Abstract
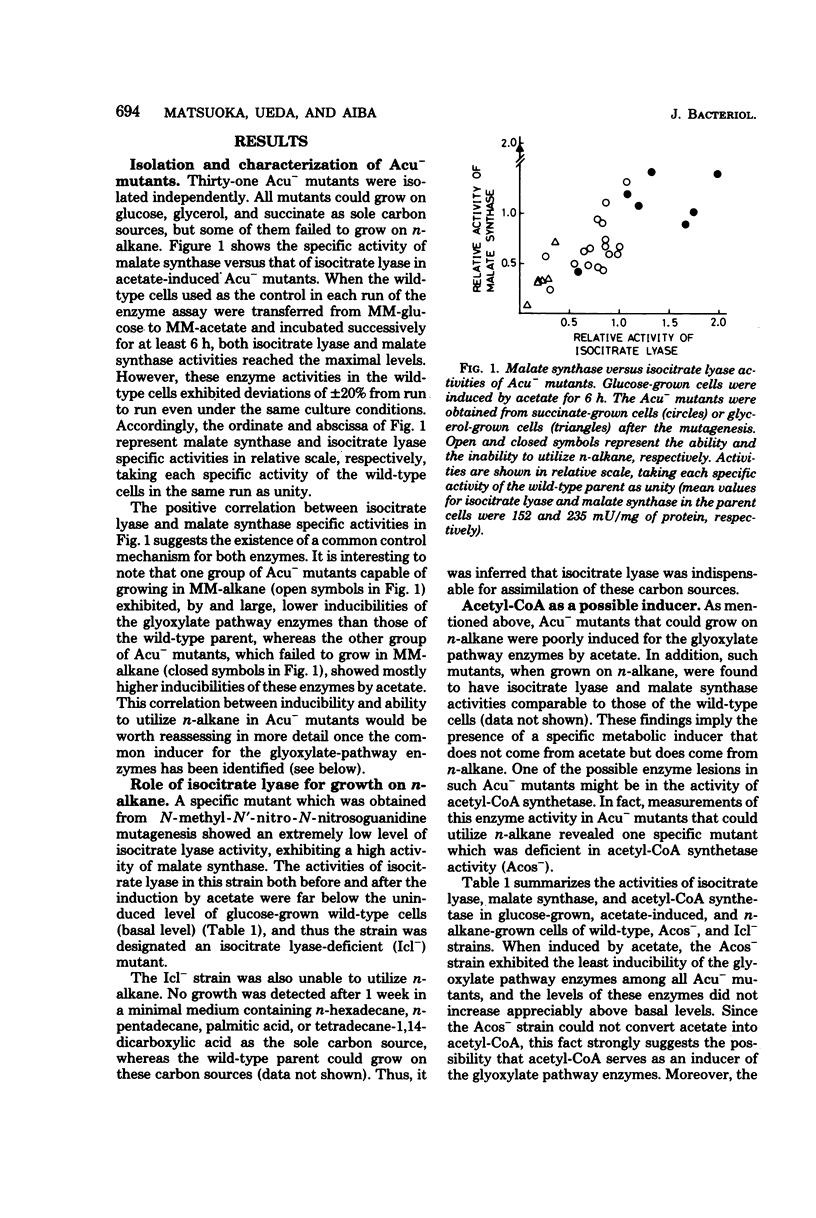
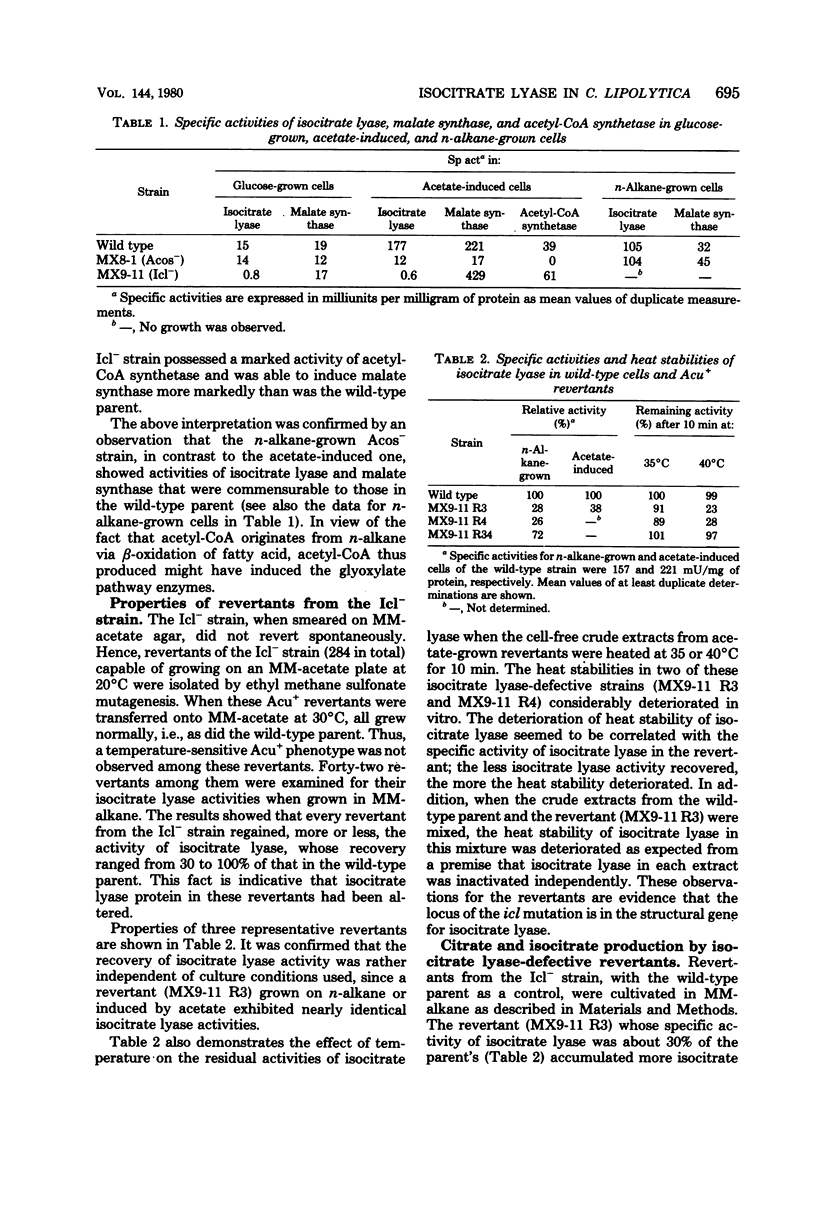
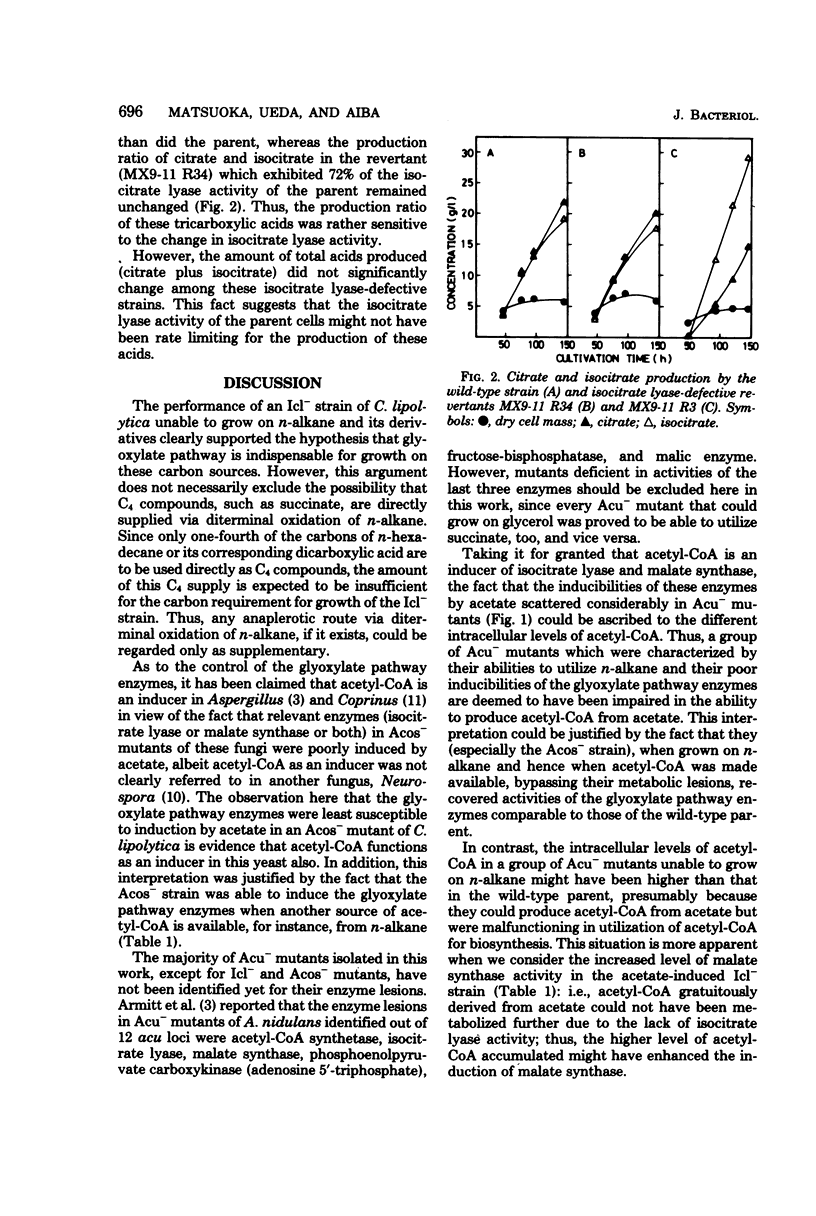
Mutants of Candida lipolytica that were unable to grow on acetate but able to utilize succinate or glycerol as a sole carbon source were isolated. Amongst the mutants isolated, one strain (Icl-) was specifically deficient in isocitrate lyase activity, whereas another strain (Acos-) was deficient in acetyl coenzyme A synthetase activity. Since the Icl- mutant could not grow either on n-alkane or its derivatives, such as fatty acid and long-chain dicarboxylic acid, any anaplerotic route other than the glyoxylate pathway was inconceivable as far as growth on these carbon sources was concerned. Acetyl coenzyme A is most likely a metabolic inducer of isocitrate lyase and malate synthase, because the Acos- mutant was characterized by the least susceptibility to induction of these enzymes by acetate. The structural gene for isocitrate lyase was most probably impaired in the Icl- mutant, since revertants (Icl-) produced thermolabile isocitrate lyase. The production of isocitrate from n-alkane by the revertants was enhanced in comparison with the parental strain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHWORTH J. M., KORNBERG H. L. THE ROLE OF ISOCITRATE LYASE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Aug 26;89:383–384. doi: 10.1016/0926-6569(64)90237-8. [DOI] [PubMed] [Google Scholar]
- Armitt S., McCullough W., Roberts C. F. Analysis of acetate non-utilizing (acu) mutants in Aspergillus nidulans. J Gen Microbiol. 1976 Feb;92(2):263–282. doi: 10.1099/00221287-92-2-263. [DOI] [PubMed] [Google Scholar]
- Brice C. B., Kornberg H. L. Genetic control of isocitrate lyase activity in Escherichia coli. J Bacteriol. 1968 Dec;96(6):2185–2186. doi: 10.1128/jb.96.6.2185-2186.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. B., Fincham J. R. Acetate-nonutilizing mutants of Neurospora rassa. II. Biochemical deficiencies and the roles of certain enzymes. J Bacteriol. 1968 Mar;95(3):1063–1068. doi: 10.1128/jb.95.3.1063-1068.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. B., Fincham J. R. Acetate-onutilizing mutants of Neurospora crassa. I. Mutant isolation, complementation studies, and linkage relationships. J Bacteriol. 1968 Mar;95(3):1056–1062. doi: 10.1128/jb.95.3.1056-1062.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King H. B., Casselton L. A. Genetics and function of isocitrate lyase in Coprinus. Mol Gen Genet. 1977 Dec 9;157(3):319–325. doi: 10.1007/BF00268669. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L. The role and control of the glyoxylate cycle in Escherichia coli. Biochem J. 1966 Apr;99(1):1–11. doi: 10.1042/bj0990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leckie B. J., Fincham J. R. A structural gene for Neurospora crassa isocitrate lyase. J Gen Microbiol. 1971 Jan;65(1):35–43. doi: 10.1099/00221287-65-1-35. [DOI] [PubMed] [Google Scholar]
- Snow R. An enrichment method for auxotrophic yeast mutants using the antibiotic 'nystatin'. Nature. 1966 Jul 9;211(5045):206–207. doi: 10.1038/211206a0. [DOI] [PubMed] [Google Scholar]


