dim flash responses across 15 cells from 3 mice. (C) The total amount of Gαo and Gαo2 proteins in WT and Gαo+/− retinas were compared using Western blot analysis. The amount of Gαo2 proteins in Gαo2−/− retinas was also examined to check the specificity of the antibody. The protein level of Gαo+/− retina was normalized to that of WT retina for a pair of WT and Gαo+/− mice used in one experiment, and the collected results are shown in the bar graph. The error bars are the SEM. The Gαo protein levels were 1 vs. 0.52 ± 0.02 (n = 4) and the Gαo2 protein levels were 1 vs. 0.46 ± 0.04 (n = 3) (mean ± SEM, WT vs. Gαo+/−). (D) Normalized response amplitudes from individual families were averaged across cells for WT rod bipolar cells (n = 14) and Gαo+/− rod bipolar cells (n = 15) and plotted as a function of flash strength. Half-maximal flash strengths estimated from the Hill curve fits were 2.5 ± 0.13 vs. 2.5 ± 0.17 Rh*/rod, and the Hill exponents were 1.54 ± 0.04 vs. 1.62 ± 0.06 (mean ± SEM, WT vs. Gαo+/−).
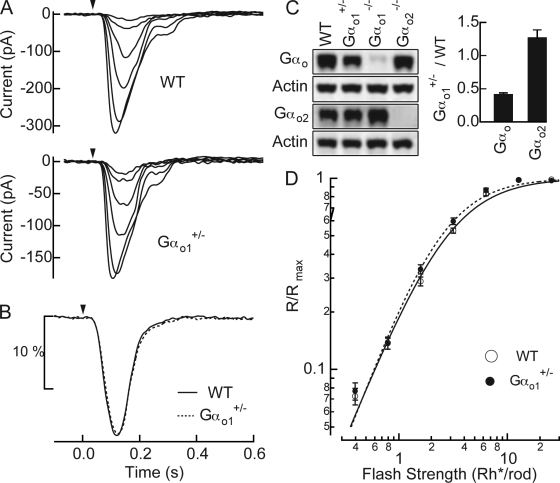
Altering the balance of expression between Gαo1 and Gαo2 does not alter rod bipolar responses
Splice variants of G proteins typically display alterations in cellular functions, and frequently act on different effectors in the same cell. Gαo1 and Gαo2 both mediate depolarizing light responses in rod bipolar cells (Figs. 1 and 2), suggesting in the simplest scheme that they act on a common effector in the mGluR6-signaling cascade, although actions on different effectors cannot be ruled out. Regulation of the effector might then be dependent on the relative ratios of each of these splice variants. We tested how the ratio of Gαo1 to Gαo2 influences the properties of rod bipolar light responses in Gαo1+/− mice. Fig. 5 C shows that the total Gαo expression was decreased by ∼60% in these mice, whereas Gαo2 expression was increased by ∼25% compared with WT retinas. Overall, the ratio of Gαo2 expression over Gαo1 increased approximately threefold in Gαo1+/− retinas compared with WT. Since the presence of Gαo2 increased the sensitivity of the light response (Fig. 3), increasing the relative ratio of Gαo2 to Gαo1 might further increase the sensitivity of rod bipolar cells. However, neither the half-maximal flash strength nor the nonlinearity of flash response family in Gαo1+/− rod bipolar cells differed from those in WT rod bipolar cells (Fig. 5, A and D; Table I). The time-to-peak and the integration time of the dim flash response were also statistically indistinguishable from WT (Table I). Thus, the balance of expression levels between two splice variants cannot explain the coordinated action of these splice variants in increasing the sensitivity of the rod bipolar cell response.
Figure 5.
Altered Gαo1 vs. Gαo2 expression in Gαo1+/− mice does not alter rod bipolar responses (A) Responses to a family of flashes producing 0.4, 0.8, 1.6, 3.2, 6.4, and 13 Rh*/rod were recorded in a WT (Gαo1+/+ littermate) and a Gαo1+/− rod bipolar cell. (B) Normalized rod bipolar response to dim flashes producing 1 Rh*/rod was estimated by averaging normalized responses to dim flashes casing 5–25% of maximal responses and dividing it by the average dim flash strength, which was 0.70 Rh*/rod for WT and 0.72 Rh*/rod for Gαo1+/−. The WT response is the average of 351 dim flash responses across 16 cells from 4 mice and the Gαo1+/− response is the average of 331 dim flash responses across 17 cells from 4 mice. (C) The total amount of Gαo and Gαo2 proteins in WT, Gαo1+/−, Gαo1−/−, and Gαo2−/− retinas were compared with Western blot analysis. The protein levels were normalized to those of WT retinas for a group of mice used in one experiment, and the results of repeated experiments are shown in the bar graph. The error bars show SEM. The Gαo protein levels were 1 vs. 0.42 ± 0.02 vs. 0.05 ± 0.01 vs. 0.95 ± 0.22 (n = 3), and the Gαo2 protein levels were 1 vs. 1.27 ± 0.11 vs. 1.84 ± 0.16 vs. 0.01 ± 0.01 (n = 3) (mean ± SEM, WT vs. Gαo1+/− vs. Gαo1−/− vs. Gαo2−/−). (D) Normalized response amplitudes from individual families were averaged across cells for WT rod bipolar cells (n = 16) and Gαo1+/− rod bipolar cells (n = 17) and plotted as a function of flash strengths. Half-maximal flash strengths estimated from the Hill curve fits were 2.81 ± 0.12 vs. 2.47 ± 0.14 Rh*/rod, and the Hill exponents were 1.43 ± 0.05 vs. 1.54 ± 0.07 (mean ± SEM, WT vs. Gαo1+/−), and are statistically indistinguishable (P = 0.12 for half-maximal flash intensity values, and P = 0.19 for Hill exponents between WT and Gαo1+/− rod bipolar cells).
DISCUSSION
G proteins are essential signaling molecules that connect hundreds of G protein–coupled receptors with a relatively limited number of downstream effectors. In particular, G-protein signaling cascades play fundamental roles in early visual processing where they generate the response to light exposure in both rod and cone photoreceptor cells, and in ON bipolar cells. In ON bipolar cells, relatively little is know about the intermediate components of the signaling cascade that allow mGluR6 receptors through the action of Gαo to close TRPM1 transduction channels (for reviews see Okawa and Sampath, 2007; Snellman et al., 2008). Here we have studied how Gαo activity influences the dark-adapted light response in mouse rod bipolar cells and found the following: (a) the coordinated action of two splice variants of Gαo (Gαo1 and Gαo2) maximizes light sensitivity, (b) reductions in the concentration of Gαo do not influence the open probability of transduction channels, and (c) the nonlinear threshold due to the saturation of the transduction cascade does not depend on the Gαo concentration.
Coordinated actions of Gαo1 and Gαo2 improve rod bipolar sensitivity
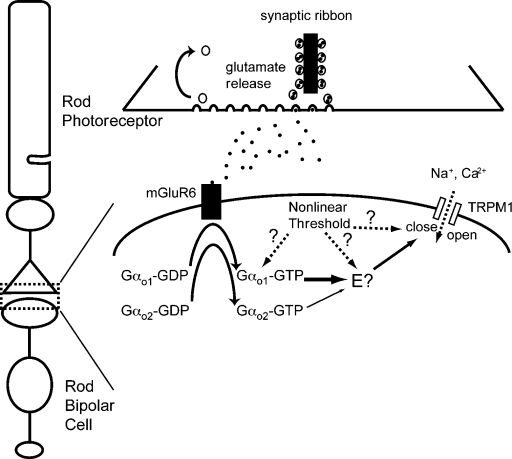
The α subunit of Go is expressed as two splice variants (Gαo1 and Gαo2) that differ by 26 amino acids in the GTPase domain near the C-terminal end (Hsu et al., 1990; Strathmann et al., 1990; Tsukamoto et al., 1991), a region known to link αo subunits to their receptors and effectors (for review see Clapham and Neer, 1997). Gαo splice variants have typically been assigned with different or redundant functions. For instance, in the rat pituitary GH3 cells, Gαo1 and Gαo2 mediate Ca2+ channel inhibition through muscarinic and somatostatin receptors, respectively (Kleuss et al., 1991, 1993; Degtiar et al., 1997). In rod bipolar cells, both Gαo1 and Gαo2 are controlled by the mGluR6 receptor and mediate the depolarizing light response (Figs. 1 and 2) without occluding each other’s actions (Fig. 5). The most parsimonious explanation for these two facts are that both splice variants act independently on a common effector, as diagrammed in Fig. 6, however, we cannot rule out actions on different effectors.
Figure 6.
Proposed mGluR6-signaling cascade in rod bipolar cells. mGluR6 receptors activated upon binding glutamate released from rods exchange GTP for GDP on both Gαo1 and Gαo2, which leads to the closure of nonselective cation channels (TRPM1) through an unknown downstream cascade. The efficiency of the Gαo2 pathway is lower than that of the Gαo1 pathway, as represented by the thin arrow leading to the putative effector (E?). While a single effector is shown, this work does not exclude the possibility that Gαo1 and Gαo2 act on separate effectors that lead to the coordinated closure of TRPM1 gating. Arrows show that nonlinearity in the signaling cascade might reside at several locations.
Although Gαo2-mediated signals are much less efficient than Gαo1-mediated signals, a feature that may result from differing affinities of each splice variant for mGluR6 or the effector, the reduced efficiency likely reflects the relatively low expression of Gαo2 compared with Gαo1 (Dhingra et al., 2002). However, given that AIIACs are highly sensitive to rod bipolar cell input (Dunn and Rieke, 2008; Tian et al., 2010), any subtle variation in the rod bipolar response should result in detectable changes in AIIAC activity. We find that the amplitude of dim flash responses per photon in rod bipolar cells of Gαo2−/− mice is ∼25% smaller on average than in WT rod bipolar cells (Fig. 3 B; Fig. S1 A; Table I). This reduced sensitivity is attributable to a Gαo2-specific effect because it cannot be explained either by the total Gαo concentration or the balance of expression between Gαo1 and Gαo2. Thus, the light response in rod bipolar cells is primarily mediated by Gαo1, but Gαo2 is necessary to increase the magnitude of the response without increasing the dark noise, thereby increasing the signal-to-noise ratio. Such coordination between two splice variants of a single Gα may represent a novel mechanism that fine tunes the functional properties of signaling cascades.
TRPM1 channels remain closed in the absence of Gαo activity
A surprising finding of this work is that reductions of Gαo concentration (Figs. 4 and 5), or even the elimination of Gαo entirely (Fig. 1), does not appear to influence the amplifier holding current for voltage-clamped (Vm = −60 mV) rod bipolar cells (see Table I). The interpretation of this result is that reductions in Gαo concentration do not correspond to increases in the nonselective cation current of TRPM1 channels. Previous studies for TRPM1 channels expressed in CHO cells (Koike et al., 2009) suggest that these channels are constitutively open, with the presumed role of Gαo to close them (Nawy, 1999; Dhingra et al., 2000, 2002; Koike et al., 2009). The lack of influence of Gαo on the open probability of TRPM1 channels argues that this scheme is more complicated in situ, and may require additional factors for TRPM1 opening (Fig. 6). Alternatively, strong Ca2+-dependent reductions in TRPM1 open probability (Nawy 2000, 2004; Berntson et al., 2004b) may relegate these channels closed even in the absence of Gαo.
Gαo concentration does not set the nonlinear thresholding of rod signals
Our most sensitive vision is encoded in a specialized retinal circuit that pools rod signals, known as the rod bipolar pathway (see Fig. 1 A; Dacheux and Raviola, 1986; Smith et al., 1986). Under conditions where few rod photoreceptors receive photons, downstream cells must discriminate between rods that absorb light from those that do not. The optimization of signal transfer in this pathway requires a nonlinear threshold in rod bipolar cells that separates the single photon response from noise (van Rossum and Smith, 1998; Field and Rieke, 2002), which is generated by saturation of the postsynaptic signaling cascade in the rod bipolar cell dendrites and not by mGluR6 receptor saturation (Sampath and Rieke, 2004). The molecular mechanism that underlies the nonlinear threshold is not well defined, largely due to the uncertain identity of components of this signaling cascade downstream of Gαo.
Here we show that the nonlinear threshold is not influenced by an ∼50% reduction in concentration of retinal Gαo (Fig. 4), providing insight into where saturation may occur in the mGluR6 signaling cascade. If the rate of G-protein activation is saturated, such that the reduced Gαo expression does not cause an equivalent reduction in G-protein activity, these results indicate that the binding of Gαo to mGluR6 does not cause this saturation. However, we cannot eliminate the possibility that the exchange of GTP for GDP on Gαo, or the dissociation of Gαo from mGluR6, places a bottleneck on the dark steady-state G-protein activity. Experimental evidence from rod outer segment preparations indicate that transducin (Gαt) activation can occur very quickly (>1000 s−1 at physiological temperatures; Bruckert et al., 1992; Heck and Hofmann, 2001), perhaps not totally surprising given the high concentration of transduction elements. However, relatively little is known about G-protein activation rates in other intact systems. It remains to be seen whether GTP exchange and Gα dissociation limit Gαo activation on the ∼120 ms integration time of dark-adapted rod bipolar light responses.
If the rate of G-protein activation by mGluR6 is not saturated in darkness, then these results would indicate that the position of the nonlinear threshold must reside downstream of Gαo activation (see Fig. 6), or alternatively that G-protein activity is sufficient to saturate a downstream component of the signaling cascade even under conditions where this activity is reduced (i.e., Gαo+/−). Saturation could potentially be in the activity of the effector molecule that controls the gating particle of TRPM1, or in the open probability of TRPM1 itself (compare Sampath and Rieke, 2004). For the level of saturation to be optimized with respect to the rod signal and noise, it must be set high enough to eliminate most of the continuous noise produced by spontaneous PDE activation, but not to eliminate too many single photon responses (Field and Rieke, 2002). Thus a delicate trade-off between noise and sensitivity must exist, giving great importance to identifying the component of the signaling cascade mediating this nonlinear step.
Acknowledgments
We thank Dr. David Paul for providing connexin 36−/− mice, Dr. Jeannie Chen’s laboratory for help with Western blotting, Josh Miyagishima for critical review of the manuscript, and Vadim Arshavsky for helpful discussions.
This work was supported by National Institutes of Health grants EY17606 (A.P. Sampath) and EY11850 (F. Rieke), the Intramural Research Program of the National Institutes of Health Z01 ES101643 (L. Birnbaumer), the Karl Kirchgessner Foundation (A.P. Sampath), and the McKnight Endowment Fund for Neurosciences (A.P. Sampath).
Edward N. Pugh Jr. served as editor.
Footnotes
Abbreviation used in this paper:
- APB
- l-2-aminophosphonobutyric acid
References
- Bellone R.R., Brooks S.A., Sandmeyer L., Murphy B.A., Forsyth G., Archer S., Bailey E., Grahn B. 2008. Differential gene expression of TRPM1, the potential cause of congenital stationary night blindness and coat spotting patterns (LP) in the Appaloosa horse (Equus caballus). Genetics. 179:1861–1870 10.1534/genetics.108.088807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson A., Smith R.G., Taylor W.R. 2004a. Transmission of single photon signals through a binary synapse in the mammalian retina. Vis. Neurosci. 21:693–702 [DOI] [PubMed] [Google Scholar]
- Berntson A., Smith R.G., Taylor W.R. 2004b. Postsynaptic calcium feedback between rods and rod bipolar cells in the mouse retina. Vis. Neurosci. 21:913–924 [DOI] [PubMed] [Google Scholar]
- Boos R., Schneider H., Wassle H. 1993. Voltage- and transmitter-gated currents of all-amacrine cells in a slice preparation of the rat retina. J. Neurosci. 13:2874–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckert F., Chabre M., Vuong T.M. 1992. Kinetic analysis of the activation of transducin by photoexcited rhodopsin. Influence of the lateral diffusion of transducin and competition of guanosine diphosphate and guanosine triphosphate for the nucleotide site. Biophys. J. 63:616–629 10.1016/S0006-3495(92)81650-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Song H., Okawa H., Sampath A.P., Sokolov M., Martemyanov K.A. 2008. Targeting of RGS7/Gbeta5 to the dendritic tips of ON-bipolar cells is independent of its association with membrane anchor R7BP. J. Neurosci. 28:10443–10449 10.1523/JNEUROSCI.3282-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D.E., Neer E.J. 1997. G protein beta gamma subunits. Annu. Rev. Pharmacol. Toxicol. 37:167–203 10.1146/annurev.pharmtox.37.1.167 [DOI] [PubMed] [Google Scholar]
- Dacheux R.F., Raviola E. 1986. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J. Neurosci. 6:331–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans M.R., Volgyi B., Goodenough D.A., Bloomfield S.A., Paul D.L. 2002. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 36:703–712 10.1016/S0896-6273(02)01046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtiar V.E., Harhammer R., Nurnberg B. 1997. Receptors couple to L-type calcium channels via distinct Go proteins in rat neuroendocrine cell lines. J. Physiol. 502:321–333 10.1111/j.1469-7793.1997.321bk.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A., Lyubarsky A., Jiang M., Pugh E.N., Jr., Birnbaumer L., Sterling P., Vardi N. 2000. The light response of ON bipolar neurons requires G[alpha]o. J. Neurosci. 20:9053–9058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A., Jiang M., Wang T.L., Lyubarsky A., Savchenko A., Bar-Yehuda T., Sterling P., Birnbaumer L., Vardi N. 2002. Light response of retinal ON bipolar cells requires a specific splice variant of Galpha(o). J. Neurosci. 22:4878–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A., Sulaiman P., Xu Y., Fina M.E., Veh R.W., Vardi N. 2008. Probing neurochemical structure and function of retinal ON bipolar cells with a transgenic mouse. J. Comp. Neurol. 510:484–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn F.A., Doan T., Sampath A.P., Rieke F. 2006. Controlling the gain of rod-mediated signals in the Mammalian retina. J. Neurosci. 26:3959–3970 10.1523/JNEUROSCI.5148-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn F.A., Rieke F. 2008. Single-photon absorptions evoke synaptic depression in the retina to extent the operational range of rod vision. Neuron. 57:894–904 10.1016/j.neuron.2008.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field G.D., Rieke F. 2002. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron. 34:773–785 10.1016/S0896-6273(02)00700-6 [DOI] [PubMed] [Google Scholar]
- Hartveit E., Veruki M.L. 1997. AII amacrine cells express functional NMDA receptors. Neuroreport. 8:1219–1223 10.1097/00001756-199703240-00032 [DOI] [PubMed] [Google Scholar]
- Heck M., Hofmann K.P. 2001. Maximal rate and nucleotide dependence of rhodopsin-catalyzed transducin activation: initial rate analysis based on a double displacement mechanism. J. Biol. Chem. 276:10000–10009 10.1074/jbc.M009475200 [DOI] [PubMed] [Google Scholar]
- Hsu W.H., Rudolph U., Sanford J., Bertrand P., Olate J., Nelson C., Moss L.G., Boyd A.E., Codina J., Birnbaumer L. 1990. Molecular cloning of a novel splice variant of the alpha subunit of the mammalian Go protein. J. Biol. Chem. 265:11220–11226 [PubMed] [Google Scholar]
- Jiang M., Gold M.S., Boulay G., Spicher K., Peyton M., Brabet P., Srinivasan Y., Rudolph U., Ellison G., Birnbaumer L. 1998. Multiple neurological abnormalities in mice deficient in the G protein Go. Proc. Natl. Acad. Sci. USA. 95:3269–3274 10.1073/pnas.95.6.3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleuss C., Scherubl H., Hescheler J., Schultz G., Wittig B. 1993. Selectivity in signal transduction determined by gamma subunits of heterotrimeric G proteins. Science. 259:832–834 10.1126/science.8094261 [DOI] [PubMed] [Google Scholar]
- Kleuss C., Hescheler J., Ewel C., Rosenthal W., Schultz G., Wittig B. 1991. Assignment of G-protein subtypes to specific receptors inducing inhibition of calcium currents. Nature. 353:43–48 10.1038/353043a0 [DOI] [PubMed] [Google Scholar]
- Koike C., Obara T., Uriu Y., Numata T., Sanuki R., Miyata K., Koyasu T., Ueno S., Funabiki K., Tani A., et al. 2009. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc. Natl. Acad. Sci. USA. 107:332–337 10.1073/pnas.0912730107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M., Iwakabe H., Tagawa Y., Miyoshi T., Yamashita M., Fukuda Y., Sasaki H., Hiroi K., Nakamura Y., Shigemoto R., et al. 1995. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell. 80:757–765 10.1016/0092-8674(95)90354-2 [DOI] [PubMed] [Google Scholar]
- Morgans C.W., Zhang J., Jeffrey B.G., Nelson S.M., Burke N.S., Duvoisin R.M., Brown R.L. 2009. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc. Natl. Acad. Sci. USA. 106:19174–19178 10.1073/pnas.0908711106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y., Iwakabe H., Akazawa C., Nawa H., Shigemoto R., Mizuno N., Nakanishi S. 1993. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J. Biol. Chem. 268:11868–11873 [PubMed] [Google Scholar]
- Nawy S. 1999. The metabotropic receptor mGluR6 may signal through G(o), but not phosphodiesterase, in retinal bipolar cells. J. Neurosci. 19:2938–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S. 2000. Regulation of the On bipolar cell mGluR6 pathway by Ca2+. J. Neurosci. 20:4471–4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S. 2004. Desensitization of the mGluR6 transduction current in tiger salamander On bipolar cells. J. Physiol. 558:137–146 10.1113/jphysiol.2004.064980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. 1982. AII amacrine cells quicken time course of rod signals in the cat retina. J. Neurophysiol. 47:928–947 [DOI] [PubMed] [Google Scholar]
- Nomura A., Shigemoto R., Nakamura Y., Okamoto N., Mizuno N., Nakanishi S. 1994. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 77:361–369 10.1016/0092-8674(94)90151-1 [DOI] [PubMed] [Google Scholar]
- Okawa H., Miyagishima K.J., Arman A.C., Hurley J.B., Field G.D., Sampath A.P. 2010. Optimal processing of photoreceptor signals is required to maximize behavioral sensitivity. J. Physiol. 588:1947–1960 10.1113/jphysiol.2010.188573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okawa H., Sampath A.P. 2007. Optimization of single-photon response transmission at the rod-to-rod bipolar synapse. Physiology (Bethesda). 22:279–286 [DOI] [PubMed] [Google Scholar]
- Pang J.J., Gao F., Wu S.M. 2004. Light-evoked current responses in rod bipolar cells, cone depolarizing bipolar cells and AII amacrine cells in dark-adapted mouse retina. J. Physiol. 558:897–912 10.1113/jphysiol.2003.059543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath A.P., Rieke F. 2004. Selective transmission of single photon responses by saturation at the rod-to-rod bipolar synapse. Neuron. 41:431–443 10.1016/S0896-6273(04)00005-4 [DOI] [PubMed] [Google Scholar]
- Sampath A.P., Strissel K.J., Elias R., Arshavsky V.Y., McGinnis J.F., Chen J., Kawamura S., Rieke F., Hurley J.B. 2005. Recoverin improves rod-mediated vision by enhancing signal transmission in the mouse retina. Neuron. 46:413–420 10.1016/j.neuron.2005.04.006 [DOI] [PubMed] [Google Scholar]
- Shen Y., Heimel J.A., Kamermans M., Peachey N.S., Gregg R.G., Nawy S. 2009. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J. Neurosci. 29:6088–6093 10.1523/JNEUROSCI.0132-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.G., Freed M.A., Sterling P. 1986. Microcircuitry of the dark-adapted cat retina: functional architecture of the rod-cone network. J. Neurosci. 6:3505–3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellman J., Kaur T., Shen Y., Nawy S. 2008. Regulation of ON bipolar cell activity. Prog. Retin. Eye Res. 27:450–463 10.1016/j.preteyeres.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathmann M., Wilkie T.M., Simon M.I. 1990. Alternative splicing produces transcripts encoding two forms of the alpha subunit of GTP-binding protein Go. Proc. Natl. Acad. Sci. USA. 87:6477–6481 10.1073/pnas.87.17.6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M., Jarsky T., Murphy G.J., Rieke F., Singer J.H. 2010. Voltage-gated Na channels in AII amacrine cells accelerate scotopic light responses mediated by the rod bipolar cell pathway. J. Neurosci. 30:4650–4659 10.1523/JNEUROSCI.4212-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T., Toyama R., Itoh H., Kozasa T., Matsuoka M., Kaziro Y. 1991. Structure of the human gene and two rat cDNAs encoding the alpha chain of GTP-binding regulatory protein Go: two different mRNAs are generated by alternative splicing. Proc. Natl. Acad. Sci. USA. 88:2974–2978 10.1073/pnas.88.8.2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum M.C., Smith R.G. 1998. Noise removal at the rod synapse of mammalian retina. Vis. Neurosci. 15:809–821 10.1017/S0952523898155037 [DOI] [PubMed] [Google Scholar]
- Vardi N. 1998. Alpha subunit of Go localizes in the dendritic tips of ON bipolar cells. J. Comp. Neurol. 395:43–52 [DOI] [PubMed] [Google Scholar]
- Vardi N., Matesic D.F., Manning D.R., Liebman P.A., Sterling P. 1993. Identification of a G-protein in depolarizing rod bipolar cells. Vis. Neurosci. 10:473–478 10.1017/S0952523800004697 [DOI] [PubMed] [Google Scholar]
- Wassle H., Puller C., Muller F., Haverkamp S. 2009. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J. Neurosci. 29:106–117 10.1523/JNEUROSCI.4442-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter S., Brunk I., Walther D.J., Holtje M., Jiang M., Peter J.U., Takamori S., Jahn R., Birnbaumer L., Ahnert-Hilger G. 2005. Galphao2 regulates vesicular glutamate transporter activity by changing its chloride dependence. J. Neurosci. 25:4672–4680 10.1523/JNEUROSCI.0549-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]