Abstract
Background
Methamphetamine (MA) use has been linked anecdotally to rampant dental disease. The authors sought to determine the relative prevalence of dental comorbidities in MA users, verify whether MA users have more quantifiable dental disease and report having more dental problems than nonusers and establish the influence of mode of MA administration on oral health outcomes.
Methods
Participating physicians provided comprehensive medical and oral assessments for adults dependent on MA (n = 301). Trained interviewers collected patients' self-reports regarding oral health and substance-use behaviors. The authors used propensity score matching to create a matched comparison group of nonusers from participants in the the Third National Health and Nutrition Examination Survey (NHANES III).
Results
Dental or oral disease was one of the most prevalent (41.3 percent) medical cormorbidities in MA users who otherwise were generally healthy. On average, MA users had significantly more missing teeth than did matched NHANES III control participants (4.58 versus 1.96, P < .001) and were more likely to report having oral health problems (P < .001). Significant subsets of MA users expressed concerns with their dental appearance (28.6 percent), problems with broken or loose teeth (23.3 percent) and tooth grinding (bruxism) or erosion (22.3 percent). The intravenous use of MA was significantly more likely to be associated with missing teeth than was smoking MA (odds ratio = 2.47; 95 percent confidence interval = 1.3-4.8).
Conclusions
Overt dental disease is one of the key distinguishing comorbidities in MA users. MA users have demonstrably higher rates of dental disease and report long-term unmet oral health needs. Contrary to common perception, users who smoke or inhale MA have lower rates of dental disease than do those who inject the drug. Many MA users are concerned with the cosmetic aspects of their dental disease, and these concerns could be used as behavioral triggers for targeted interventions.
Clinical Implications
Dental disease may provide a temporally stable MA-specific medical marker with discriminant utility in identifying MA users. Dentists can play a crucial role in the early detection of MA use and participate in the collaborative care of MA users.
Keywords: Methamphetamine use, dental disease, oral health, general health, temporomandibular joint disorders
Methamphetamine (MA), a powerful psychostimulant, has established itself rapidly as a leading drug of abuse. Easy to produce and relatively inexpensive to purchase, MA produces an intense rush of pleasure and a prolonged sense of euphoria1-4 matched by few other illicit substances. Thus, it is not surprising that this highly addictive stimulant rapidly is replacing marijuana and crack cocaine as a preferred “drug of choice” for recreational drug users in many areas of the United States.5 According to the 2003 National Survey on Drug Use and Health, 12.3 million Americans (5.2 percent of the population) had tried MA at least once, with the majority of users being between 18 and 34 years of age.6 The rapid spread of MA addiction across the socioeconomic spectrum and the compelling narratives of its personal and societal consequences highlight the importance of viewing MA use as a long-term and widespread public health problem.
Anecdotal reports have suggested that MA use exacts a unique, accelerated toll inside its users' mouths.7-15 Described variously as blackened, stained, rotting or crumbling teeth,16 the purported dental manifestations of MA use have given rise to the moniker “meth mouth” used to describe the phenomenon.17,18 Various explanations, ranging from MA-induced xerostomia to the acidic nature of smoked MA, have been suggested as precipitators of the increased caries.11 However, outside of media coverage and individual case reports, there is a surprising lack of systematic studies to corroborate MA's putative relationship to substantial dental disease. Organized investigations of the meth mouth phenomenon by the dental community have been hindered by a disconnect between the dental community and substance use researchers, dental researchers' lack of access to MA using populations and inadequate resources or infrastructure to conduct field studies in drug using populations.
The infrastructure of a multisite clinical trial called the Methamphetamine Treatment Project (MTP)19 provided us with an opportunity to examine the relationship between chronic MA use and dental disease and to develop a scientific basis for this phenomenon. One of the largest randomized clinical trials of treatment approaches for MA dependence, the MTP compared standard psychosocial treatment to treatment-as-usual in eight treatment programs located in Montana, Hawaii and California between 1999 and 2002.20,21 By comparing the nature and rates of dental disease in MA users from the MTP cohort with the dental status of a sociodemographically similar group of participants in the Third National Health and Nutrition Examination Survey (NHANES III),22 we attempted to verify the increased dental burden that has been attributed to MA use. We hypothesized that people who used MA would manifest substantially greater rates of oral consequences (such as missing teeth, self-reported dental problems, temporomandibular joint [TMJ] problems) than would propensity score-matched nonusers from NHANES III. Specifically, our study addressed the following questions:
▬ What is the relative prevalence of dental comorbidities in MA users compared with nonusers?
▬ Do MA users report having higher rates of dental problems than do nonusers?
▬ Do MA users have quantifiably more dental disease than do nonusers?
▬ Does the mode of MA administration (for example, intravenous [IV], intranasal or smoking) influence oral health outcomes in MA users?
PARTICIPANTS, METHODS AND MATERIALS
Participants
We recruited our study participants (n = 301) from the larger cohort (N = 1,016) of MA-dependent adults (18 years or older) who had participated in the MTP. In our follow-up study to the MTP, physician examiners administered comprehensive health assessments to a subset of the original cohort an average of 3.1 years (standard deviation [SD] = 0.48) after they had completed the initial MTP intervention. We obtained informed consent from the study participants in accordance with protocols approved by the institutional review boards of the Friends Research Institute, Baltimore, and the University of California, Los Angeles.
Assessments
All study participants completed a self-administered follow-up health status survey that elicited detailed information about past and current dental and medical conditions. Physician examiners performed comprehensive physical examinations of the participants and recorded their findings for each body system. Oral health assessments (number of missing teeth, condition of oral mucosa and presence of dentures) were augmented by blood pressure, heart rate, height, weight, hematologic and biochemical measurements. The examiners recorded lesions or irregularities of the oral mucosa as “abnormal mouth condition” and summarized obvious dental caries as “abnormal dental condition.” In addition, trained interviewers conducted face-to-face assessments of substance-use behaviors with study participants. They used the Addiction Severity Index23 to elicit the frequency and patterns of use of various illegal drugs that participants may have used in the 30 days preceding the interview. Primary route of drug administration was determined as the “usual or most recent” route; for patients who reported using more than one route, the most severe route (in descending order, IV, intranasal and smoking) was abstracted from their records. Finally, the interviewers used the Life Experience Timeline interview24 to quantify MA use in the period after the initial MTP study.
Statistical analysis
We used descriptive summaries to characterize the most common comorbidities among MA users. To mimic some of the characteristics of a randomized controlled trial and create a control group of comparable nonusers of MA, we used the propensity score–matching strategy described by Rosenbaum and Rubin.25,26
We compared the MTP participants with a subset of respondents (aged 18-60 years) from the NHANES III study who were similar to the MTP participants in all relevant background characteristics except the use of MA. We estimated individual propensity scores by using a logistic regression model that included 85 predictor variables such as marital status, race and ethnicity, sex, age, education, household income, weight, height, general health self-assessment and number of days since last dental visit. We grouped propensity scores by splitting the entire sample at the median propensity score and then iteratively performing median splits on the groups. We did this until statistical tests indicated that the background characteristics of participants from the MTP and NHANES III were similar within propensity score groups. Within each group, we compared continuously scaled outcomes using independent-sample t tests, and we investigated categorical outcomes using χ2 tests of independence and Fisher exact tests. Owing to missing data, we had propensity scores for 250 MA users.
To gain insight into connections between characteristics of MA use and a broader set of oral health outcomes, we used logistic regression to estimate relationships between background characteristics and the probability of experiencing a particular oral health condition. Specifically, we sought to estimate the relationships between oral health conditions and route of MA administration, lifetime years of MA use and the number of days of MA use within the preceding 30 days while adjusting analyses for age and sex. Dependent variables included both physician-reported and participant-reported outcomes. Our scientific interest focused on inferences for main effects in logistic regression models; our investigation of sensitivity in main-effect inferences appears in an appendix that is available as supplemental data to the online version of this article (found at “http://jada.ada.org”). We carried out all statistical analyses by using commercially available software (SAS Version 9, SAS Institute, Cary, N.C.) and the publicly available R software.27
RESULTS
Sample characteristics
Table 1 summarizes the sociodemographic and substance-use characteristics of the participants in our follow-up study. Generally, the participants were young (mean age = 36.5 years, SD ± 7.9), and most had completed high school (mean = 12.5 years of education, SD ± 1.6). Smoking appeared to be the preferred route of MA administration (64.2 percent, n = 190) with participants reporting MA use for an average of 4.5 days of the preceding 30 days (SD ± 8.6). Comparison of results from the original MTP sample (N = 1,016) and those from the follow-up subset (n = 301) indicated no significant differences with regard to age, sex, level of education, employment, marital status or route of administration. In terms of ethnicity, white participants were represented at a significantly higher rate in the follow-up subset than in the original MTP sample (70 percent versus 60 percent, respectively; χ2 [1] = 9.64; P < .01). Also, the two groups differed in the number of days of MA use in the preceding 30 days (t[1315] = −11.44; P < .001), as would be expected given that the follow-up cohort represented participants who previously had undergone intervention.
TABLE 1.
Demographics and participant's characteristics.
| CHARACTERISTIC (N = 301) | N | % |
|---|---|---|
| Sex | ||
| Male | 114 | 37.9 |
| Female | 187 | 62.1 |
|
| ||
| Marital Status | ||
| Married | 70 | 23.3 |
| Widowed, separated or divorced | 105 | 34.9 |
| Never Married | 126 | 41.9 |
|
| ||
| Employment | ||
| Unemployed | 88 | 29.2 |
| Employed | 213 | 70.8 |
|
| ||
| Race or Ethnicity | ||
| White | 211 | 70.1 |
| African American | 4 | 1.3 |
| Asian | 37 | 12.3 |
| Hispanic | 39 | 13.0 |
| Other | 10 | 3.3 |
|
| ||
|
Route of Methamphetamine Administration* |
||
| Intranasal | 44 | 14.0 |
| Intravenous | 62 | 20.9 |
| Smoking | 190 | 64.2 |
|
| ||
|
Methamphetamine Use During Follow-up Period |
||
| Abstinent | 66 | 22.0 |
| Low | 75 | 25.0 |
| Moderate | 75 | 25.0 |
| Heavy | 84 | 28.0 |
|
| ||
| MEAN | SD † | |
|
| ||
| Age in Years at Follow-up | 36.5 | 7.9 |
|
| ||
| Years of Education at Follow-up | 12.5 | 1.6 |
|
| ||
|
No. of Days Using Methamphetamine in the Preceding 30 Days, Measured at Follow-up |
4.5 | 8.6 |
Information regarding route of administration was missing for five of the 301 Methamphetamine Treatment Project participants.
SD: Standard deviation
MA use and comorbid conditions
The most frequent physical examination findings were elevated body mass index (BMI > 25, 65.7 percent, n = 195), abnormal dental or oral findings (41.3 percent, n = 213), hypertension (21.6 percent, n = 64) and mental status abnormalities (16.3 percent, n = 49). A small subset of the sample had abnormal neurological findings; 6.7 percent (n = 20) evidenced disorders of movement (that is, tremor, tic, akathesia or choreoathetosis), 11 percent (n = 33) had abnormalities on sensory examination and 13.6 percent (n = 41) had other neurological problems (that is, cranial nerve disorders, abnormal deep-tendon reflexes or problems with gait, coordination, motor strength or tone). The prevalence rates of self-reported medical conditions and clinical laboratory abnormalities generally were unremarkable. However, we found elevated rates of hepatitis C antibody and hepatitis B core antibody, indicating prior exposure to or current infection with these viruses, in 16.3 percent (n = 45) and 12.9 percent (n = 24) of participants, respectively. Participants who injected MA were more likely to report having hepatitis (odds ratio [OR] = 15.3; 95 percent confidence interval [CI], 6.4-36.8) and sexually transmitted diseases (OR = 2.1; 95 percent CI, 1.2-3.9) than were participants who smoked MA. Other medical conditions were not related significantly to route of administration.
Self-rated oral health in MA users
At the time of the oral assessment, a substantial proportion of the participants reported experiencing one or more dental problems (Table 2). Problems with dental appearance (28.6 percent, n = 86), broken or loose teeth (23.3 percent, n = 70) and tooth grinding or erosion (22.3 percent, n = 67) were the most commonly reported dental conditions. About 8 percent of the participants reported having TMJ problems. Study participants who reported having dental problems also manifested unmet oral care needs for extended periods (Table 2). The average period during which participants experienced a dental problem ranged from nearly 18 months (for swollen or bleeding gingiva) to nearly 77 months (for TMJ disorders).
TABLE 2.
Participants' oral health outcomes.
| OUTCOME | EVER EXPERIENCED |
CURRENTLY EXPERIENCING | MONTHS EXPERIENCING |
|||
|---|---|---|---|---|---|---|
| n | % | n | % of Ever Experienced* |
% of Total† | Mean | |
| As Reported by Participant | ||||||
| Toothache | 212 | 70.4 | 43 | 20.3 | 14.3 | 30.75 |
| Swollen, inflamed and/or bleeding gums |
102 | 33.9 | 45 | 44.1 | 15 | 17.82 |
| Broken or loose teeth | 159 | 52.8 | 70 | 44 | 23.3 | 32.43 |
| Problems with cap, restoration or other prosthesis |
81 | 26.9 | 36 | 44.4 | 12.0 | 19.34 |
| Cosmetic problems | 115 | 38.2 | 86 | 74.8 | 28.6 | 61.79 |
| Temporomandibular joint disorders |
39 | 13 | 25 | 64.1 | 8.3 | 76.68 |
| Tooth grinding/Enamel erosion | 92 | 30.6 | 67 | 72.8 | 22.3 | 72.15 |
|
| ||||||
| As Assessed by Physician | Mean | SD ‡ | ||||
| Abnormal mouth condition | 13 | 4.3 | ||||
| Abnormal dental condition | 93 | 30.9 | Not applicable | |||
| Dentures | 40 | 13.3 | ||||
| Missing teeth | 180 | 59.8 | 4.58 | 7.11 | ||
Calculated as the number of participants who currently were experiencing the problem divided by the number who had ever experienced the same problem.
Calculated as the number of participants who currently were experiencing the problem divided by the total number of participants who used methamphetamine.
SD: Standard deviation.
Physicians' assessment of participants' oral health
Table 2 shows the pervasiveness of participants' abnormal oral health conditions as reported by the physical examiners. Almost 60 percent (n = 180) of the participants who used MA had one or more missing teeth (excluding third molars) and the mean number of missing teeth was 4.58 (SD ± 7.11). The physician assessors reported abnormal dental conditions (such as overtly carious and broken-down teeth) in 30.9 percent (n = 93) of the patients and noted that 4.3 percent (n = 13) manifested a lesion or abnormality of the oral mucosa. Despite their relative youth (mean age = 36.5 years, SD ± 7.9 years), 13.3 percent (n = 40) of the patients already were wearing dentures (partial or complete).
MA use and dental disease in propensity score–matched participants
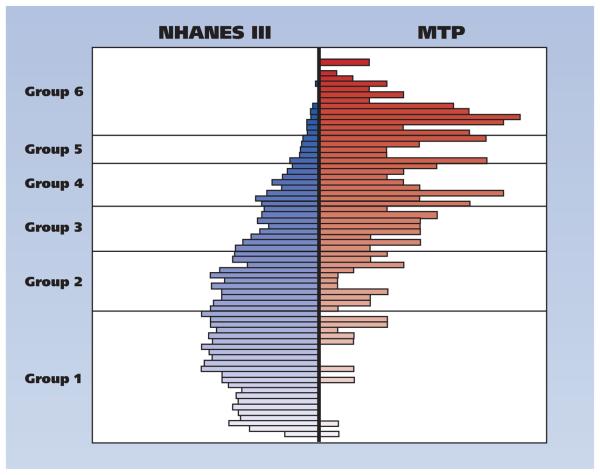
The figure (page 312) summarizes the distribution of the estimated propensity scores for the MA users and matched NHANES III participants. The dissimilar shapes of the overall distributions, typical in observational-study settings, reflect differences in the distributions of baseline characteristics between the two samples. However, the overlap of the distributions and the availability of matched participants within each propensity score group allow comparisons between MA users and matched control participants from the NHANES III sample.
Figure.
Propensity score grouping of the Methamphetamine Treatment Project (MTP) and Third National Health and Nutrition Examination Survey (NHANES III) participants.
Table 3 (page 313) emphasizes the effectiveness of the propensity score–matching strategy. As evident in the column summarizing the data for the total sample, there are significant underlying differences between the NHANES III and MA groups. Yet, when we sorted the data by propensity score groups (groups 1-6) and compared the MTP participants with NHANES III participants within each stratum, we found that the two groups were equivalent, with negligible statistical differences, on all of the matching variables. Despite the similarities, with the exception of substance-use behaviors, MA users had significantly more missing teeth on average than did participants in the NHANES III group (Table 4, pages 314-315). Similarly, MA users were significantly more likely to report having poor oral health conditions than were matched NHANES III control participants (Table 4).
TABLE 3.
Comparisons between the MTP* and NHANES III† participants overall and by propensity score group, in P values.
| CHARACTERISTIC | PARTICIPANT GROUP | ||||||
|---|---|---|---|---|---|---|---|
| Entire Sample (n = 7,880) |
Group 1 (n = 4,112) |
Group 2 (n = 1,877) |
Group 3 (n = 962) |
Group 4 (n = 583) |
Group 5 (n = 186) |
Group 6 (n = 160) |
|
| Age | < .001‡ | .835 | .049‡ | .146 | .088 | .673 | .106 |
| Last Visit to a Dentist | .406 | .83 | .235 | .202 | .124 | < .001‡ | .782 |
| Time at Current Address | < .001‡ | .962 | .803 | .419 | .49 | .751 | .736 |
| Weight | < .001‡ | .967 | .314 | .711 | .419 | .679 | .607 |
| Height | .051‡ | .73 | .314 | .732 | .432 | .203 | .625 |
| Sex | .004‡ | .942 | .167 | .766 | .594 | .421 | .322 |
| Race or Ethnicity | .013 | .569 | .536 | .815 | .439 | .866 | .159 |
| Marital Status | < .001‡ | .539 | .531 | .982 | .933 | .067 | .038 |
| Self-Rated Health | .002‡ | .763 | .278 | .658 | .75 | .529 | .651 |
| Household Income | < .001‡ | .635 | .95 | .473 | .181 | .846 | .725 |
| Last Visit to a Physician | .166 | .315 | .209 | .859 | .978 | .152 | .964 |
| Nights in Hospital | .853 | .901 | .267 | .089 | .358 | .796 | .862 |
| Education Level | < .001‡ | .461 | .62 | .515 | .909 | .346 | .399 |
MTP: Methamphetamine Treatment Project.
NHANES III: Third National Health and Nutrition Examination Survey.
Indicates a statistically significant difference at the α = .05 level.
TABLE 4.
| OUTCOME | ENTIRE SAMPLE | PROPENSITY SCORE GROUP | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||||||||
| NHANES III | MTP | NHANES III | MTP | NHANES III | MTP | NHANES III | MTP | NHANES III | MTP | NHANES III | MTP | NHANES III | MTP | |
| (n = 7,630) | (n = 250)‡ | (n = 4,092) | (n = 20) | (n = 1,847) | (n = 30) | (n = 912) | (n = 50) | (n = 532) | (n = 51) | (n = 153) | (n = 33) | (n = 94) | (n = 66) | |
|
Condition of Teeth, As Reported by Participant (%) |
||||||||||||||
| Very good | 22 | 11 | 26 | 20 | 23 | 14 | 24 | 12 | 20 | 8 | 22 | 9 | 18 | 5 |
| Good | 32 | 24 | 35 | 30 | 33 | 17 | 32 | 28 | 36 | 25 | 35 | 27 | 30 | 26 |
| Fair | 28 | 31 | 25 | 25 | 29 | 31 | 28 | 24 | 26 | 29 | 23 | 33 | 33 | 36 |
| Poor | 14 | 22 | 12 | 15 | 12 | 24 | 14 | 20 | 16 | 16 | 16 | 21 | 11 | 23 |
| No natural teeth | 4 | 13 | 3 | 10 | 3 | 14 | 2 | 16 | 2 | 22 | 5 | 9 | 9 | 11 |
| P Value | < .0001§ | .348 | .006§ | < .001§ | < .001§ | .201 | .038§ | |||||||
|
| ||||||||||||||
|
Mean Number of Missing Teeth, As Assessed by Physician |
1.96 | 4.58 | 1.81 | 2 | 2.02 | 3.66 | 2.02 | 4.55 | 1.71 | 5.16 | 1.49 | 4.88 | 1.55 | 4.38 |
| P Value | < .001§ | .979 | .015§ | .154 | .028§ | < .001§ | < .001§ | |||||||
MTP: Methamphetamine Treatment Project.
NHANES III: Third National Health and Nutrition Examination Survey.
Values for some MTP propensity scores were missing, so these comparisons were made using the 250 participants for whom propensity scores were available.
Indicates a statistically significant difference at the α = .05 level.
MA use characteristics and oral health outcomes
To determine the differential effect of frequency and mode of MA use, we compared the oral health conditions of IV MA users, intranasal MA users and MA smokers. Analyses showed that IV users had significantly higher rates of dental illness than did smokers and intranasal users (47.7 percent versus 28.9 percent and 21.9 percent respectively; P < .05). At the physical examination, significantly more IV users had missing teeth than did smokers (73.3 percent versus 57.2 percent; P < .05), and the mean number of missing teeth within these two groups was 6.2 (SD ± 2.35) and 3.8 (SD ± 2.1), respectively.
Table 5 (page 316) displays results from logistic regression analyses in which we examined the relationship between the characteristics of MA use and several dental outcomes while adjusting for other potential confounders. Notably, IV MA users were significantly more likely to report having cosmetic problems (OR = 1.95; 95 percent CI, 1.06-3.56) than were users who smoked MA. The IV use of MA was significantly more likely to be associated with missing teeth than was smoking MA (OR = 2.47; 95 percent CI, 1.26-4.83), as was the recent use of MA (OR = 1.42; 95 percent CI, 1.02-1.99) (data not shown).
TABLE 5.
Factors significantly relating to presence of dental conditions as determined by logistic regressions controlling for background characteristics.
| DENTAL CONDITION |
CHARACTERISTIC, IN ODDS RATIOS (95% CONFIDENCE INTERVALS) |
|||||
|---|---|---|---|---|---|---|
| Female Sex | Age in Years | Route of Administration* | Methamphetamine Use | |||
| Intravenous | Nasal | Lifetime years of use† |
Days of use in past 30 days‡ |
|||
|
As Reported by Participant |
||||||
| Toothache (n = 212) | 1.54 (0.9-2.62) | 1.04 (0.99-1.08) | 1.08 (0.56-2.1) | 0.59 (0.29-1.21) | 0.95 (0.74-1.21) | 1.43 (0.99-2.07)§ |
| Swollen, inflamed and/or bleeding gums (n = 102) |
0.96 (0.57-1.6) | 1.02 (0.98-1.07) | 1.49 (0.8-2.76) | 1.35 (0.67-2.70) | 1.15 (0.92-1.43) | 0.94 (0.70-1.27) |
| Broken or loose teeth (n = 159) |
0.74 (0.45-1.21) | 1.05 (1-1.09)¶ | 1.6 (0.87-2.94) | 1.02 (0.51-2.0) | 0.88 (0.70-1.09) | 1.26 (0.94-1.69) |
| Problems with cap, restoration or other prosthesis (n = 81) |
1.65 (0.93-2.91)§ | 1.02 (0.98-1.07) | 1.65 (0.86-3.16) | 1.15 (0.54-2.43) | 1.16 (0.92-1.47) | 1.22 (0.90-1.65) |
| Cosmetic problems (n = 115) |
1.17 (0.71-1.95) | 1.03 (0.99-1.08)§ | 1.95 (1.06-3.56)¶ | 1.52 (0.77-2.99) | 0.96 (0.77-1.19) | 1.15 (0.86-1.53) |
| Temporomandibular joint disorders (n = 39) |
3.43 (1.44-8.2)¶ | 1 (0.95-1.06) | 0.97 (0.4-2.37) | 1.29 (0.5-3.34) | 1.06 (0.78-1.44) | 1.15 (0.77-1.71) |
| Tooth grinding or enamel erosion (n = 92) |
1.43 (0.84-2.45) | 0.98 (0.94-1.03) | 1.49 (0.79-2.79) | 1.36 (0.66-2.79) | 1.23 (0.97-1.56)§ | 1.13 (0.84-1.52) |
|
| ||||||
|
As Assessed by Physician |
||||||
| Abnormal mouth condition (n = 13) |
0.27 (0.08-0.95)¶ | 1.08 (0.98-1.2) | 0.83 (0.16-4.31) | 2.07 (0.56-7.63) | 0.88 (0.54-1.42) | 0.98 (0.52-1.87) |
| Abnormal dental condition (n = 93) |
0.45 (0.26-0.77)¶ | 1.07 (1.02-1.12)¶ | 1.72 (0.9-3.31) | 1.03 (0.49-2.14) | 0.94 (0.75-1.19) | 1.2 (0.89-1.62) |
| Dentures (n = 40) | 2.22 (1.01-4.88)¶ | 1.06 (0.99-1.12)§ | 0.79 (0.31-2.01) | 1.48 (0.60-3.63) | 1.28 (0.96-1.72)§ | 0.82 (0.52-1.28) |
| Missing teeth (n = 180) | 1.28 (0.76-2.18) | 1.08 (1.04-1.13)¶ | 2.47 (1.26-4.83)¶ | 1.31 (0.63-2.73) | 1.03 (0.81-1.30) | 1.42 (1.02-1.99)¶ |
Compared with those who smoked methamphetamine.
Odds ratios are for an additional five years of use.
Odds ratios are for an additional 10 days of use.
Statistically significant at the α = .10 level.
Statistically significant at the α = .05 level.
DISCUSSION
Key findings
The results of our study, one of the first systematic investigations of the meth mouth phenomenon, reveal four key findings:
▬ overt dental disease is one of the key distinguishing medical comorbidities in MA users who otherwise generally are young and healthy;
▬ a significant subset of MA users reported having current dental problems as well as unmet oral health needs;
▬ MA users, per physician assessment, have quantifiably higher rates of dental disease and oral health problems than do matched control participants;
▬ MA users who inject the drug have rates of dental disease higher than those of users who smoke or inhale MA.
Taken together, our findings substantiate the anecdotal reports associating MA use with extensive dental disease and underscore the growing concern about the personal and public health implications of the oral health consequences of using MA.
Overt dental disease as a distinguishing marker of MA use
Three years after receiving treatment for MA dependence, a substantial proportion of the patient sample manifested oral or dental disease as a particularly prominent physical finding. Although the rates of elevated BMI and hypertension appear relatively high, they are consistent with patterns noted in the general population.28 Similarly, the prevalence rates of participant-reported medical conditions as well as clinical laboratory abnormalities generally were unremarkable except for increased evidence of hepatitis B or hepatitis C coinfections, which are related to the IV use of MA and risky sexual behaviors. Although other researchers have documented psychiatric symptoms and movement disorders in MA users29 and we observed them in small subsets of our patient sample, these symptoms often are present only transiently in the context of MA intoxication. Thus, overt dental disease may provide a temporally stable MA-specific medical marker with discriminant utility in a variety of clinical settings. With adequate elaboration through larger clinical studies involving trained dentists using calibrated technique, the rates and specificity of the dental caries experience may be useful in distinguishing covert MA users and helping clinicians initiate timely dental treatment and substance-use interventions.30
High rates of current dental problems and unmet oral health needs among MA users
Our findings reveal that a significant subset of MA users is likely to be experiencing dental pain and discomfort. Articulated variously as toothache, broken or loose teeth and problems with dental restorations, the reports of the participants in our study illustrate the pervasiveness of acute dental problems among MA users. The prevalence of bruxism and TMJ problems was consistent with the descriptions provided by Donaldson and Goodchild,31 who attributed bruxism to drug-induced hyperactivity. Bruxism is an activity of particular concern in MA users because of its potential consequences for an already compromised dentition: tooth destruction, breakage of dental restorations and exacerbation of TMJ disorders. As is true of patterns in the general population, we determined that female MA users were much more likely than were their male counterparts to report having TMJ problems.
Many of the MA users in our study were concerned about the cosmetic aspects of their dental disease. This finding challenges the conventional perspective that substance users are focused on their acute dental problems to the exclusion of any long-term dental care. Our data suggest that many MA users are conscious of the oral effects of their drug addiction. Restoration of their dental self-image may be central to the emergence of reconstructed self-identities and could be used as a behavioral trigger for substance-use interventions.32 Despite the pervasiveness of dental problems and symptoms, most of the study participants did not appear to have received needed care for extended periods. As we noted earlier, the mean period during which participants experienced a dental problem ranged from nearly 18 months (for swollen or bleeding gingiva) to nearly 77 months (for TMJ disorders). Our finding is consistent with those of other studies that have shown substance users to be at a disadvantage in terms of receiving needed health services.33,34 Systemic barriers such as lack of dental insurance and fragmented health care systems may explain the unmet need. Alternatively, the findings also may be attributable in part to the drug users' lifestyle, which encourages procrastination until a crisis condition occurs. Although our study design did not allow us to probe the barriers to dental care that are specific to MA patients, such information would be relevant to the development of focused and effective interventions.
MA users' demonstrably higher rates of dental disease and oral health problems than those of control participants
Our physician-collected data confirmed that MA users have significantly higher rates of dental and oral disease than do matched population-based control participants. Nearly 60 percent of the MA-using participants had one or more missing teeth, and more than 13 percent (n = 40) already were wearing prosthodontic appliances—a striking discovery given that our study population was relatively young (mean age = 36.5 years, SD ± 7.9). Our findings echo and reinforce those of Morio and colleagues,35 who examined a small group of 18 MA users and determined that they had fewer molars and more dental caries than did a corresponding group of age-matched and sex-matched nonusers. We should point out that their simple strategy of matching MA users to nonusers in a small group of participants may have resulted in biased estimates of MA's dental effects owing to differences in observed covariates. By using a much larger sample size, we specifically addressed, via our propensity score–matching methods, any naturally occurring imbalances between the groups (MA users and NHANES III participants) and minimized the possibility of bias caused by systematic differences.
Our propensity score–matching strategy was successful in creating a control group of people who did not use MA but who had baseline characteristics similar to those of the cohort of participants who used MA. Within the propensity score–matched groups, we established that MA users have significantly more missing teeth, on average, than do members of a comparable sample from the general population. It is reasonable to assume that the missing teeth represent the consequences of advanced dental disease. Also, MA users were significantly more likely to report having poor oral health conditions than were the matched NHANES III control participants. Even though our physician-conducted oral assessments corroborated the anecdotal descriptions of increased dental disease associated with MA use, we were unable to verify the caries patterns reported to be distinctive of meth mouth. Detailed, tooth-surface–specific examinations by dental examiners using calibrated techniques are necessary to refine our understanding of the dental consequences distinctive of MA use.
Higher rates of dental disease among IV users of MA than among users who smoke or inhale MA
One of our salient findings was the higher rates of dental disease associated with injected MA use compared with those associated with smoked or inhaled MA. Our logistic regression analyses highlighted significant differences in oral health outcomes between IV MA users and MA smokers and also suggested that IV MA use is associated with a higher prevalence of dental conditions than is intranasal MA use. The findings contradict prevailing beliefs that the local effects of smoked MA result in greater levels of dental disease. Whereas investigators such as Shaner and colleagues8 implicated the xerostomic side effects of MA use, others, including McGrath and Chan,36 speculated that the caustic nature of the inhaled drug directly contributes to tooth destruction. We did not investigate MA's effect on salivary flow, but our data linking higher dental disease to IV MA use belie common notions about the corrosive effects of MA. Local dental effects associated with the acidity of MA would be minimal with IV use. We believe it is more likely that the IV MA users have a higher level of addiction than those who smoke or inhale MA and, thus, are less likely to pursue oral hygiene. Those who use MA may make the transition from noninjection methods of drug use to injection methods as their dependence on MA becomes more severe. IV drug use is a popular route of administration because the injected substance has almost 100 percent bioavailability and the onset of the drug high is fairly rapid, generally 15 to 30 seconds.37
Limitations
The results of our study, however, must be viewed in the light of several limitations. First, we derived the oral health outcomes in the MA-using cohort from participant self-reports and brief oral assessments. Admittedly, our strategy of relying on self-reports and a concise oral assessment was dictated by the time and resource constraints attendant on the comprehensive health assessments performed. Our primary objective was to verify the hitherto anecdotal reports of MA-associated dental disease and not to provide a detailed insight into the meth mouth condition or into the mechanistic pathways that might account for this association. But now that our study results provide scientific evidence linking MA use to adverse oral health outcomes, it would be valuable to pursue detailed investigations with more refined oral health assessments.
Second, physicians performed all assessments of oral health variables in the MTP cohort, whereas the NHANES III assessments derive from comprehensive dentist-conducted examinations. While this would not suggest any predictable bias in measurements, our findings justify the pursuit of additional research by dental examiners using calibrated tooth-surface–specific assessment techniques. Future findings from studies that build on our research findings would only bolster the validity of our inferences. A related issue is the absence of any effort to calibrate the oral assessments of the various physicians involved in the MTP study. It is possible that some of the variation in oral health measurements could be explained by differences in how our examiners performed assessments. While we do not expect such disparities to account for all of the observed differences in oral health outcomes between MA users and nonusers, we suggest that quality-control strategies incorporated into future research protocols would enhance the reliability of measurement.
One of the features of our MTP cohort was that it was composed primarily of long-term MA users. It would be useful to assess the extent to which dental problems emerge in MA users who have different levels of substance-use severity and varying durations of lifetime substance use. One concern could be the interaction of several illegal substances used concomitantly with MA. Our analysis did not address this, inasmuch as our MA cohort exhibited low rates of use of other illicit drugs. An implicit assumption of our statistical-analysis strategy was that none of the NHANES III survey participants was a user of MA. Although we would expect violation of this assumption to have some effect on our results, we did not believe there necessarily was a great risk of bias in our current estimates—for even though MA use has been growing, MA users still do not represent a large proportion of the U.S. population.38 Nevertheless, future research strategies could address this potential concern, through either study design or data analysis methods.
Strengths
The limitations notwithstanding, the salience of our findings and their agreement with the results of a wider body of individual reports relating extensive dental disease to MA use underscore the need to address the consequential, but poorly studied, dental effects of MA use. Our study does have several strengths, including a substantial sample size of usually difficult-to-reach MA users, comprehensive medical data to provide a context for the dental comorbidity, use of patient-centered self-reports, use of a sociodemographically similar comparison group and innovative statistical strategies to address any bias caused by covariate imbalances between the MA and comparison groups. By systematically outlining the disproportionate dental disease in MA users, their substantial unmet needs for dental care and their concerns about their dental appearance, we hope to attract the attention and concerted efforts of the dental community toward the end of developing ameliorative dental strategies that prove both effective and practical. Greater recognition of the dental consequences of MA use will ensure that our advocacy for improvements in oral health care for MA users is linked to broader health initiatives. Our findings offer a framework for research that can help guide the response of the dental community to the epidemic of MA use. As difficult as it is to address the hidden aspects of drug use, the personal and public health effects of MA's use and its long-term claim on scarce health care resources make it imperative that we as a society develop a well-coordinated response. From this perspective, we envision the dental community's playing a crucial role in mounting a broader strategy to address the health burden of MA use.
Given that dental disease is a prominent comorbidity of MA use, dental professionals are in a unique position to help in the early detection of undisclosed MA use and participate as integral members of a collaborative care team tending to those who use MA. One practical response may be to develop screening protocols that use the differential rates and patterns of dental caries experience to identify covert MA users39 and connect or reconnect them to substance-use treatment programs. Concerns about dental appearance may provide a stimulus for engaging patients in stepped, motivational, dental clinic–based interventions patterned after similar tobacco-use cessation programs40-43 or, alternatively, referral to specialty drug treatment. For MA users seeking to change, the dental deterioration may be symbolic of their drug-using identity and a visible embodiment of the “drug user” persona they seek to escape. Helping these patients regain oral function and a positive oral self-image through dental reconstruction could become an important part of the recovery process and one of the first steps to helping them recover their lives entirely.44 Thus, having an expanded role in the management of MA users would afford dental specialists a tremendous opportunity to staunch the loss of identity, sense of life, and health and happiness caused by this addiction.
CONCLUSIONS
Overt dental disease is one of the key distinguishing comorbidities in MA users who otherwise generally are healthy. Unlike the psychiatric and neurological symptoms of acute intoxication that tend to be transient, dental disease may provide a temporally stable MA-specific medical marker that could help clinicians identify MA users in a variety of clinical settings. MA users have quantifiably higher rates of dental disease than those found in the general population, and a significant subset report having current dental problems as well as long-term unmet oral health needs. Contrary to common perception, people who smoke or inhale MA have lower rates of dental disease than do those who inject MA. Further elaboration of the differential rates and patterns of dental caries experience may help clinicians develop screening protocols to identify covert MA users in the dental setting. Many MA users are concerned about the cosmetic aspects of their dental disease, and clinicians could use dental self-image as a behavioral trigger for targeted interventions in the dental office or for referral to substance-use treatment programs. Given that dental comorbidities are a prominent feature of MA use and that many MA users are concerned about their dental appearance, dentists can play a crucial role in the early detection of MA use and participate as integral members of a collaborative care team tending to the MA user. ■
Supplementary Material
Acknowledgments
The study described in this article was supported by grant 1R01DA0256801 from the National Institutes of Health, National Institute on Drug Abuse, Rockville, Md., and enabled by Methampheta-mine Abuse Treatment—Special Studies (MAT-SS) contract 270-01-7089 and grants TI 11440–01, TI 11427–01, TI 11425–01, TI 11443–01, TI 11484–01, TI 11441–01, TI 11410–01 and TI 11411–01 from the Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services, Rockville, Md.
The authors thank the clinicians who participated in this study.
ABBREVIATION KEY
- BMI
Body mass index
- IV
Intravenous
- MA
Methamphetamine
- MTP
Methamphetamine Treatment Project
- NHANES III
Third National Health and Nutrition Examination Survey
- TMJ
Temporomandibular joint
Footnotes
Disclosure. None of the authors reported any disclosures.
Contributor Information
Dr. Vivek Shetty, Section of Oral and Maxillofacial Surgery, 23-009 School of Dentistry, University of California, Los Angeles, 10833 Le Conte Ave., Los Angeles, Calif. 90095-1668..
Dr. Larissa J. Mooney, Integrated Substance Abuse Programs, University of California, Los Angeles..
Mr. Corwin M. Zigler, Section of Oral and Maxillofacial Surgery, School of Dentistry, University of California, Los Angeles..
Dr. Thomas R. Belin, Department of Biostatistics, School of Public Health, University of California, Los Angeles..
Dr. Debra Murphy, Integrated Substance Abuse Programs, University of California, Los Angeles..
Dr. Richard Rawson, Integrated Substance Abuse Programs, University of California, Los Angeles..
References
- 1.Cho AK, Melega WP. Patterns of methamphetamine abuse and their consequences. J Addict Dis. 2002;21(1):21–34. doi: 10.1300/j069v21n01_03. [DOI] [PubMed] [Google Scholar]
- 2.Itzhak Y, Achat-Mendes C. Methamphetamine and MDMA (ecstasy) neurotoxicity: “of mice and men.”. IUBMB Life. 2004;56(5):249–255. doi: 10.1080/15216540410001727699. [DOI] [PubMed] [Google Scholar]
- 3.Thompson PM, Hayashi KM, Simon SL, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24(26):6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sulzer D, Sonders MS, Poulsen N, Galli A. Mechanisms of neuro-transmitter release by amphetamines: a review. Prog Neurobiol. 2005;75(6):406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Maxwell JC, Rutkowski BA. The prevalence of methamphetamine and amphetamine abuse in North America: a review of the indicators, 1992-2007. Drug Alcohol Rev. 2008;27(3):229–235. doi: 10.1080/09595230801919460. [DOI] [PubMed] [Google Scholar]
- 6.Wright D, Sathe N. State Estimates of Substance Use From the 2003-2004 National Surveys on Drug Use and Health. Substance Abuse and Mental Health Services Administration, Office of Applied Studies; Rockville, Md.: 2006. (U.S. Department of Health and Human Services Publication SMA 06-4142, NSDUH Series H-29). “ www.oas.samhsa.gov/2k4State/toc.htm”. Accessed Jan. 18, 2010. [Google Scholar]
- 7.Curtis EK. Meth mouth: a review of methamphetamine abuse and its oral manifestations. Gen Dent. 2006;54(2):125–129. [PubMed] [Google Scholar]
- 8.Shaner JW, Kimmes N, Saini T, Edwards P. “Meth mouth”: rampant caries in methamphetamine abusers. AIDS Patient Care STDS. 2006;20(3):146–150. doi: 10.1089/apc.2006.20.146. [DOI] [PubMed] [Google Scholar]
- 9.Rhodus NL, Little JW. Methamphetamine abuse and “meth mouth.”. Northwest Dent. 2005;84(5):29,31,33–37. [PubMed] [Google Scholar]
- 10.McGrath C, Chan B. Oral health sensations associated with illicit drug abuse. Br Dent J. 2005;198(3):159–162. doi: 10.1038/sj.bdj.4812050. [DOI] [PubMed] [Google Scholar]
- 11.Saini T, Edwards PC, Kimmes NS, Carroll LR, Shaner JW, Dowd FJ. Etiology of xerostomia and dental caries among methamphetamine abusers. Oral Health Prev Dent. 2005;3(3):189–195. [PubMed] [Google Scholar]
- 12.Mallatt ME. Meth mouth: a national scourge. J Indiana Dent Assoc. 2005;84(3):28–29. [PubMed] [Google Scholar]
- 13.Klasser G, Epstein J. Methamphetamine and its impact on dental care. J Can Dent Assoc. 2005;71(10):759–762. [PubMed] [Google Scholar]
- 14.Stein WE. Rotten times are here again! Northwest Dent. 2005;84(5):10. [PubMed] [Google Scholar]
- 15.Smart RJ, Rosenberg M. Methamphetamine abuse: medical and dental considerations. J Mass Dent Soc. 2005;54(2):44–46. 48–49. [PubMed] [Google Scholar]
- 16.American Dental Association Methamphetamine use: meth mouth. “ www.ada.org/prof/resources/topics/methmouth.asp”. Accessed Jan. 18, 2010.
- 17.Rhodus NL, Little JW. Methamphetamine abuse and “meth mouth.”. Pa Dent J (Harrisb) 2008;75(1):19–29. [PubMed] [Google Scholar]
- 18.Padilla R, Ritter AV. Meth mouth: methamphetamine and oral health. J Esthet Restor Dent. 2008;20(2):148–149. doi: 10.1111/j.1708-8240.2008.00167.x. [DOI] [PubMed] [Google Scholar]
- 19.Huber A, Lord RH, Gulati V, Marinelli-Casey P, Rawson R, Ling W. The CSAT methamphetamine treatment program: research design accommodations for “real world” application. J Psychoactive Drugs. 2000;32(2):149–156. doi: 10.1080/02791072.2000.10400223. [DOI] [PubMed] [Google Scholar]
- 20.Herrell JM, Taylor JA, Gallagher C, Dawud-Noursi S. A multisite study of the effectiveness of methamphetamine treatment: an initiative of the Center for Substance Abuse Treatment. J Psychoactive Drugs. 2000;32(2):143–147. doi: 10.1080/02791072.2000.10400222. [DOI] [PubMed] [Google Scholar]
- 21.Rawson RA, Marinelli-Casey P, Anglin MD, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99(6):708–717. doi: 10.1111/j.1360-0443.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- 22.Dye BA, Nowjack-Raymer R, Barker LK, et al. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2003-04. J Public Health Dent. 2008;68(4):218–226. doi: 10.1111/j.1752-7325.2007.00076.x. [DOI] [PubMed] [Google Scholar]
- 23.McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: the Addiction Severity Index. J Nerv Ment Dis. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Hillhouse M, Marinelli-Casey P, Rawson R. The LET (Life Experience Timeline): a new instrument for collecting time-anchored natural history data; Paper presented at the 67th Annual Meeting of the College on Problems of Drug Dependence; Orlando, Fla. June 2005. [Google Scholar]
- 25.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 26.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79(387):516–524. [Google Scholar]
- 27.The R project for statistical computing. “ www.r-project.org”. Accessed Jan. 18, 2010.
- 28.Pleis JR, Lethbridge-Çejku M. Summary health statistics for U.S. adults: National Health Interview Survey, 2005. Vital Health Stat. 2006;10(232):1–153. [PubMed] [Google Scholar]
- 29.Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson R, Methamphetamine Treatment Project Identifying methamphetamine users at risk for major depressive disorder: findings from the methamphetamine treatment project at three-year follow-up. Am J Addict. 2008;17(2):99–102. doi: 10.1080/10550490701861110. [DOI] [PubMed] [Google Scholar]
- 30.Richards JR, Brofeldt BT. Patterns of tooth wear associated with methamphetamine use. J Periodontol. 2000;71(8):1371–1374. doi: 10.1902/jop.2000.71.8.1371. [DOI] [PubMed] [Google Scholar]
- 31.Donaldson M, Goodchild JH. Oral health of the methamphetamine abuser (published correction appears in Am J Health Syst Pharm 2006;63[22]:2180) Am J Health Syst Pharm. 2006;63(21):2078–2082. doi: 10.2146/ajhp060198. [DOI] [PubMed] [Google Scholar]
- 32.Gibson B, Acquah S, Robinson PG. Entangled identities and psychotropic substance use. Sociol Health Illn. 2004;26(5):597–616. doi: 10.1111/j.0141-9889.2004.00407.x. [DOI] [PubMed] [Google Scholar]
- 33.Chitwood DD, McBride DC, Metsch LR, Comerford M, McCoy CB. A comparison of the need for health care and use of health care by injection-drug users, other chronic drug users, and nondrug users. Am Behav Scientist. 1998;8(41):1107–1122. [Google Scholar]
- 34.Chitwood DD, McBride DC, Metsch LR, et al. Health care need and utilization: a preliminary comparison of injection drug users, other chronic drug users, and nondrug users. J Substance Use Misuse. 1999;34(4-5):727–746. doi: 10.3109/10826089909037240. [DOI] [PubMed] [Google Scholar]
- 35.Morio KA, Marshall TA, Qian F, Morgan TA. Comparing diet, oral hygiene and caries status of adult methamphetamine users and nonusers: a pilot study. JADA. 2008;139(2):171–176. doi: 10.14219/jada.archive.2008.0133. [DOI] [PubMed] [Google Scholar]
- 36.McGrath C, Chan B. Oral health sensations associated with illicit drug abuse. Br Dent J. 2005;198(3):159–162. doi: 10.1038/sj.bdj.4812050. [DOI] [PubMed] [Google Scholar]
- 37.Baciewicz GJ. Injecting drug use. “ http://emedicine.medscape.com/article/286976-overview”. Accessed Feb. 8, 2010.
- 38.Anglin MD, Burke C, Perrochet B, Stamper E, Dawud-Noursi S. History of the methamphetamine problem. J Psychoactive Drugs. 2000;32(2):137–141. doi: 10.1080/02791072.2000.10400221. [DOI] [PubMed] [Google Scholar]
- 39.McDaniel TF, Miller D, Jones R, Davis M. Assessing patient willingness to reveal health history information (published correction appears in JADA 1995;126[5]:560) JADA. 1995;126(3):375–379. doi: 10.14219/jada.archive.1995.0183. [DOI] [PubMed] [Google Scholar]
- 40.Stevens VJ, Severson H, Lichtenstein E, Little SJ, Leben J. Making the most of a teachable moment: a smokeless-tobacco cessation intervention in the dental office. Am J Public Health. 1995;85(2):231–235. doi: 10.2105/ajph.85.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lichtenstein E, Hollis JF, Severson HH, et al. Tobacco cessation interventions in health care settings: rationale, model, outcomes. Addict Behav. 1996;21(6):709–720. doi: 10.1016/0306-4603(96)00030-5. [DOI] [PubMed] [Google Scholar]
- 42.Cohen SJ, Stookey GK, Katz BP, Drook CA, Christen AG. Helping smokers quit: a randomized controlled trial with private practice dentists. JADA. 1989;118(1):41–45. doi: 10.14219/jada.archive.1989.0018. [DOI] [PubMed] [Google Scholar]
- 43.Smith SE, Warnakulasuriya KA, Feyerabend C, Belcher M, Cooper DJ, Johnson NW. A smoking cessation programme conducted through dental practices in the UK. Br Dent J. 1998;185(6):299–303. doi: 10.1038/sj.bdj.4809796. [DOI] [PubMed] [Google Scholar]
- 44.McIntosh J, McKeganey N. Addicts' narratives of recovery from drug use: constructing a non-addict identity. Soc Sci Med. 2000;50(10):1501–1510. doi: 10.1016/s0277-9536(99)00409-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.