Abstract
Suppressing unwanted immune responses without compromising host immunity against pathogens is considered the holy grail of immunology. Lack of responsiveness to self-antigens is normally maintained by multiple mechanisms, including the suppressive activities of several T cell subsets. In this issue of the JCI, Jiang and colleagues define a CD8+ suppressor T cell subset in humans that recapitulates a regulatory pathway previously described in mice. These investigators further show that patients with type 1 diabetes have defects in their CD8+ suppressor T cells, thus identifying these cells as potential therapeutic targets in human disease.
A cardinal feature of the immune system is its capacity to distinguish self from nonself, a property referred to as immunological self-tolerance. Breakdown of the immune mechanisms that normally maintain self-tolerance is a factor contributing to the etiology of many human diseases, including type 1 diabetes (T1D) and MS. Devising means to promote self-tolerance while maintaining effective immune defenses against infectious agents and cancer has been a goal of immunologists ever since the pioneering studies by Medewar and colleagues on immune tolerance (1).
Immune tolerance is normally maintained by both central mechanisms, which are active during lymphocyte development in central lymphoid organs (2), and peripheral mechanisms, which are active after lymphocyte emigration to peripheral lymphoid organs (3). Developing T cells in the thymus undergo a rigorous selection process that purges potentially harmful T cells through a process known as negative selection. Through positive selection, the thymus promotes survival of T cells that recognize antigens in the context of the MHC molecules expressed by the individual, a phenomenon referred to as self-MHC restriction. However, due to the delicate balance between the need for self-MHC restriction and elimination of potentially harmful T cells, a substantial proportion of T cells with low or intermediate affinity for self-antigens manage to escape to the periphery. These potentially harmful T cells are normally kept in check by a variety of peripheral tolerance mechanisms, including the induction of unresponsiveness, regulation by immunosuppressive cytokines, and suppression by Tregs (3). Although early studies on T cell suppression focused on cells expressing CD8, studies in recent years have centered on CD4+CD25+ Tregs and NKT cells (4). However, in this issue of the JCI, Jiang and colleagues now show that CD8+ suppressor T cells have a role in maintaining self-tolerance in humans and provide a potential means to harness these cells for therapeutic purposes (5).
CD8+ T cells with immune suppressive activities
The existence of T cells with suppressive capacity was first proposed in the early 1970s by Gershon and colleagues (6). These cells, most of which expressed CD8, were extensively investigated during the next decade, but subsequent studies failed to identify the molecular basis of suppression, which brought the field to an abrupt halt in the mid-1980s. The field was eventually revived in the mid-1990s by the identification of natural CD4+CD25+ T cells as potent suppressors of autoimmunity (7). Since then, CD4+ Tregs have taken center stage, and CD8+ Tregs have fallen by the wayside. Nevertheless, elegant studies from die-hard proponents of the CD8+ T cell suppressor hypothesis have provided strong evidence that CD8+ Tregs have a role that is complementary to that of CD4+ Tregs (8–10). A key finding was that vaccination with irradiated, antigen-activated autoreactive T cell clones provided protection against the subsequent induction of experimental autoimmune disease in a manner that involved suppression mediated by CD8+ T cells (11, 12). These studies suggested that a subset of CD8+ T cells with suppressive activity reacted with antigenic determinants on the vaccinating cells and subsequently recognized these same determinants on the pathogenic T cells. Studies using the EAE animal model of MS further demonstrated a critical role of CD8+ T cells in suppressing disease following secondary challenge with autoantigens and during disease relapse (13, 14). The regulatory functions of CD8+ T cells were also observed in other models of autoimmune diseases, including collagen-induced arthritis, autoimmune myocarditis, and herpes simplex virus–induced stromal keratitis (15). CD8+ T cells with suppressor activities have also been implicated in human autoimmune diseases, including MS (16) and inflammatory bowel disease (17). Nevertheless, acceptance of the CD8+ T cell suppressor hypothesis required demonstration of the molecular mechanisms involved.
The Qa-1/HLA-E system
Early studies suggested a role for the Qa-1 molecule in the mechanisms underlying suppression of CD4+ T cells by CD8+ Tregs (18). Mouse Qa-1 and its human homolog HLA-E belong to a large group of unconventional MHC class Ib molecules that are related to conventional MHC class Ia molecules (19). Qa-1 and HLA-E are best known for their capacity to bind the inhibitory receptor CD94/NKG2A on NK cells (20). These proteins are transiently upregulated on activated T cells and bind hydrophobic peptides derived from self or foreign proteins. They are predominantly occupied by peptides, Qdm in mice and B7sp in humans, derived from the signal sequences of other MHC class I molecules. The association of CD8+ Tregs with Qa-1 was most clearly demonstrated using Qa-1–deficient mice, which develop exaggerated immune responses to self-antigens (21). Furthermore, it was shown that vaccination with irradiated, antigen-activated CD4+ T cells expressing Qa-1 induced the generation of Qa-1–restricted CD8+ T cells that suppressed autoimmunity (12). The new studies by Jiang and colleagues (5) provide strong evidence that this suppressor T cell pathway is also active in humans, as clearly demonstrated by the ability of HLA-E–restricted CD8+ T cell lines to suppress the activities of autoreactive CD4+ T cells in vitro.
Sensing the signal strength of CD4+ T cells
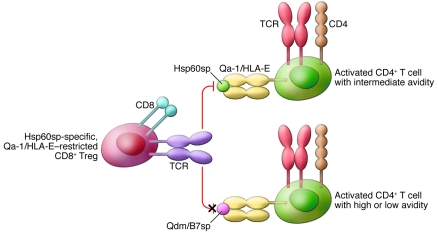
Studies with Qa-1–restricted CD8+ Tregs showed that these cells do not suppress CD4+ T cell responses indiscriminately. CD8+ Tregs can sense the strength (or avidity) of target CD4+ T cells for their cognate MHC/peptide complexes (22). Thus, Qa-1–restricted CD8+ T cells suppress activated CD4+ T cells exhibiting intermediate but not high or low avidity for cognate antigen (Figure 1). Qa-1–restricted T cells accomplish this feat by sensing the self peptides that are displayed by Qa-1 at the surface of intermediate-avidity but not high- or low-avidity CD4+ T cells. In molecular terms, the CD8+ Tregs react with complexes between Qa-1 molecules and peptides derived from the signal sequence of heat shock protein 60 (Hsp60sp), which are enriched on intermediate-avidity CD4+ T cells and thus outnumber Qa-1/Qdm complexes (23) (Figure 1). Again, the article by Jiang and colleagues (5) provides strong evidence that this molecular mechanism of suppression characterized in mice is conserved in humans. Specifically, they showed that Hsp60sp-specific, but not B7sp-specific, HLA-E–restricted CD8+ T cells were able to suppress the reactivity of CD4+ T cells against self-antigens (the T1D-related self-antigen glutamic acid decarboxylase [GAD] and the MS-related self-antigen myelin basic protein [MBP]) in vitro.
Figure 1. Signal strength model for the ability of Qa-1/HLA-E–restricted CD8+ T cells to suppress activated CD4+ T cells.
Activated CD4+ T cells with intermediate avidity for their cognate MHC class II/peptide complexes express Qa-1/HLA-E molecules that are predominantly occupied by Hsp60sp. These T cells are effectively suppressed by Hsp60sp-specific CD8+ Tregs. In contrast, activated CD4+ T cells with high or low avidity for their cognate MHC class II/peptide complexes express Qa-1/HLA-E molecules that are predominantly occupied by Qdm/B7sp peptides. These T cells are not under the control of Hsp60sp-specific CD8+ Tregs. The ultimate outcome of these interactions is inhibition of autoreactive T cell responses and enrichment of high-avidity responses against foreign antigens.
Comparison with other Treg subsets
Although Qa-1/HLA-E–restricted CD8+ Tregs exhibit many similarities to other Treg subsets, there are important differences as well (4). Most notably, CD4+ Tregs suppress immune responses indiscriminately, whereas CD8+ Tregs can distinguish self from nonself. For example, using an ex vivo coculture system, Jiang and colleagues showed that Hsp60sp-specific, HLA-E–restricted CD8+ T cells suppressed CD4+ T cell responses against the self-antigens GAD and MBP but enhanced responses against the foreign antigens tetanus toxoid and purified protein derivative (5). This was not observed for B7sp-specific CD8+ T cells, which left both types of CD4+ T cells undisturbed. Autoreactive T cells predominantly exhibit intermediate or low avidity for self-antigens, whereas T cells reactive against foreign antigens exhibit a wide range of avidities. Because the CD8+ Tregs generated by Jiang and colleagues recognized HLA-E/Hsp60sp on intermediate-avidity T cells, they suppressed T cell responses against self-antigens but enriched for high-avidity responses against foreign antigens (5). Although incompletely understood, this suppression likely involves both lytic and cytokine-mediated mechanisms (5, 10).
Another important difference is that CD4+CD25+ Tregs and NKT cells manifest their natural suppressive activities during the early phases of an immune response, whereas the suppressive activities of CD8+ Tregs are only observed during the late phases of an immune response. Thus, development of CD8+ T cell suppression requires priming, something that was clearly illustrated by the vaccination studies with irradiated, self-reactive CD4+ T cell clones (12). Furthermore, CD8+ Tregs could be induced in mice by vaccination with DCs loaded with Hsp60sp but not Qdm, and this protected against autoimmunity (23).
Relevance to human autoimmunity and its therapy
The studies by Jiang and colleagues show that individuals with recent-onset T1D display defects in the ability of CD8+ T cells to suppress autoreactive CD4+ T cells in vitro (5). However, for most of the patients, this deficit could be restored by an in vitro stimulation with Hsp60sp-loaded DCs. Therefore, these findings identify HLA-E–restricted CD8+ Tregs as potential new targets for immunotherapy of T1D and other autoimmune diseases. One way to harness the therapeutic activities of these cells would be to inject in vitro expanded CD8+ suppressor T cells back into the patients. Alternatively, patients could be vaccinated with their own DCs loaded ex vivo with Hsp60sp, a method that has proven effective in animal studies (23). A major advantage of these proposed therapeutic modalities, as compared with antigen-specific tolerance strategies, is that they could be performed independently of detailed knowledge of the self-antigens that are targeted by the autoimmune process.
Perspectives
Many advances in the field of CD8+ Treg biology are needed if the therapeutic potential of these cells, suggested by the studies of Jiang and colleagues (5), is to be fully exploited. For example, it will be necessary to develop better means to identify these cells with specific markers; to improve understanding of the mechanisms that induce these cells, either naturally or during therapy; to enhance understanding of the mechanism(s) of suppression; and to improve insight into the signaling events that underlie the selective induction of Hsp60 in intermediate-avidity CD4+ T cells. It will also be important to better define the role of CD94/NKG2A, the receptor on NK cells and a subset of CD8+ T cells that is inhibited by Qa-1/Qdm or HLA-E/B7sp complexes (20), in conferring self/nonself tolerance. Other outstanding questions are whether Qa-1/HLA-E–restricted CD8+ Tregs represent a separate lineage of T lymphocytes and whether HLA-E polymorphisms are associated with human disease, as has been suggested for T1D (24). Finally, it will be important to determine how these cells impact immune responses during different immunological situations such as infection, allograft rejection, cancer, and allergies. With more studies like those performed by Jiang and colleagues (5), answers to many of these questions should be within reach, bringing us closer to attaining the holy grail of immunology.
Acknowledgments
The author is funded by the NIH.
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2010;120(10):3432–3434. doi:10.1172/JCI44395.
See the related article beginning on page 3641.
References
- 1.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 2.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 3.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11(1):21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 4.Jiang H, Chess L. Regulation of immune responses by T cells. New Engl J Med. 2006;354(11):1166–1176. doi: 10.1056/NEJMra055446. [DOI] [PubMed] [Google Scholar]
- 5.Jiang H, et al. HLA-E–restricted regulatory CD8+ T cells are involved in development and control of human autoimmune type 1 diabetes. . J Clin Invest. 2010;120(10):3641–3650. doi: 10.1172/JCI43522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18(5):723–737. [PMC free article] [PubMed] [Google Scholar]
- 7.Shevach EM. Regulatory T cells in autoimmmunity. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 8.Jiang H, Chess L. The specific regulation of immune responses by CD8+ T cells restricted by the MHC class Ib molecule, Qa-1. . Annu Rev Immunol. 2000;18:185–216. doi: 10.1146/annurev.immunol.18.1.185. [DOI] [PubMed] [Google Scholar]
- 9.Smith TR, Kumar V. Revival of CD8+ Treg-mediated suppression. . Trends Immunol. 2008;29(7):337–342. doi: 10.1016/j.it.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Sarantopoulos S, Lu L, Cantor H. Qa-1 restriction of CD8+ suppressor T cells. . J Clin Invest. 2004;114(9):1218–1221. doi: 10.1172/JCI23152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun D, Qin Y, Chluba J, Epplen JT, Wekerle H. Suppression of experimentally induced autoimmune encephalomyelitis by cytolytic T-T cell interactions. Nature. 1988;332(6167):843–845. doi: 10.1038/332843a0. [DOI] [PubMed] [Google Scholar]
- 12.Jiang H, et al. T cell vaccination induces T cell receptor Vβ-specific Qa-1-restricted regulatory CD8+ T cells. . Proc Natl Acad Sci U S A. 1998;95(8):4533–4537. doi: 10.1073/pnas.95.8.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang H, Zhang SI, Pernis B. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. . Science. 1992;256(5060):1213–1215. doi: 10.1126/science.256.5060.1213. [DOI] [PubMed] [Google Scholar]
- 14.Koh DR, Fung-Leung WP, Ho A, Gray D, Acha-Orbea H, Mak TW. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8–/– mice. . Science. 1992;256(5060):1210–1213. doi: 10.1126/science.256.5060.1210. [DOI] [PubMed] [Google Scholar]
- 15.Lu L, Cantor H. Generation and regulation of CD8+ regulatory T cells. . Cell Mol Immunol. 2008;5(6):401–406. doi: 10.1038/cmi.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tennakoon DK, Mehta RS, Ortega SB, Bhoj V, Racke MK, Karandikar NJ. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. . J Immunol. 2006;176(11):7119–7129. doi: 10.4049/jimmunol.176.11.7119. [DOI] [PubMed] [Google Scholar]
- 17.Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. . J Immunol. 2005;174(9):5814–5822. doi: 10.4049/jimmunol.174.9.5814. [DOI] [PubMed] [Google Scholar]
- 18.Cantor H, et al. Immunoregulatory circuits among T-cell sets. Identification of a subpopulation of T-helper cells that induces feedback inhibition. . J Exp Med. 1978;148(4):871–877. doi: 10.1084/jem.148.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol. 2005;5(6):459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- 20.Jensen PE, Sullivan BA, Reed-Loisel LM, Weber DA. Qa-1, a nonclassical class I histocompatibility molecule with roles in innate and adaptive immunity. Immunol Res. 2004;29(1–3):81–92. doi: 10.1385/IR:29:1-3:081. [DOI] [PubMed] [Google Scholar]
- 21.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5(5):516–523. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 22.Jiang H, et al. An affinity/avidity model of peripheral T cell regulation. J Clin Invest. 2005;115(2):302–312. doi: 10.1172/JCI23879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, et al. Perceiving the avidity of T cell activation can be translated into peripheral T cell regulation. Proc Natl Acad Sci U S A. 2007;104(51):20472–20477. doi: 10.1073/pnas.0709878104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodgkinson AD, Millward BA, Demaine AG. The HLA-E locus is associated with age at onset and susceptibility to type 1 diabetes mellitus. Hum Immunol. 2000;61(3):290–295. doi: 10.1016/S0198-8859(99)00116-0. [DOI] [PubMed] [Google Scholar]