Abstract
In mammalian cells, MCTs (monocarboxylate transporters) require association with an ancillary protein to enable plasma membrane expression of the active transporter. Basigin is the preferred binding partner for MCT1, MCT3 and MCT4, and embigin for MCT2. In rat and rabbit erythrocytes, MCT1 is associated with embigin and basigin respectively, but its sensitivity to inhibition by AR-C155858 was found to be identical. Using RT (reverse transcription)–PCR, we have shown that Xenopus laevis oocytes contain endogenous basigin, but not embigin. Co-expression of exogenous embigin was without effect on either the expression of MCT1 or its inhibition by AR-C155858. In contrast, expression of active MCT2 at the plasma membrane of oocytes was significantly enhanced by co-expression of exogenous embigin. This additional transport activity was insensitive to inhibition by AR-C155858 unlike that by MCT2 expressed with endogenous basigin that was potently inhibited by AR-C155858. Chimaeras and C-terminal truncations of MCT1 and MCT2 were also expressed in oocytes in the presence and absence of exogenous embigin. L-Lactate Km values for these constructs were determined and revealed that the TM (transmembrane) domains of an MCT, most probably TM7–TM12, but not the C-terminus, are the major determinants of L-lactate affinity, whereas the associated ancillary protein has little or no effect. Inhibitor titrations of lactate transport by these constructs indicated that embigin modulates MCT2 sensitivity to AR-C155858 through interactions with both the intracellular C-terminus and TMs 3 and 6 of MCT2. The C-terminus of MCT2 was found to be essential for its expression with endogenous basigin.
Keywords: basigin, embigin, erythrocyte, lactate transport, monocarboxylate transporter 1 (MCT1), monocarboxylate transporter 2 (MCT2)
Abbreviations: BCECF, 2′,7′-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein; CFP, cyan fluorescent protein; EST, expressed sequance tag; FRET, fluorescence resonance energy transfer; HA, haemagglutinin; MCT, monocarboxylate transporter; MCT1trn, MCT1 without C-terminus; MCT1/2c, MCT1 with MCT2 C-terminus; MCT2trn, MCT2 without C-terminus; MCT2/1c, MCT2 with MCT1 C-terminus; pCMBS, p-chloromercuribenzene sulfonate; RT, reverse transcription; TM, transmembrane; WT, wild-type; YFP, yellow fluorescent protein
INTRODUCTION
The transport of L-lactate across the plasma membrane of mammalian cells is catalysed by proton-linked MCTs (monocarboxylate transporters) of which four isoforms have been characterized: MCT1, MCT2, MCT3 and MCT4 [1–5]. These transporters are encoded by separate genes and belong to the MCT family [SLC16 (solute carrier 16)] that contains 14 members in humans and mice [6]. MCT1 is widely expressed and is important for the uptake of lactic acid by heart and red skeletal muscle, where it acts as a major respiratory fuel, and for gluconeogenesis in the liver and kidney of some species [7–11]. MCT2 is a higher-affinity transporter [2] and may facilitate the uptake of lactic acid for gluconeogenesis in the liver and kidney where it is the dominant MCT isoform in some, but not all, species [6,9,12]. MCT2 is also expressed in neurons, especially at the postsynaptic density, where it may facilitate the uptake of lactic acid, produced by glycolysis in astrocytes, for oxidation as a respiratory fuel [13,14]. The expression of MCT3 is limited to the basal membrane of the retinal pigment epithelium and choroid plexus epithelia [15,16], whereas MCT4 is primarily expressed in highly glycolytic cells, such as white muscle fibres, where it is used to facilitate lactic acid efflux from the tissue [17,18], and is up-regulated in other cells under hypoxic conditions [19].
All members of the MCT family are predicted to have 12 TM (transmembrane) α-helices with a large loop between TMs 6 and 7 that faces the intracellular side of the membrane as do the C- and N-termini. For most MCT isoforms, the N-terminus is relatively short, whereas the C-terminus and TM6/7 loop are quite long and show little sequence identity between isoforms [6,11]. For MCT1, the proposed structure has been confirmed by proteolytic cleavage and labelling studies, as well as by molecular modelling [20–23]. An ancillary protein, either basigin or embigin, is required for MCT1–MCT4 to be properly expressed at the plasma membrane [21–24]. Embigin and basigin are single-TM glycoproteins with two or three extracellular Ig domains [25–28], and the continued interaction between the MCT and its ancillary protein is essential to maintain transporter activity [21,22]. Basigin is the normal binding partner for MCT1, MCT3 and MCT4 [22,29–34], although MCT1 may be expressed with embigin in some tissues such as rat erythrocytes [24]. In contrast, MCT2 prefers embigin as its binding partner [22].
In order to elucidate the importance of each MCT isoform in particular metabolic pathways and disease states, it would be highly desirable to develop isoform-specific inhibitors. A new class of specific and extremely high-affinity inhibitors of MCT1 have been discovered by AstraZeneca [35–37]. We have confirmed the ability of one of these inhibitors, AR-C155858, to inhibit MCT1 in rat erythrocytes with a Ki value of approx. 2 nM and demonstrated similar high-affinity inhibition of both MCT1 and MCT2 when they are expressed in Xenopus laevis oocytes. In contrast, MCT4 was not inhibited, and by studying MCT1/MCT4 chimaeric transporters, we were able to locate the inhibitor binding site to TMs 7–10 of the C-terminal half of MCT1 [38]. In studies directed towards establishing the relationship between the structure and function of MCT2, we found that co-expressing MCT2 with embigin in X. laevis oocytes significantly increased plasma membrane expression and activity of the transporter. However, when we investigated the inhibition of MCT2 activity by AR-C155858 under these conditions, we found a major reduction in inhibitor sensitivity. In the present paper, we report the results of these studies and provide evidence for an interaction of embigin with both the C-terminus and TM3 and TM6 of MCT2, but not MCT1, that plays an important role in mediating this reduced inhibitor sensitivity.
EXPERIMENTAL
Materials
All reagents were obtained from Sigma unless stated otherwise, and most antibodies were obtained from the sources cited in [38]. Rabbit polyclonal antibodies against the C-terminus of rat embigin were raised in-house as described previously [22], and the anti-HA (haemagglutinin) antibody was purchased from Covance. Restriction enzymes were obtained from Roche Applied Science. Rat and rabbit blood were purchased from Harlan SeraLabs. X. laevis toads were obtained from Xenopus Express and oocytes were harvested as described previously [21]. L-[14C]Lactate was obtained from GE Healthcare. AR-C155858 was obtained from AstraZeneca and made up as a 10 mM stock in DMSO.
Detection of basigin and embigin in Xenopus oocytes by RT (reverse transcription)–PCR
A BLAST search of the EST (expressed sequence tag) database with the protein sequence for rat basigin and embigin identified a full-length mRNA sequence for X. laevis basigin (BC099064.1) and a partial mRNA sequence (853 bp) that was highly homologous with embigin (EB645817). Whereas the former was common in the EST database, the embigin sequence gave only three hits (thymus cDNA library). These sequences were used to design primers (see Supplementary Table S1 at http://www.BiochemJ.org/bj/431/bj4310217add.htm) for PCR detection of embigin and basigin in X. laevis oocytes using Xenopus thymus tissue as a positive control. RNA was extracted from the oocytes and thymus using TRIzol® reagent (Invitrogen) following the manufacturer's protocol. cDNA was synthesized with Expand Reverse Transcriptase (Roche) and used in PCRs. Thermocycling was performed using the following parameters: 1 min at 95 °C, 1 min at 55 °C and 1 min at 72 °C for 5 cycles, and 1 min at 95 °C, 1 min at 50 °C and 1 min at 72 °C for 30 cycles. PCR products were analysed by agarose gel electrophoresis.
Generation of MCT chimaeras and truncations of rat MCT1 and MCT2
Chimaeras of MCT1 and MCT2 were created in which the N- and C-terminal halves either side of the TM6/7 loop (MCT2/1 and MCT1/2) or just the C-terminal tails (MCT1/2c and MCT2/1c) were swapped. The rationale and methodology used was the same as that described previously [38]. The MCT1/2 and MCT2/1 loop chimaeras were produced based upon a stretch of nucleotide sequence similarity near the end of the TM6/7 large intracellular loop consisting of residues (P/K)(K/R)(G/L)(E/S)K(L/V)S (MCT1/MCT2). Similarly the MCT1/2c and MCT2/1c C-terminal chimaeras were based on a conserved YRL (Tyr-Arg-Leu) sequence one residue downstream of the end of TM12 for both MCT1 and MCT2. Sequences for all primers used are given in Supplementary Table S1 and were designed to be between 15 and 30 bases in length. The C-terminal truncation of MCT1 (MCT1trn) was produced as described previously [38]. For C-terminal truncation of MCT2 (MCT2trn), PCR was used to produce MCT2 lacking the sequence C-terminal of the end of TM12, just as was performed when making the MCT2/1c chimaera, but the product was ligated into the pGEM-T Easy vector system (Promega). From here, it was extracted by EcoRI digestion and ligated into EcoRI-linearized oocyte pGHJ vector with a stop codon within the plasmid sequence downstream of MCT2trn. Since the anti-MCT2 antibodies were raised against the C-terminus, in order to monitor plasma membrane expression of MCT2trn, an HA tag (a short peptide sequence, YPYDVPDYA) was added to the N-terminus of MCT2. This was performed by insertion of the MCT2trn construct into a linearized pcDNA3 vector (Invitrogen) previously modified by Dr Harry Mellor (Department of Biochemistry, University of Bristol) to include the HA tag upstream of the inserted DNA (HA-pcDNA3), while maintaining the reading frame between the HA tag and MCT sequence. Subsequently, the HA–MCT2trn construct was extracted from the pcDNA3 vector using PCR with the primers given in Supplementary Table S1. These contained XbaI restriction enzyme sites both upstream and downstream of the HA–MCT2trn cDNA, enabling insertion of the HA-tagged construct into the appropriately linearized pGHJ vector. Confirmation that all of the desired changes had been correctly engineered was provided by sequencing (DNA Sequencing & Services, University of Dundee, Dundee, U.K.).
Measurement of MCT1 activity in erythrocytes
L-Lactate transport into rat and rabbit erythrocytes was measured by monitoring the change in extracellular pH with a pH-sensitive electrode as described previously [22,38]. The erythrocytes (5% haematocrit) were pre-incubated for 1 h at room temperature (22 °C) with or without AR-C155858 at the required concentration before assaying transport of L-lactate (10 mM) at 6 °C. Initial rates of transport were calculated by first-order regression analysis of the time course of pH change and converted into nmol of H+/min by determining the pH change induced by small additions of standardized NaOH.
Measurement of MCT transport activity in Xenopus oocytes
cRNA was prepared and injected into X. laevis oocytes as described previously [21,38]. For all assays, 10 ng of MCT cRNA ±10 ng of rat embigin cRNA in 9.2 nl of water were injected, with controls receiving just water. MCT and embigin expression at the plasma membrane of oocytes was confirmed by immunofluorescence microscopy as described previously [21,38]. MCT kinetic assays were performed by monitoring intracellular pH with H+-sensitive dye BCECF [2′,7′-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein] or by determining the uptake of L-[14C]lactate (7.4 MBq/ml) [5,21,23,38]. The uptake buffer contained 75 mM NaCl, 2 mM KCl, 0.82 mM MgCl2, 1 mM CaCl2 and 20 mM Tris/Hepes (pH 7.4). AR-C155858 inhibitor titrations were performed at pH 6 with oocytes pre-incubated for 45 min in a different uptake buffer (75 mM NaCl, 2 mM KCl, 0.82 mM MgCl2, 1 mM CaCl2 and 20 mM Mes, pH 6) containing the required concentration of AR-C155858 prior to measuring the uptake of L-[14C]lactate (0.5 mM) as described previously [38]. Unless stated otherwise, uptake was determined over 2.5 min for all MCT constructs except for MCT2trn with or without embigin and MCT2/1 with or without embigin, where 5 and 10 min were used respectively. We determined that these conditions represented the longest period over which uptake was linear with time (results not shown).
RESULTS
Embigin enhances MCT2 expression in Xenopus oocytes
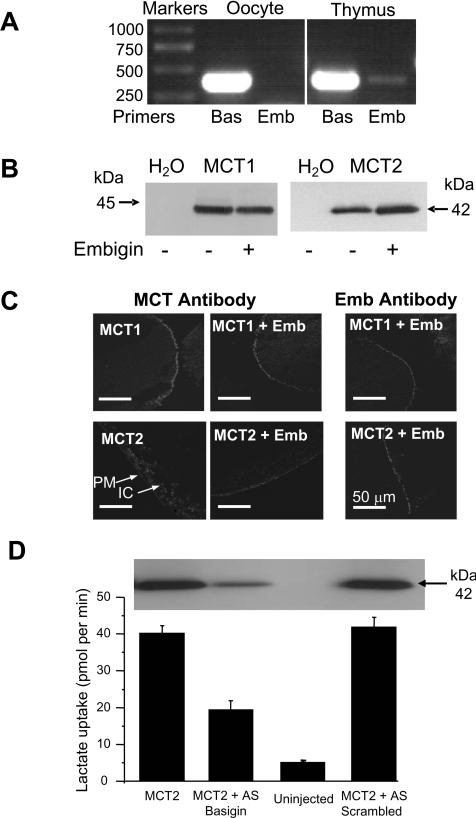
We have shown previously that MCT2 usually associates with embigin rather than basigin [22]. If Xenopus oocytes express endogenous basigin, but not embigin, this might explain the poor expression of exogenous MCT2 relative to MCT1 [38]. In order to determine whether or not this was the case, we performed RT–PCR analysis of mRNA extracted from Xenopus oocytes using primers designed against Xenopus basigin and embigin as described in the Experimental section. Results are reported in Figure 1(A). As anticipated, a strong signal was found for basigin, but not for embigin. As a positive control, we were able to detect embigin using mRNA from Xenopus thymus in agreement with the presence of embigin in a Xenopus thymus cDNA library (EST database accession number EB645817). Since we do not have suitable antibodies to detect endogenous Xenopus embigin or basigin, we were unable to confirm these results at the protein level. However, using Western blot analysis of a crude membrane fraction (Figure 1B) and confocal microscopy of sections of Xenopus oocytes (Figure 1C), we were able to confirm that, when oocytes were co-injected with cRNA for rat embigin and MCT2, the plasma membrane expression of MCT2 was increased relative to the MCT2 expressed in the absence of embigin. Coincident with this, the co-expression of embigin reduced the amount of MCT2 located in a compartment beneath the plasma membrane in the absence of embigin (Figure 1C) and increased the rate of L-lactate transport into the oocytes (Figure 2). In contrast, embigin failed to enhance the plasma membrane expression of MCT1 (Figures 1B and 1C) and produced no increase in the rate of lactate transport (Figure 2). The modest expression of MCT2 in the absence of embigin is likely to reflect its association with endogenous Xenopus basigin or a currently unidentified ancillary protein. We confirmed this by demonstrating that when this endogenous basigin was knocked down by microinjecting an antisense cDNA against basigin, expression of MCT2 was greatly reduced (Figure 1D). This was associated with rates of lactate transport a little higher than in non-injected controls. Scrambled antisense cDNA was without effect on either MCT2 expression or lactate transport.
Figure 1. Importance of embigin for MCT2 expression in oocytes.
(A) Expression of endogenous basigin (Bas) and embigin (Emb) in X. laevis oocytes and thymus determined by RT–PCR. Sizes are indicated in bp. (B) Membrane preparations of oocytes expressing MCT1 or MCT2 in the presence or absence of embigin were subject to SDS/PAGE and Western blotting with the anti-MCT1 or the anti-MCT2 antibody as indicated. Sizes are indicated in kDa. (C) Immunofluorescence confocal microscopy on sections of the oocytes using the appropriate antibody against rat MCT1 or MCT2 with or without embigin (Emb). For MCT2 in the absence of embigin, arrows show weak plasma membrane (PM) and stronger intracellular (IC) expression. (D) L-[14C]Lactate uptake (mean+S.E.M., n=20–30) for water-injected oocytes and those expressing MCT2 in the presence or absence of antisense oligonucleotide against endogenous basigin or a scrambled antisense (AS) oligonucleotide. A Western blot of a crude membrane preparation is shown to confirm knockdown of MCT2.
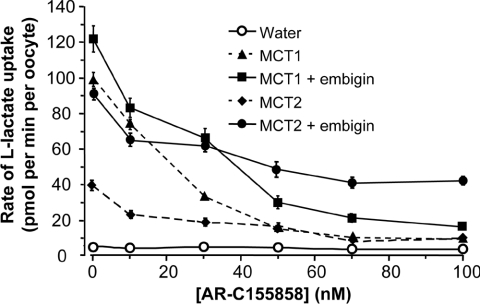
Figure 2. The sensitivity of MCT2 to inhibition by AR-C155858 is decreased by co-expression of embigin.
Inhibition of L-lactate transport activity at pH 6 by increasing concentrations of AR-C155858 was determined using 0.5 mM L-[14C]lactate (pH 6). Results are means±S.E.M. for 27–107 separate oocytes at each inhibitor concentration.
Embigin reduced the sensitivity of MCT2, but not MCT1, to inhibition by AR-C155858
In Figure 2, we show that co-expression of embigin with MCT1 had no effect on the high sensitivity of this isoform to inhibition by AR-C155858. However, the situation with MCT2 was more complex. In the absence of embigin, the first addition of the inhibitor (10 nM) reduced the rate of transport by approx. 20 pmol/min per oocyte, which represented a 70% inhibition of transport. With further inhibitor additions, there was a progressive inhibition that reached nearly 100% at 100 nM AR-C155858 as described previously [38]. In the presence of embigin, the absolute reduction in transport rate by 10 nM AR-C155858 was again approx. 20 pmol/min per oocyte, but further additions of AR-C155858 gave little additional inhibition. Indeed, the difference between the rate of lactate uptake in the presence and absence of embigin remained almost constant at approx. 40 pmol/min per oocyte concentrations as the AR-C155858 concentration was increased from 10 nM to 100 nM. Thus approx. 50% of transport mediated by MCT2 co-expressed with embigin appears to be resistant to inhibition by AR-C155858. It would seem probable that this inhibitor-insensitive transport represents the activity of MCT2 that is associated with embigin, whereas the remaining 50% that is inhibitor-sensitive represents transport mediated by MCT2 associated with endogenous basigin. No such inhibitor-insensitive transport was observed when MCT1 was co-expressed with embigin.
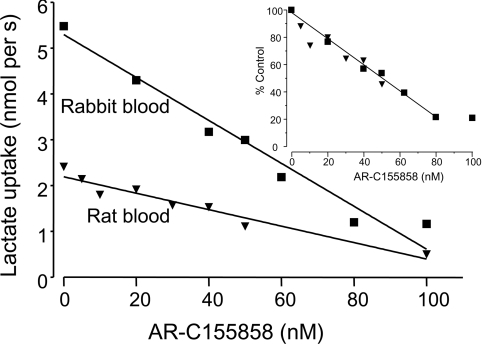
Although it is quite possible that the MCT1 continues to utilize endogenous basigin rather than embigin under these conditions, we have shown previously that in rat erythrocytes, where MCT1 is naturally expressed in association with embigin, AR-C155858 still inhibits lactate transport potently with a Ki value of 2.3 nM [38]. However, to confirm that MCT1 sensitivity to AR-C155858 is unaffected by its choice of ancillary protein, we compared inhibitor titrations of lactate transport into rabbit erythrocytes, where MCT1 is naturally expressed in association with basigin [22], with those of rat erythrocytes, where embigin acts as the ancillary protein. The results are shown in Figure 3. As described previously for rat erythrocytes [38], the potency of inhibition is such that a linear relationship between [AR-C155858] and rate of transport is observed for both species. The extrapolation to zero rate gives an approximate estimate of the number of binding sites for the inhibitor which is similar for both rat and rabbit erythrocytes despite the 2-fold higher absolute rate of transport seen in the rabbit erythrocytes at the same pH. When rates of transport are expressed as a percentage of control (zero inhibitor) the two plots superimpose (inset), also implying that there are a similar number of binding sites with the same affinity for AR-C155858 in both species. Thus the turnover number for MCT1 in rabbit erythrocytes must be approx. 2-fold higher than for rat erythrocytes under these conditions. Our findings do not allow us to determine with certainty whether this reflects an effect of the ancillary protein on the kcat of MCT1 or whether it is a property of the MCT itself.
Figure 3. Sensitivity to inhibition by AR-C155858 of MCT1 associated with basigin or embigin in rabbit and rat erythrocytes respectively.
The rate of transport of 10 mM L-lactate (pH 7) into rabbit or rat blood at 5% haematocrit was monitored by extracellular pH following 1 h of pre-incubation with the concentration of AR-C155858. The inset shows the superimposed data for both species with rates expressed as percentages of control (no inhibitor). Linear regression was used to calculate the plot.
Using MCT1/MCT2 chimaeras to investigate how embigin co-expression desensitizes MCT2 to inhibition by AR-C155858
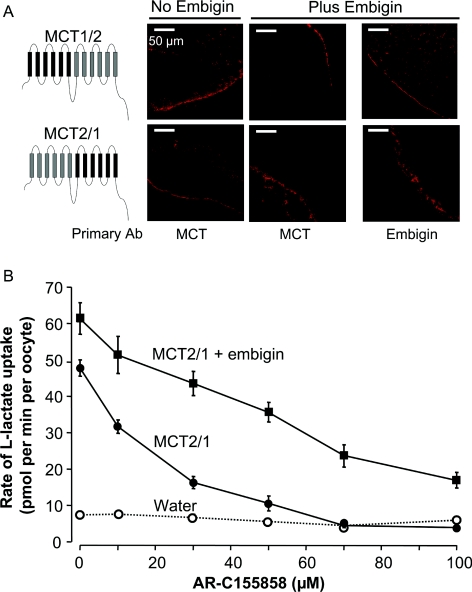
Since potent inhibition of MCT1 by AR-C155858 is observed whether MCT1 is associated with embigin or basigin, we sought to establish what regions of MCT2 are responsible for the desensitizing effect of embigin. We have previously created chimaeric MCTs utilizing different segments of MCT1 and MCT4 in order to identify the region of MCT1 that is likely to be the binding site for AR-C155858 [38]. These results identified the binding site to be in the C-terminal half of the molecule. Thus we adopted a similar approach with MCT1/MCT2 chimaeras. We successfully created a chimaera in which the N-terminal half of MCT2 (up to the end of the intracellular loop between TMs 6 and 7) was combined with the C-terminal half of MCT1. This chimaera (MCT2/1) was strongly expressed at the plasma membrane of the oocyte (Figure 4A) and gave kinetics of L-lactate transport, determined with the BCECF method, indistinguishable from those for MCT1 (Table 1). Co-expression of embigin had no effect on the Km for L-lactate (Table 1), neither did it have a profound effect on either the level of expression at the plasma membrane (Figure 4A) or the Vmax of transport (Figure 4B). Inhibition of MCT2/1 in the absence of embigin was similar to that of MCT1 (Figure 4B), although when co-expressed with embigin, the chimaera appeared slightly less sensitive to the inhibitor. However, if association with embigin rather than basigin decreases the kcat of MCT2/1, then this difference may merely reflect a greater expression of a less active transporter rather than a decrease in inhibitor binding affinity. We were also able to express the equivalent MCT1/2 chimaera at the plasma membrane of oocytes independently of embigin (Figure 4), but these oocytes showed no increase in the rates of L-lactate transport, suggesting this chimaera was inactive for some reason.
Figure 4. The sensitivity of MCT1/2 and MCT2/1 chimaeras to inhibition by AR-C155858.
(A) Immunofluorescence confocal microscopy data with appropriate C-terminal antibodies (Ab) of sections of oocytes expressing MCT1/2 or MCT2/1 with or without exogenous embigin. (B) Inhibition of lactate transport activity by increasing concentrations of AR-C155858 determined using 0.5 mM L-[14C]lactate (pH 6) over 10 min. Results are means±S.E.M. for ten separate oocytes at each inhibitor concentration.
Table 1. Summary of the L-lactate Km values and sensitivity to inhibition by AR-C155858 of MCT chimaeras expressed in the presence and absence of exogenous embigin.
The Km values reported were derived either by monitoring changes in intracellular pH with BCECF or using L-[14C]lactate uptake (asterisk) as described in the Experimental section. The majority of the kinetic data was obtained using the BCECF technique which was preferred over the radioactive technique since it allowed a single egg to be used for a complete Km estimation, reducing errors associated with the various levels of expression. However, this technique could not be used for MCT2 and chimaeras that were poorly expressed and have low Km values because the pH changes associated with uptake were too small to detect accurately with BCECF. In these cases, we needed to use the radioactive technique as we have done previously [2]. Results are means±S.E.M. derived from the fit of the mean data to the Michaelis–Menten equation by non-linear least-squares analysis. For L-[14C]lactate uptake, L-lactate concentrations used were 0.2, 0.5, 1, 2 and 5 mM, whereas for BCECF measurements, the concentrations were 2.5, 5, 10, 20 and 50 mM. The n values given in parentheses represent the number of separate eggs used at each substrate concentration. The expression at the plasma membrane is a qualitative indicator only, with +++ for good expression (as for MCT1 with or without embigin), ++ for modest expression (as for MCT2/1c without embigin), + for poor expression (as for MCT2 without embigin) and − for no expression (as for MCT2trn without embigin). The transporter activity represents the activity in the absence of inhibitor as monitored by L-[14C]lactate (0.5 mM, pH 6) uptake with results as means±S.E.M. (as for Figure 5). Initial sensitivity to AR-C155858 describes the decrease in rate between control and 10 nM AR-C155858 as a percentage of the control rate, whereas the inhibitor-insensitive component is the rate remaining at 100 nM AR-C155858 as a percentage of the control rate±S.E.M. Inhibitor data are taken from Figures 2, 4 and 6 and are expressed with or without (in parentheses) correction for rates of uptake in water-injected oocytes. n/a, not applicable.
| MCT chimaera | Embigin co-expressed | Expression at plasma membrane | Activity (pmol/min per oocyte) | Lactate Km (mM) | Initial sensitivity to AR-C155858 (%) | Inhibitor-insensitive component (%) |
|---|---|---|---|---|---|---|
| MCT1 | No | +++ | 85±6.4 (30) | 4.4±1.5 (4) | 28±3 (26±3) | 1.9±0.6 (8±0.6) |
| MCT1 | Yes | +++ | 119±7.8 (30) | 6.1±1.7 (4) | 34±5 (32±5) | 7.4±1.3 (12±1) |
| MCT2 | No | + | 32±3 (30) | 1.0±0.2 (8)* | 51±7 (42±6) | 12±2 (25±2) |
| MCT2 | Yes | +++ | 83±3.3 (106) | 2.3±0.3 (8)* | 31±4 (28±4) | 43±3 (47±3) |
| MCT1/2 | No | +++ | 1.8±0.3 (10) | n/a | n/a | n/a |
| MCT1/2 | Yes | +++ | 2.3±0.3 (10) | n/a | n/a | n/a |
| MCT1/2c | No | +++ | 143±2.7 (20) | 4.4±1.4 (6) | 21±2 (20±2) | 6±0.4 (10±0.4) |
| MCT1/2c | Yes | +++ | 127±3.7 (20) | 4.3±1.2 (6) | 6±6 (6±6) | 44±4 (46±4) |
| MCT1trn | No | +++ | 149±10.2 (20) | 4.5±2.1 (7) | 25±7 (24±7) | 10±2 (13±1) |
| MCT1trn | Yes | +++ | 139±5.4 (20) | 4.5±1.0 (5) | 27±5 (26±5) | 12±1 (15±1) |
| MCT2/1 | No | +++ | 40±2.2 (10) | 5.04±0.60 (4) | 40±5 (34±4) | 0±3 (8±2) |
| MCT2/1 | Yes | +++ | 54±4.4 (10) | 4.70±0.96 (4) | 34±13 (30±11) | 20±4 (28±4) |
| MCT2/1c | No | ++ | 74±6.1 (29) | 2.4±2.2 (4) | 33±6 (30±6) | 21±2 (26±2) |
| MCT2/1c | Yes | +++ | 103±7 (28) | 1.5±0.5 (7) | 10±8 (9±8) | 73±6 (74±6) |
| MCT2trn | No | − | 2.4±1.3 (15) | n/a | n/a | n/a |
| MCT2trn | Yes | + | 44±2.7 (28) | 1.4±0.2 (8)* | 46±3 (39±2) | 37±3 (44±2) |
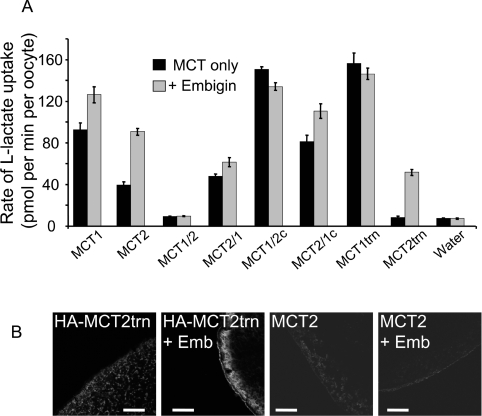
We next created chimaeras in which just the C-terminal tail was swapped between MCT1 and MCT2 (MCT1/2c and MCT2/1c) or truncated MCT1 and MCT2 without the C-terminal tail (MCT1trn and MCT2trn). Figure 5 shows that all of these constructs facilitated lactate transport whether or not embigin was co-expressed, with the exception of MCT2trn whose activity required embigin co-expression. As might be predicted, MCT1/2c and MCT1trn exhibited similar rates of transport to that of MCT1 with no stimulation of transport activity (Figure 5A) or expression (Figure 5B and Supplementary Figure S1 at http://www.BiochemJ.org/bj/431/bj4310217add.htm) when co-expressed with embigin. More surprising was that the MCT2/1c chimaera gave significantly higher rates of lactate transport than MCT2 itself with a less pronounced effect of co-expressed embigin. However, when the C-terminal tail of MCT2 was totally removed (MCT2trn), little, if any, transport was observed in the absence of co-expressed embigin, whereas co-expression with embigin bought about rates approx. 60% of full-length MCT2 plus embigin. Interestingly, the absolute increase in rate of transport by MCT2trn induced by embigin was similar to that observed for full-length MCT2. Expression data were consistent with these transport data. Thus MCT2trn showed no detectable expression at the oocyte plasma membrane unless co-expressed with embigin (Figure 5B), whereas full-length MCT2 showed a small expression at the plasma membrane that was enhanced by embigin as described above (Figure 1C). It should be noted that, in order to detect MCT2trn expression, we attached an HA tag to the N-terminus of MCT2 as described in the Experimental section and used anti-HA antibodies. This was necessary because our own anti-MCT2 antibody was raised against the C-terminus of MCT2 [9], which is not present in MCT2trn. The activity of all other constructs also correlated with their plasma membrane expression (see Supplementary Figure S1).
Figure 5. The C-terminus of MCT2 plays a role in its association with endogenous basigin, but not co-expressed embigin.
(A) Rates of L-[14C]lactate (0.5 mM) uptake into oocytes expressing the MCT or MCT chimaera indicated, in the presence of absence of co-expressed embigin. Uptake was assayed at a time where uptake is linear with time: 2.5 min for all except 5 min for MCT1/2 and MCT2trn, and 10 min for MCT2/1. Results are means±S.E.M. for the number of separate oocytes shown in Table 1. (B) Immunofluorescence microscopy data of the oocytes using C-terminal antibodies against MCT2 or, in the case of the MCT2trn construct, against the HA tag inserted at the N-terminus. Emb, embigin.
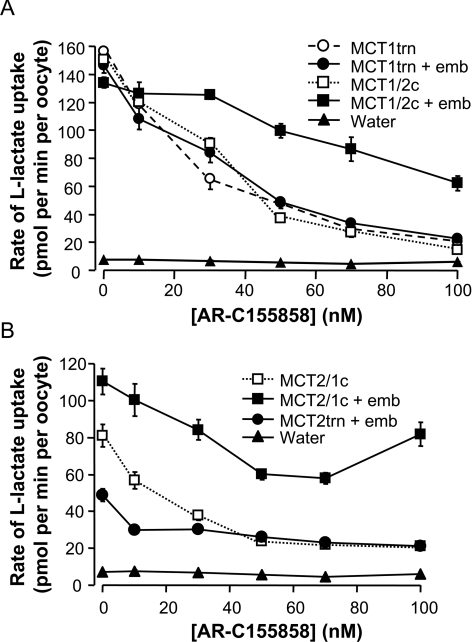
Having established the expression of the different C-terminal constructs in the presence and absence of embigin, we investigated their sensitivity to inhibition by AR-C155858. Figure 6(A) shows that MCT1trn and MCT1/2c exhibit potent inhibition by AR-C155858, similar to that observed for MCT1 itself (Figure 2). Co-expression of embigin did not affect the sensitivity of the MCT1trn to AR-C155858, consistent with its effects on full-length MCT1 (Figure 2). However, the MCT1/2c chimaera showed an altered sensitivity to inhibition by AR-C155858 when co-expressed with embigin (Figure 6A), similar to that observed for the MCT2/1 chimaera (Figure 4). These results imply that the presence of the C-terminus of MCT2 led to expression of some of the chimaera with embigin rather than endogenous basigin and that this interaction caused the observed reduction in sensitivity to AR-C155858. Desensitization was characterized by a shallower slope of the inhibition plot compared with that for MCT1 and is distinct from the effect of embigin on the AR-C155858 inhibition profile of MCT2. In the case of MCT2, the initial profile of the inhibition plot was similar to that for MCT1 at low inhibitor concentrations, but then reached a plateau phase in which little additional inhibition occurred (Figure 2). The shallow slope of the inhibition profile of MCT1/2c in the presence of embigin could be the result of an interaction between the C-terminus of MCT2 with the C-terminus of embigin. However, an alternative explanation would be that MCT1/2c co-expressed with embigin showed a greater expression, but lower kcat than when expressed with endogenous basigin. This would give the same rate of transport despite greater transporter expression, and thus a shallower inhibitor titration as observed.
Figure 6. The sensitivity to inhibition by AR-C155858 of MCT1/MCT2 C-terminal chimaeras and truncations in the presence and absence of co-expressed embigin.
Inhibition of lactate-transport activity by increasing concentrations of AR-C155858 was determined at pH 6 using 0.5 mM L-[14C]lactate. Results are means±S.E.M. of 10–20 (A) or 16–30 (B) separate oocytes for each inhibitor concentration. Slight variations in the control rates from those reported in Figure 5 are typical of those seen between different batches of oocytes and cRNA preparations. Immunofluorescence confocal microscopy of oocyte sections are presented in Supplementary Figure S1 at http://www.BiochemJ.org/bj/431/bj4310217add.htm to confirm plasma membrane expression of the different constructs. emb, embigin.
Help in discriminating between these two possibilities was sought by studying a chimaera in which the C-terminus of MCT2 was replaced with the MCT1 C-terminus (MCT2/1c). This chimaera was active in the absence of co-expressed embigin and was very sensitive to AR-C155858 as predicted, but with a small inhibitor-insensitive component similar to that seen for full-length MCT2. However, the presence of the MCT1 C-terminus appeared to enhance association with endogenous basigin since the stimulation of transport seen by co-expression with embigin was less for MCT2/1c than MCT2 (Figure 5A). Nevertheless, when MCT2/1c was co-expressed with embigin the inhibitor-insensitive component of transport was greater, implying that, when associated with embigin rather than endogenous basigin, MCT2/1c becomes inhibitor-insensitive. Consistent with this, the majority of transport mediated by the C-terminally truncated MCT2 (MCT2trn) co-expressed with embigin was also insensitive to inhibition by AR-C155858.
DISCUSSION
The present study involved the use of a variety of MCT1/MCT2 chimaeras expressed in oocytes in the presence and absence of embigin. To aid interpretation of the data, Table 1 provides L-lactate Km values for all of the chimaeras and truncated MCTs when expressed in the presence or absence of exogenous embigin as well as summarizing the effects of embigin on MCT expression and inhibitor sensitivity. Figure 7 is a schematic diagram that provides a tentative explanation of our data. Swapping or truncation of the C-terminal tails had no effect on the L-lactate Km values, but the MCT2/1 chimaera had a Km value more similar to that of MCT1 (4–5 mM) than that of MCT2 (1–2 mM). This is consistent with previous work that has implicated the C-terminal half of MCT1 and MCT4 in their substrate affinity [7,21,23,39,40]. The presence or absence of exogenous embigin had no significant effect on Km values, suggesting that the kinetic properties of an MCT are not greatly influenced by the ancillary protein with which it is associated.
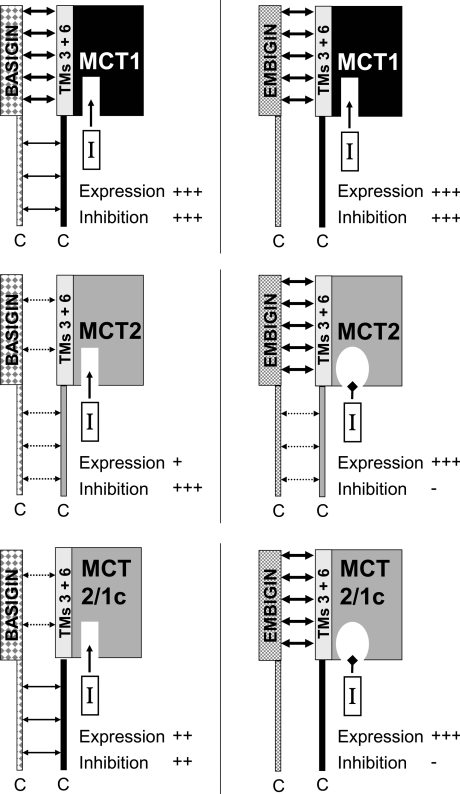
Figure 7. Schematic diagram of how MCT–ancillary protein interactions may affect expression and AR-C155858 binding.
Interactions between the TM of the ancillary protein and TMs 3 and 6 of the MCT are shown as being either strong (thick arrows) or weak (broken arrows), whereas interactions between the two C-terminal tails are shown as either moderate (thin arrow) or weak (broken arrows). Inhibition by AR-C155858 is indicated by an arrow directing the inhibitor into its binding site. The loss of this inhibition is indicated by an occlusion of the AR-C155858-binding site.
MCT2 requires embigin for optimal expression in Xenopus oocytes
We have shown previously that MCT2 is active when expressed in Xenopus oocytes [4], but that rates of lactate transport are substantially lower than for either MCT1 or MCT4 [38]. In mammalian cells, we have shown that MCT2 preferentially associates with embigin and that co-expression of embigin rather than basigin with MCT2 is required to obtain plasma membrane expression of MCT2 [22]. In the present study, we have demonstrated that co-expression of embigin with MCT2 also enhances the plasma membrane expression and activity of MCT2 in Xenopus oocytes which contain endogenous basigin, but not embigin (Figures 1 and 2). The expression of MCT2 in the absence of embigin is assumed to reflect an association with endogenous Xenopus basigin, since knockdown of this basigin using antisense cDNA greatly reduced MCT2 expression and the associated lactate transport (Figure 1D). It would appear that the C-terminal tail of MCT2 must enable this interaction with endogenous basigin because its removal prevented plasma membrane expression of MCT2 unless embigin was co-expressed. In contrast, MCT1 without its C-terminal tail (MCT1trn) was expressed to an extent similar to both full-length MCT1 and the MCT1/2c construct, and embigin was without effect on their expression. The MCT2/1c construct was also expressed in the absence of embigin, but, in this case, expression was enhanced when embigin was co-expressed (Figure 5A). However, the stimulation of transport seen by co-expression with embigin was less for MCT2/1c than MCT2, implying that the presence of the MCT1 C-terminus on MCT2 enhanced its association with endogenous basigin relative to full-length MCT2.
Taken together, these data suggest that the interaction of MCT2 with its ancillary protein involves two components: the primary association of the TM domain of the ancillary protein with TMs 3 and 6 of MCT2 as proposed previously [21,22,31] and a secondary interaction between the C-terminal tail of MCT2 and the C-terminal tail of the ancillary protein. When embigin is the ancillary protein, the interaction with TMs 3 and 6 is sufficient to allow some expression, but this is enhanced by addition of the C-terminal tail of either MCT1 or MCT2 to allow additional interactions with the C-terminus of exogenous embigin. However, the interaction of TMs 3 and 6 of MCT2 with endogenous basigin may be relatively weak compared with that of embigin and thus, in the absence of embigin, the C-terminal tail of either MCT2 or MCT1 is required to enhance the interaction with endogenous basigin sufficiently to allow the plasma membrane expression of the two proteins. The C-terminal tail of MCT1 is more effective than that of MCT2 in this regard (Figure 5A) and it may be significant that the amino acid sequences of these C-terminal tails show little sequence conservation. Such an interaction between the C-terminal tail of MCT1 and MCT2 with their ancillary protein is consistent with previous data from this laboratory using FRET (fluorescence resonance energy transfer) between either MCT1 or MCT2 tagged at the C-terminus with YFP (yellow fluorescent protein) and basigin or embigin tagged at their C-terminus with CFP (cyan fluorescent protein). FRET was observed when MCT1–CFP was co-expressed with basigin–YFP [31] and when MCT2–YFP was co-expressed with embigin–CFP [22].
Embigin decreases the affinity of MCT2, but not MCT1, for AR-C155858
Although our data do not allow determination of absolute Ki values for AR-C155858, Figures 2 and 3 show that, when associated with embigin, the inhibitor sensitivity of MCT2 decreased relative to that of MCT2 associated with endogenous basigin. This is not without precedent, since we have demonstrated previously that inhibition of MCT1-mediated lactate transport by the organomercurial reagent pCMBS (p-chloromercuribenzene sulfonate) occurs when MCT1 associates with basigin, but not embigin [22]. Unlike pCMBS, AR-C155858 binds to an intracellular site on the MCT [38] and thus the insensitivity of MCT2/embigin to inhibition by AR-C155858 is likely to involve an effect of the embigin on either the access to or shape of the intracellular inhibitor-binding domain. The former could involve the intracellular tail of embigin interacting with the intracellular face of MCT2 in a manner that is not possible with MCT1. The latter could indicate that the interaction of the TM domain of embigin, but not basigin, with TMs 3 and 6 of MCT1 and MCT2 causes a subtle change in the shape of the intracellular binding pocket of the MCT sufficient to prevent inhibitor binding in MCT2, but not MCT1. Such an effect might also account for the lower kcat of MCT1 in rat erythrocytes, where it associates with embigin, compared with MCT1 in rabbit erythrocytes, where basigin is the ancillary protein. Interestingly, the binding affinity of AR-C155858 to MCT1 and MCT2 expressed in yeast, where there is no basigin or embigin, is also different, with Kd values of 4.9 and 100 nM respectively [38]. Thus it is possible that association of MCT2 with basigin enhances AR-C155858 binding, whereas association with embigin has either no effect or reduces binding affinity. No such effects are apparent with MCT1.
Conclusions
In order to reconcile all of the results of the present study, we propose the scheme shown in Figure 7. The key features of this proposal are as follows.
(i) MCT2 binds preferentially to embigin over endogenous basigin, with the binding to the latter requiring an interaction between the C-termini of both proteins that is not required by MCT1. Thus MCT1trn, but not MCT2trn, expresses well in the absence of co-expressed embigin.
(ii) MCT1 associates with embigin in the absence of basigin, but prefers the latter as binding partner when both are present, unless the C-terminal tail of MCT2 replaces the MCT1 C-terminus (MCT1/2c) when binding to embigin is promoted.
(iii) When bound to embigin, the binding affinity of AR-C155858 to MCT2, but not MCT1, is greatly reduced and this effect is independent of the presence of the C-terminal tail of MCT2.
Our data emphasize that the potency with which AR-C155858 inhibits MCT2 is dependent on the ancillary protein with which it associates. In mammalian cells, embigin is the preferred endogenous binding partner of MCT2 [22] and thus MCT2-mediated lactate transport is likely to be considerably less sensitive to inhibition by AR-C155858 than that mediated by MCT1. This may justify the cautious use of AR-C155858 to dissect out the different roles of MCT1 (very sensitive to AR-C155858), MCT2 (less sensitive to AR-C155858) and MCT4 (insensitive to AR-C155858) in the metabolism of tissues such as the brain, as has been reported by Bröer and colleagues [41]. However, the development of totally isoform-specific inhibitors of MCTs that are not influenced by the associated ancillary protein is clearly desirable.
Online data
AUTHOR CONTRIBUTION
Matthew Ovens did the majority of the experimental work building on preliminary data of Christine Manoharan. Marieangela Wilson generated some of the constructs employed and provided training and experimental guidance. Clare Murray gave advice on the use of AR-C155858 and helped in the design of the study. Andrew Halestrap directed the research and, with Matthew Ovens, wrote the paper.
ACKNOWLEDGEMENT
We thank Agnieszka Bierzynska for skilled technical support.
FUNDING
This work was funded by The Wellcome Trust [grant number 079792/z/06/]. M.J.O. was supported by a Ph.D. studentship from the Medical Research Council.
References
- 1.Bröer S., Schneider H. P., Bröer A., Rahman B., Hamprecht B., Deitmer J. W. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem. J. 1998;333:167–174. doi: 10.1042/bj3330167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bröer S., Bröer A., Schneider H. P., Stegen C., Halestrap A. P., Deitmer J. W. Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochem. J. 1999;341:529–535. doi: 10.1042/0264-6021:3410529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grollman E. F., Philp N. J., McPhie P., Ward R. D., Sauer B. Determination of transport kinetics of chick MCT3 monocarboxylate transporter from retinal pigment epithelium by expression in genetically modified yeast. Biochemistry. 2000;39:9351–9357. doi: 10.1021/bi000464+. [DOI] [PubMed] [Google Scholar]
- 4.Dimmer K. S., Friedrich B., Lang F., Deitmer J. W., Bröer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem. J. 2000;350:219–227. [PMC free article] [PubMed] [Google Scholar]
- 5.Manning Fox J. E., Meredith D., Halestrap A. P. Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. J. Physiol. 2000;529:285–293. doi: 10.1111/j.1469-7793.2000.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halestrap A. P., Meredith D. The SLC16 gene family: from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Arch. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 7.Kim-Garcia C., Goldstein J. L., Pathak R. K., Anderson R. G. W., Brown M. S. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994;76:865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 8.McCullagh K. J. A., Poole R. C., Halestrap A. P., O'Brien M., Bonen A. Role of the lactate transporter (MCT1) in skeletal muscles. Am. J. Physiol. 1996;34:E143–E150. doi: 10.1152/ajpendo.1996.271.1.E143. [DOI] [PubMed] [Google Scholar]
- 9.Jackson V. N., Price N. T., Carpenter L., Halestrap A. P. Cloning of the monocarboxylate transporter isoform MCT2 from rat testis provides evidence that expression in tissues is species-specific and may involve post-transcriptional regulation. Biochem. J. 1997;324:447–453. doi: 10.1042/bj3240447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halestrap A. P., Wang X. M., Poole R. C., Jackson V. N., Price N. T. Lactate transport in heart in relation to myocardial ischemia. Am. J. Cardiol. 1997;80:A17–A25. doi: 10.1016/s0002-9149(97)00454-2. [DOI] [PubMed] [Google Scholar]
- 11.Halestrap A. P., Price N. T. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem. J. 1999;343:281–299. [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia C. K., Brown M. S., Pathak R. K., Goldstein J. L. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J. Biol. Chem. 1995;270:1843–1849. doi: 10.1074/jbc.270.4.1843. [DOI] [PubMed] [Google Scholar]
- 13.Pierre K., Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J. Neurochem. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- 14.Bergersen L. H. Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience. 2007;145:11–19. doi: 10.1016/j.neuroscience.2006.11.062. [DOI] [PubMed] [Google Scholar]
- 15.Yoon H. Y., Fanelli A., Grollman E. F., Philp N. J. Identification of a unique monocarboxylate transporter (MCT3) in retinal pigment epithelium. Biochem. Biophys. Res. Commun. 1997;234:90–94. doi: 10.1006/bbrc.1997.6588. [DOI] [PubMed] [Google Scholar]
- 16.Philp N. J., Yoon H. Y., Lombardi L. Mouse MCT3 gene is expressed preferentially in retinal pigment and choroid plexus epithelia. Am. J. Physiol. Cell Physiol. 2001;280:C1319–C1326. doi: 10.1152/ajpcell.2001.280.5.C1319. [DOI] [PubMed] [Google Scholar]
- 17.Wilson M. C., Jackson V. N., Heddle C., Price N. T., Pilegaard H., Juel C., Bonen A., Montgomery I., Hutter O. F., Halestrap A. P. Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. J. Biol. Chem. 1998;273:15920–15926. doi: 10.1074/jbc.273.26.15920. [DOI] [PubMed] [Google Scholar]
- 18.Juel C., Halestrap A. P. Lactate transport in skeletal muscle: role and regulation of the monocarboxylate transporter. J. Physiol. 1999;517:633–642. doi: 10.1111/j.1469-7793.1999.0633s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ullah M. S., Davies A. J., Halestrap A. P. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1 α-dependent mechanism. J. Biol. Chem. 2006;281:9030–9037. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 20.Poole R. C., Sansom C. E., Halestrap A. P. Studies of the membrane topology of the rat erythrocyte H+/lactate cotransporter (MCT1) Biochem. J. 1996;320:817–824. doi: 10.1042/bj3200817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manoharan C., Wilson M. C., Sessions R. B., Halestrap A. P. The role of charged residues in the transmembrane helices of monocarboxylate transporter 1 and its ancillary protein basigin in determining plasma membrane expression and catalytic activity. Mol. Membr. Biol. 2006;23:486–498. doi: 10.1080/09687860600841967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson M. C., Meredith D., Fox J. E. M., Manoharan C., Davies A. J., Halestrap A. P. Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is embigin (gp70) J. Biol. Chem. 2005;280:27213–27221. doi: 10.1074/jbc.M411950200. [DOI] [PubMed] [Google Scholar]
- 23.Wilson M. C., Meredith D., Bunnun C., Sessions R. B., Halestrap A. P. Studies on the DIDS binding site of monocarboxylate transporter 1 suggest a homology model of the open conformation and a plausible translocation cycle. J. Biol. Chem. 2009;284:20011–20021. doi: 10.1074/jbc.M109.014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poole R. C., Halestrap A. P. Interaction of the erythrocyte lactate transporter (monocarboxylate transporter 1) with an integral 70-kDa membrane glycoprotein of the immunoglobulin superfamily. J. Biol. Chem. 1997;272:14624–14628. doi: 10.1074/jbc.272.23.14624. [DOI] [PubMed] [Google Scholar]
- 25.Nabeshima K., Iwasaki H., Koga K., Hojo H., Suzumiya J., Kikuchi M. Emmprin (Basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol. Int. 2006;56:359–367. doi: 10.1111/j.1440-1827.2006.01972.x. [DOI] [PubMed] [Google Scholar]
- 26.Guenette R. S., Sridhar S., Herley M., Mooibroeck M., Wong P., Tenniswood M. Embigin, a developmentally expressed member of the immunoglobulin super family, is also expressed during regression of prostate and mammary gland. Dev. Genet. 1997;21:268–278. doi: 10.1002/(SICI)1520-6408(1997)21:4<268::AID-DVG4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Muramatsu T., Miyauchi T. Basigin (CD147): a multifunctional transmembrane protein involved in reproduction, neural function, inflammation and tumor invasion. Histol. Histopathol. 2003;18:981–987. doi: 10.14670/HH-18.981. [DOI] [PubMed] [Google Scholar]
- 28.Iacono K. T., Brown A. L., Greene M. I., Saouaf S. J. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp. Mol. Pathol. 2007;83:283–295. doi: 10.1016/j.yexmp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirk P., Wilson M. C., Heddle C., Brown M. H., Barclay A. N., Halestrap A. P. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000;19:3896–3904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao C., Wilson M. C., Schuit F., Halestrap A. P., Rutter G. A. Expression and distribution of lactate/monocarboxylate transporter isoforms in pancreatic islets and the exocrine pancreas. Diabetes. 2001;50:361–366. doi: 10.2337/diabetes.50.2.361. [DOI] [PubMed] [Google Scholar]
- 31.Wilson M. C., Meredith D., Halestrap A. P. Fluorescence resonance energy transfer studies on the interaction between the lactate transporter MCT1 and CD147 provide information on the topology and stoichiometry of the complex in situ. J. Biol. Chem. 2002;277:3666–3672. doi: 10.1074/jbc.M109658200. [DOI] [PubMed] [Google Scholar]
- 32.Philp N. J., Ochrietor J. D., Rudoy C., Muramatsu T., Linser P. J. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Invest. Ophthalmol. Visual Sci. 2003;44:1305–1311. doi: 10.1167/iovs.02-0552. [DOI] [PubMed] [Google Scholar]
- 33.Deora A. A., Philp N., Hu J., Bok D., Rodriguez-Boulan E. Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia. Proc. Natl. Acad. Sci. U.S.A. 2005;102:16245–16250.. doi: 10.1073/pnas.0504419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallagher S. M., Castorino J. J., Wang D., Philp N. J. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res. 2007;67:4182–4189. doi: 10.1158/0008-5472.CAN-06-3184. [DOI] [PubMed] [Google Scholar]
- 35.Murray C. M., Hutchinson R., Bantick J. R., Belfield G. P., Benjamin A. D., Brazma D., Bundick R. V., Cook I. D., Craggs R. I., Edwards S., et al. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat. Chem. Biol. 2005;1:371–376. doi: 10.1038/nchembio744. [DOI] [PubMed] [Google Scholar]
- 36.Guile S. D., Banticka J. R., Cheshire D. R., Cooper M. E., Davis A. M., Donald D. K., Evans R., Eyssade C., Ferguson D. D., Hill S., et al. Potent blockers of the monocarboxylate transporter MCT1: Novel immunomodulatory compounds. Bioorg. Med. Chem. Lett. 2006;16:2260–2265. doi: 10.1016/j.bmcl.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 37.Ekberg H., Qi Z., Pahlman C., Veress B., Bundick R. V., Craggs R. I., Holness E., Edwards S., Murray C. M., Ferguson D., et al. The specific monocarboxylate transporter-1 (MCT-1) inhibitor, AR-C117977, induces donor-specific suppression, reducing acute and chronic allograft rejection in the rat. Transplantation. 2007;84:1191–1199. doi: 10.1097/01.tp.0000287541.53389.be. [DOI] [PubMed] [Google Scholar]
- 38.Ovens M. J., Davies A. J., Wilson M. C., Murray C. M., Halestrap A. P. AR-C155858 is a potent inhibitor of monocarboxylate transporters MCT1 and MCT2 that binds to an intracellular site involving transmembrane helices 7–10. Biochem. J. 2010;425:523–530. doi: 10.1042/BJ20091515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim C. M., Goldstein J. L., Brown M. S. cDNA cloning of MEV, a mutant protein that facilitates cellular uptake of mevalonate, and identification of a point mutation responsible for its gain in function. J. Biol. Chem. 1992;267:23113–23121. [PubMed] [Google Scholar]
- 40.Rahman B., Schneider H. P., Bröer A., Deitmer J. W., Bröer S. Helix 8 and helix 10 are involved in substrate recognition in the rat monocarboxylate transporter MCT1. Biochemistry. 1999;38:11577–11584. doi: 10.1021/bi990973f. [DOI] [PubMed] [Google Scholar]
- 41.Rae C., Nasrallah F. A., Bröer S. Metabolic effects of blocking lactate transport in brain cortical tissue slices using an inhibitor specific to MCT1 and MCT2. Neurochem. Res. 2009;34:1783–1791. doi: 10.1007/s11064-009-9973-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.