Abstract
Focal segmental glomerulosclerosis (FSGS) is a common form of idiopathic nephrotic syndrome defined by the characteristic lesions of focal glomerular sclerosis and foot process effacement; however, its etiology and pathogenesis are unknown. We used mRNA isolated from laser-captured glomeruli from archived formalin-fixed, paraffin-embedded renal biopsies, until recently considered an unsuitable source of mRNA for microarray analysis, to investigate the glomerular gene expression profiles of patients with primary classic FSGS, collapsing FSGS (COLL), minimal change disease (MCD), and normal controls (Normal). Amplified mRNA was hybridized to an Affymetrix Human X3P array. Unsupervised (unbiased) hierarchical clustering revealed two distinct clusters delineating FSGS and COLL from Normal and MCD. Class comparison analysis of FSGS + COLL combined versus Normal + MCD revealed 316 significantly differentially regulated genes (134 up-regulated, 182 down-regulated). Among the differentially regulated genes were those known to be part of the slit diaphragm junctional complex and those previously described in the dysregulated podocyte phenotype. Analysis based on Gene Ontology categories revealed overrepresented biological processes of development, differentiation and morphogenesis, cell motility and migration, cytoskeleton organization, and signal transduction. Transcription factors associated with developmental processes were heavily overrepresented, indicating the importance of reactivation of developmental programs in the pathogenesis of FSGS. Our findings reveal novel insights into the molecular pathogenesis of glomerular injury and structural degeneration in FSGS.
Focal segmental glomerulosclerosis (FSGS) is a clinicopathologic syndrome manifesting proteinuria, usually of nephrotic range, and lesions of focal and segmental glomerulosclerosis and foot process effacement. It is a heterogeneous condition that may result from diverse pathogenetic mechanisms including heritable mutations of podocyte specific proteins, viral infections, toxic agents, and adaptive structural-functional responses.1 Most patients with FSGS and heavy proteinuria have no identifiable secondary cause and are thus considered primary (idiopathic). Circulating permeability factors have been implicated in the pathogenesis of primary FSGS but remain to be defined. FSGS is a leading cause of idiopathic nephrotic syndrome in children and adults, and an important cause of end-stage renal disease. Approximately 30% of patients experience remission of proteinuria with subsequent long-term stabilization of renal function after treatment with corticosteroids.2 A better understanding of the molecular basis for disease should facilitate the design of more efficacious, disease-specific therapies for FSGS.
A working classification system recognizes five histological subtypes of FSGS (collapsing, tip, cellular, perihilar, and not otherwise specified or NOS) that can be applied to both primary and secondary forms.1 Though this subclassification has proved useful for identifying clinical, prognostic, and pathogenetic information,3,4 no clear mechanistic basis underlying the morphological differences is known. It has become clearer in recent years that the common denominator in all variants is injury either directed to or originating within the podocyte, a highly specialized, terminally differentiated epithelial cell.5 The latter mechanism is highlighted by the number of critical podocyte proteins that have been identified to be mutated or deficient in human forms of congenital nephrotic syndrome or inherited FSGS.5 The clinical signature of podocyte injury is proteinuria, but morphologically, podocyte injury produces a dysregulated phenotype that demonstrates disruption and reorganization of the actin cytoskeleton, focal microvillous transformation, loss of primary processes and effacement of foot processes. Permissive cellular proliferation and loss of mature podocyte markers are characteristic features of the collapsing form of FSGS.6 Podocyte depletion through detachment or activation of apoptotic mechanisms contributes to progressive glomerulosclerosis by promoting denudation of the glomerular basement membrane and adhesion to Bowman's capsule.7 In addition, FSGS and minimal change disease (MCD) may be related podocytopathies in that they manifest nephrotic proteinuria and foot process effacement, however in MCD, the podocyte injury is readily reversible and does not lead to podocyte depletion and subsequent tuft sclerosis.8
Renal biopsy provides key information for the diagnosis and effective therapeutic management of patients with progressive kidney disease; however the use of this resource for molecular profiling of disease is only in its early stages. The feasibility of studying gene expression profiles by microarray analysis has been demonstrated in glomeruli isolated from frozen biopsy sections of lupus nephritis,9 diabetic nephropathy,10 obesity related glomerulopathy,11 and FSGS.12 With the exception of Peterson et al,9 few cases were available for study. This highlights the challenge of obtaining an adequate number of high quality tissue samples. Extraction of RNA from formalin-fixed, paraffin-embedded (FFPE) tissue has had limited success for global gene expression profiling in part due to chemical alteration and fragmentation observed in this material,13 though some success has been reported.14 New technologies have been developed to allow the use of FFPE material, which represent the greatest stock of archived samples. Our goals in this study are to provide proof-of-principle that exceedingly small amounts of mRNA from laser-captured glomerular sections from archived biopsies can be used to identify important genes, biological processes, and candidates for the transcriptional control of glomerular phenotypes in MCD, FSGS (NOS) also known as classic FSGS, and collapsing FSGS (COLL).
Materials and Methods
Clinical Samples
FFPE renal biopsy material was obtained from the archives of the Columbia Renal Pathology Laboratory. Biopsies were fixed in 10% formalin within 1 hour of extraction, remaining in fixative no more than 1.5 days. Twenty-one patients with biopsy-proven MCD, FSGS or COLL were studied, including 19 with idiopathic nephrotic syndrome (edema, proteinuria >3.5 g/day; serum albumin <3.0 g/dl) and 2 without full nephrotic syndrome. Controls were renal biopsies that appeared normal by histological, immunofluorescence, and electron microscopic examination. These controls were either obtained from renal biopsies performed for minimal isolated proteinuria or hematuria (seven patients) or tissue from uninvolved portions of a kidney at the time of nephrectomy for tumor (two patients). Biopsies contained from 8 to 35 glomeruli by light microscopy. All paraffin blocks were stored at room temperature for several months to 5 years. Snap-frozen and FFPE tissue from three additional nephrectomy cases were used to compare RNA quality and gene expression profiles within the same case. The use of archival renal tissue and clinical data reported in this study were approved by the Columbia University Institutional Review Board.
Laser Capture Microdissection, Sample Preparation, and Microarray Hybridization
FFPE blocks were cut into two to four sections (6 μm thick) onto PALM membrane slides (PALM, Zeiss, Germany), baked at 60°C for 1 minute, deparaffinized with xylene, lightly stained with eosin, and air-dried. Frozen sections from nephrectomy specimens were cryostat-sectioned (6 μm thick), fixed in 95% ethanol, lightly stained with eosin, dehydrated, and air-dried. Glomeruli were isolated by laser capture using the PALM Laser Microdissection and Pressure Catapulting system (PALM MicroBeam IP 230V Z microscope). Approximately 20 to 25 glomerular cross-sections were captured from each case. Only glomeruli without global sclerosis were captured, including segmentally sclerotic glomeruli, glomeruli with collapsing lesions, and glomeruli without light microscopic abnormalities. Total RNA was isolated using RecoverAll Total Nucleic Acid Isolation Kit (Ambion, Austin, TX). An Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA) with an RNA 6000 LabChip kit (Agilent Technologies) was used to profile RNA quality from the nephrectomy material. Amplification and biotin-labeling was achieved using a T7 promoter-based amplification using MessageAmp and MessageAmp II, Biotin-Enhanced aRNA Amplification Kits (Ambion). Amplified target RNA was hybridized to the Human X3P Array (Affymetrix, Santa Clara, CA), which is based on the Human Genome U133 plus 2.0 Array and contains probes for 47,000 transcripts. After scanning according to standard Affymetrix protocols, raw data were analyzed with BRB-Array Tools, version 3.7.0 (http://linus.nci.nih.gov/BRB-ArrayTools.html).
Data Analysis
Analysis was performed using Robust Multichip Average normalization without a baseline. To reduce complexity, expression data were filtered to remove 74% of the genes with the lowest log intensity variation, leaving 15,953 genes that passed filtering criteria. In addition, a background filter cutoff was defined using the median signal value obtained, plus two standard deviations, from nonhuman Affymetrix-control probe sets. A hierarchical dendrogram was generated using median-centered average linkage unsupervised clustering of log2-transformed data. Combined class comparison (FSGS + COLL versus Normal + MCD) was performed with a false-discovery rate (FDR) < 0.1 (BRB-Array Tools). The FDR can be defined as the expected proportion of the null hypotheses that are falsely rejected divided by the total number of rejections. It is a more useful approach when determining a significance cutoff when a large number of experiments are tested (ie, microarray probesets) because the approach allows more claims of significant differences to be made, as long as a fraction of false-positive findings are accepted.15 For the noncombined class comparison (MCD versus Normal and COLL versus FSGS) a single probe-based microarray data analysis16 was modified to select intragene probes with highly reproducible expression patterns within the probesets. The resulting gene lists were uploaded into BiblioSphere (version 7.20; Genomatix Software, Munich, Germany) for literature mining and annotation analysis (Gene Ontology). Genomatix Gene2Promoter suite was used to search and identify transcription factors (TFs) and modules common to differentially regulated genes. The promoter region was designated by Genomatix default settings.
Quantitative RT-PCR Analysis Used for Validation
Where available, excess RNA after two rounds of amplification not applied to the Affymetrix Human X3P Array was reverse transcribed using High Capacity cDNA RT Kit (Applied Biosystems, Foster City, CA). The expression of 10 differentially regulated genes from the microarray analysis was measured by TaqMan (Applied Biosystems), quantitative real-time RT-PCR using oligonucleotide primers (300 nmol/L) and probe (100 nmol/L) designed to target within the 200 bases at the most 3′ end of the transcripts (Table 1). Glyceraldehyde-3-phosphate dehydrogenase was used as an endogenous reference for normalization and data are presented as mean ± SE Significance was set to a P ≤ 0.05 and tested by one-way t-test followed by Bonferroni correction for multiple comparisons.
Table 1.
Primer and Taqman Probe Sequences
| Gene | Forward primer | Reverse primer | TaqMan probe |
|---|---|---|---|
| ABLIM1 | 5′-TGTGTCGTGTTCCCTGTGAAAC-3′ | 5′-CACAAGCATGTGGCACTTAGC-3′ | 5′-CAGGTGTGTGTTGGC-3′ |
| CDH2 | 5′-GTGGCACTACTAAGTGTGTGTTTTTTT-3′ | 5′-CAATACAGAGGCAAAGCTGTTGTC-3′ | 5′-AAACTGGAGAGACTTC-3′ |
| CLDN5 | 5′-TCTTGGCTGCTGCCTTACTTC-3′ | 5′-TGGAGTAAAGACCAGCTGTACACATC-3′ | 5′-CTCCTGCTGACTTCG-3′ |
| GAPDH | 5′-GTCCCCCACCACACTGAATC-3′ | 5′-GCCCCTCCCCTCTTCAAG-3′ | 5′-CCCCTCCTCACAGTTGCCATGTAGACC-3′ |
| IGFBP2 | 5′-GTGGGTGCTGGAGGATTTTC-3′ | 5′-CGGTGCTGGTCTCTTTCCAA-3′ | 5′-AGTTCTGACACACGTATTT-3′ |
| INF2 | 5′-GGCGGTCTGCCTTTGCT-3′ | 5′-TGATGTGATGGCCAAGTTTCA-3′ | 5′-ACTGCCAGGCCTC-3′ |
| MET | 5′-CAGATTGTGGGAGTAAGTGATTCTTC-3′ | 5′-CCATTCAGTTCAGCTGCAGGTA-3′ | 5′-AGAATTAGATACTTGTCACTGCC-3′ |
| PAX2 | 5′-CCAGGCCTAACCTGCTAAATGT-3′ | 5′-CGAACCGACGCACTGAAAGT-3′ | 5′-CCGGACGGTTCTG-3′ |
| PTGDS | 5′-GCTTCACAGAGGATACCATTGTCTT-3′ | 5′-AGTCCTATTGTTCCGTCATGCA-3′ | 5′-TGCCCCAAACCGAT-3′ |
| SOX9 | 5′-TGTTCGTGTTTTGTTTGTTTCACTT-3′ | 5′-AAGTTTCACGGAGAGAACAAAAGG-3′ | 5′-CCCTCCCAGCCCCAA-3′ |
| VCAM1 | 5′-TGGTACGGAGATGTTTCACGAA-3′ | 5′-TTTAGGCCACATTGGGAAAGTT-3′ | 5′-TTTGTTCATCAGACTCCTG-3′ |
ABLIM1, actin-binding LIM protein 1; CDH2, cadherin 2, type 1, n-cadherin; CLDN5, claudin 5; GAPDH, glyceraldehyde phosphate dehydrogenase; IGFBP2, insulin-like growth factor-binding protein 2; INF2, inverted formin, FH2 and WH2 domain containing; MET, met proto-oncogene (hepatocyte growth factor receptor); PAX2, paired box gene 2; PTGDS, prostaglandin d2 synthase 21kda; SOX9, SRY-box 9; VCAM1, vascular cell adhesion molecule 1.
Immunohistological Analysis
SOX9 staining was performed on formalin-fixed paraffin-embedded normal kidney obtained from the archives of the Columbia Renal Pathology Laboratory. Four micrometer thick sections were deparaffinized, hydrated, and subjected to 10 minutes of heated pH 6.0 citrate. After serum-free protein block, the tissue was exposed to SOX9 mouse monoclonal antibody (catalog number H00006662-MO2; Abnova, Taipei, Taiwan) at 1/750 dilution. The EnVision+ anti-mouse HRP system with DAB was used for detection of the primary antibody.
Results
Validation of Protocol
Laser capture microdissection was used to isolate glomeruli from the surrounding tissue. Globally sclerotic glomeruli were excluded. Successful capture was easily visualized before RNA isolation (Figure 1). Gene expression profiling was determined using glomerular RNA after two rounds of amplification with a T7-promoter based method and application to the Affymetrix Human X3P Array. Because the T7-promoter binds to the polyA tail of mRNA, the amplified product has an extreme 3′ bias. The X3P Array is designed to interrogate sequences closer to the 3′ end of the transcripts and is well suited for highly fragmented RNA such as found in FFPE samples. Examination of the absolute detection signals of podocyte and tubular cell specific transcripts from captured glomeruli demonstrates the enrichment for the glomerular compartment over tubulointerstitium (Table 2).
Figure 1.
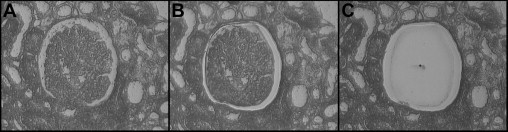
Isolation of a glomerulus by laser capture microdissection from an eosin-stained section cut 6-μm thick on a membrane-covered slide. A glomerulus is easily identified (center of A) and separated from surrounding tubulointerstitium using a laser to cut both the tissue and the clear membrane. B: A laser pulse (dot in C) catapults the liberated glomerulus and portion of membrane into a digestion buffer for RNA extraction.
Table 2.
Enrichment of Specific mRNA by Laser Capture Microdissection
| Gene | Absolute signal |
|---|---|
| Glomerular | |
| Podocalyxin | 10809 |
| Podocin | 7643 |
| Synaptopodin | 803 |
| Tubular | |
| CLCN5 | Absent |
| PKD2L2 | Absent |
| Uromodulin | Absent |
| Nonrenal | |
| TITF1 | Absent |
CLCN5, chloride channel 5; PKD2L2, polycystic kidney disease 2-like 2; TITF1, thyroid transcription factor 1.
The reproducibility between matched frozen (FRZN) and FFPE samples from three nephrectomies was assessed. As expected, a profound difference in RNA quality between FRZN and FFPE samples can be observed in the electropherograms in Figure 2A. Compared with FRZN, the FFPE samples have highly fragmented total RNA with no ribosomal peaks. Nevertheless, comparison of the gene expression profiles of glomeruli from FFPE versus FRZN kidney for the same sample revealed high correlations of 0.90, 0.92, and 0.93 demonstrating that glomerular gene expression profiles from FFPE are comparable to FRZN (Figure 2B).
Figure 2.
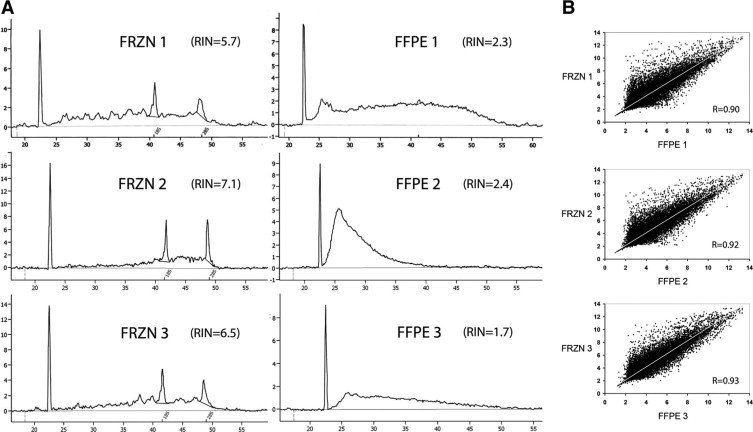
RNA quality and gene expression profiles of FFPE versus frozen FRZN tissue. A: We analyzed RNA quality using 25 to 75 ng RNA isolated from renal cortex with the Agilent 2100 Bioanalyzer. The x-axis is in seconds, the y-axis is fluorescence units. RIN denotes RNA integrity number. B: Scatter plots demonstrate high correlation (R) of FFPE versus FRZN glomerular gene expression profiles. The x- and y-axis are log-transformed array signals. Each point represents one gene.
Clinical and Pathological Characteristics of Cases
We examined glomerular gene expression signatures from a total of 30 patients in four diagnostic groups including Normal (9), MCD (7), FSGS (8), and COLL (6). Normal cases were obtained from patients with isolated hematuria or proteinuria but normal histological, immunofluorescence, and utrastructural evaluation (9), or from uninvolved cortex of tumor nephrectomy (2). Patient characteristics and biopsy findings are listed for each study group in Table 3. Clinical and histopathological characteristics for each patient can be found in Supplemental Table 1 at http://ajp.amjpathol.org. There was no difference in average age between groups and male to female ratios were comparable. The racial composition between groups was comparable with the exception of COLL which had five Black, one Hispanic, and no White patients. All but two of the cases with histological diagnosis of MCD, FSGS, and COLL presented with full nephrotic syndrome. No patient had a history of diabetes, HIV infection, hepatitis, i.v. drug abuse, vesicoureteral reflux, obesity, non-steroidal anti-inflammatory drug use, pamidronate therapy, or other known causes of secondary MCD, FSGS, or COLL. No patient was using steroids or cyclosporine at the time of biopsy, and only one patient (FSGS: ID number 1698) was treated with steroids in the past and had stopped 2 years before biopsy. The histopathological and ultrastructural findings of FSGS and foot process effacement define the diagnostic categories, thus significant differences are expected between groups. Of note, three cases of COLL also had glomeruli with lesions of classic FSGS (Supplemental Table 1 at http://ajp.amjpathol.org). The percentage of tubular atrophy/interstitial fibrosis and globally sclerotic glomeruli, markers for overall damage to the kidney parenchyma, were nearly nonexistent in the Normal and MCD groups in contrast to FSGS and COLL. There was no significant difference in these histopathological characteristics between FSGS and COLL.
Table 3.
Clinical, Pathological, and Array QC Findings
| Groups | Clinical findings |
Histopathological findings |
Hybridization quality |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age (range) | Sex | Race | NS | % Glomeruli with FSGS | % Foot process effacement | % Globally sclerotic glomeruli | % Tubular atrophy/interstitial fibrosis | Age of FFPE tissue | Scale factor | % Present calls | |
| Histopathologic diagnosis | ||||||||||||
| Normal | 9 | 31.9 (7–67) | 5F/4 mol/L | 4B/1H/4W | 0 | 0 | 0 | 0 | 0 | 0.3–5 years | 10 ± 8 | 30 ± 7 |
| MCD | 7 | 41.4 (14–64) | 4F/3 mol/L | 3B/4W | 7 | 0 | 93 ± 4 | 4 ± 4 | 0 | 0.3–2 years | 11 ± 10 | 32 ± 9 |
| FSGS | 8 | 42.9 (5–74) | 3F/5 mol/L | 2B/1A/5W | 7 | 25 ± 15 | 92 ± 8 | 22 ± 22 | 26 ± 23 | 1–3 years | 12 ± 7 | 28 ± 6 |
| COLL | 6 | 38.7 (20–57) | 4F/2 mol/L | 5B/1H | 5 | 41 ± 32 | 86 ± 21 | 12 ± 10 | 33 ± 28 | 2–4 years | 12 ± 5 | 30 ± 8 |
| Combined groups | ||||||||||||
| Normal + MCD | 16 | 36.1 (7–67) | 9F/7 mol/L | 7B/1H/8W | 7 | 0 | 37 ± 47 | 2 ± 3 | 0 | 0.3–5 years | 10 ± 8 | 31 ± 8 |
| FSGS + COLL | 14 | 41.1 (5–74) | 7F/7 mol/L | 7B/1A/1H/5W | 12 | 32 ± 24 | 90 ± 14 | 18 ± 18 | 29 ± 25 | 1–4 years | 12 ± 6 | 28 ± 7 |
MCD, minimal change disease; FSGS, focal segmental glomerulosclerosis; COLL, collapsing variant of focal segmental glomerulosclerosis. A, Asian; B, black; H, Hispanic; W, White; NS, Nephrotic Syndrome; QC, quality control.
Hybridization Quality
The scale factor and percent present call give insight into the quality of the RNA hybridization. Scale factor is a metric inversely related to the overall signal intensity from a single array and must be normalized for comparison. Percent present call is the number of detected transcripts and is sensitive to RNA sampling, scanning and data extraction. The FFPE samples demonstrated higher and more variable scale factors and lower percent present calls than would be expected from FRZN samples. However there were no significant differences in average scale factor and percent genes present between study groups (Table 3). The age of the FFPE tissue blocks, which varied from a few months to ∼5 years, did not correlate with scale factor or percent present call and the age of the sample did not impact the RNA quality as assessed by the Agilent Bioanalyzer 2100 (data not shown).
Unsupervised Clustering
Transcriptional fingerprints reflect the functional status of a tissue at time of biopsy and can be used to group patients according to their mRNA expression status. Unsupervised hierarchical clustering was used to explore the relationships between expression signatures and patient classification. The resulting dendrogram showed two distinct clusters, I and II (Figure 3). With the exception of three samples, all Normal and MCD samples clustered together in I. Similarly, all FSGS and COLL samples clustered together in II. Within cluster I, MCD did not segregate apart from Normal, nor did COLL segregate apart from FSGS in cluster II. The dendrogram indicates that global glomerular gene expression differences are detectable with our methods between biopsies that contain failing glomeruli (sclerosis) and those without. Significantly differentially regulated genes in glomeruli with dysfunction of the glomerular filter (in the form of proteinuria and foot process effacement at the ultrastructural level) or between FSGS variants required a more sensitive method of analysis (see Discussion).
Figure 3.
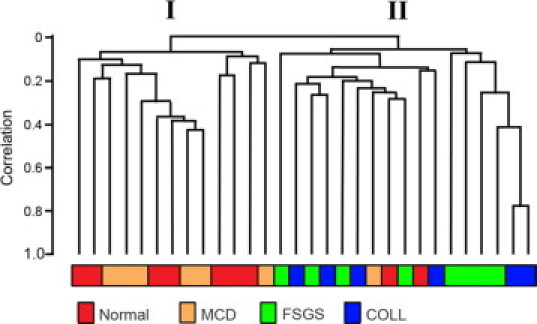
Unsupervised hierarchical clustering analysis demonstrates two main clusters of similar glomerular gene expression signatures. In cluster I, with the exception of three cases, Normal and MCD cluster together. In cluster II, FSGS and COLL cluster together.
Class Comparison (Supervised Clustering) for FSGS + COLL versus Normal + MCD
For class comparison analysis (supervised clustering), the histopathological groups FSGS and COLL (FSGS + COLL), and Normal and MCD (Normal + MCD) were combined. This was done to reflect the findings from unsupervised clustering shown above and current histopathological classification that recognizes collapsing FSGS and classical FSGS to be histological subtypes of FSGS. In addition, Normal and MCD are grouped apart from FSGS + COLL because neither normal nor MCD cases have segmentally sclerotic, failing glomeruli. Normal and MCD have been combined as a control group previously.17
Compared with Normal + MCD, 405 probesets were significantly differentially expressed in the glomeruli captured from the FSGS + COLL group (FDR < 0.10). The complete list of differentially expressed probesets can be found in Supplemental Table 2 at http://ajp.amjpathol.org. Of these, 316 unique genes could be mapped for further analysis (134 up-regulated, 182 down-regulated). A partial list of genes with the greatest fold changes is presented in Table 4. Additional class comparison analyses of FSGS versus Normal + MCD and COLL versus Normal + MCD yielded 58 and 359 probesets, respectively. These overlapped extensively and were not significantly different from each other or from the probeset list generated by comparing FSGS + COLL versus Normal + MCD (data not shown). The findings agree with known molecular changes in FSGS, especially in the podocyte, and highlight new pathogenetically relevant molecular pathways (see Discussion).
Table 4.
Partial List of Differentially Regulated Genes (FSGS + COLL versus Normal + MCD)
| Gene symbol | Description | Fold change |
|---|---|---|
| COL1A1 | Collagen, type I, α1 | 51.49 |
| SOX9 | SRY (sex determining region Y)-box 9 (campomelic dysplasia, autosomal sex-reversal) | 20.77 |
| CDH2 | Cadherin 2, type 1, N-cadherin (neuronal) | 12.99 |
| PAX8 | Paired box 8 | 12.34 |
| IL8 | Interleukin 8 | 10.00 |
| PAX2 | Paired box 2 | 9.79 |
| CD24 | CD24 molecule | 8.98 |
| DSP | Desmoplakin | 7.07 |
| ABLIM1 | Actin-binding LIM protein 1 | 5.26 |
| SCIN | Scinderin | 4.82 |
| SPP1 | Secreted phosphoprotein 1 (osteopontin) | 4.52 |
| HNF1B | HNF1 homeobox B | 4.42 |
| MAFF | v-maf musculoaponeurotic fibrosarcoma oncogene homolog F (avian) | 4.36 |
| BHLHB2 | Basic helix-loop-helix domain containing, class B, 2 | 4.01 |
| MET | Met proto-oncogene (hepatocyte growth factor receptor) | 3.73 |
| VCAM1 | Vascular cell adhesion molecule 1 | 3.45 |
| THY1 | Thy-1 cell surface antigen | 2.75 |
| NCOA2 | Nuclear receptor coactivator 2 | 2.54 |
| CORO1C | Coronin, actin binding protein, 1C | 2.51 |
| HOXC10 | Homeobox C10 | 2.39 |
| TGIF1 | TGFB-induced factor homeobox 1 | 2.25 |
| HOXB5 | Homeobox B5 | 2.24 |
| GRB2 | Growth factor receptor-bound protein 2 | 2.14 |
| CDKN1B | Cyclin-dependent kinase inhibitor 1B (p27, Kip1) | 0.58 |
| CCDC91 | Coiled-coil domain containing 91 | 0.57 |
| NFKBIA | Nuclear factor of κ light polypeptide gene enhancer in B-cells inhibitor, α | 0.57 |
| TJP1 (ZO1) | Tight junction protein 1 (zona occludens 1) | 0.56 |
| NPHS2 | Nephrosis 2, idiopathic, steroid-resistant (podocin) | 0.53 |
| FAT1 | FAT tumor suppressor homolog 1 (Drosophila) | 0.49 |
| DCN | Decorin | 0.48 |
| CR1 | Complement component (3b/4b) receptor 1 (Knops blood group) | 0.46 |
| MAFB | v-Maf musculoaponeurotic fibrosarcoma oncogene homolog B (avian) | 0.44 |
| NES | Nestin | 0.44 |
| GHR | Growth hormone receptor | 0.44 |
| CDKN1C | Cyclin-dependent kinase inhibitor 1C (p57, Kip2) | 0.44 |
| PODXL | Podocalyxin | 0.43 |
| AIF1 | Allograft inflammatory factor 1 | 0.41 |
| PLCE1 | Phospholipase C, epsilon 1 | 0.40 |
| IGFBP2 | Insulin-like growth factor-binding protein 2, 36 kd | 0.39 |
| FGF1 | Fibroblast growth factor 1 (acidic) | 0.38 |
| MME/CD10 | Membrane metallo-endopeptidase | 0.38 |
| DACH1 | Dachshund homolog 1 (Drosophila) | 0.38 |
| NPHS1 | Nephrosis 1, congenital, Finnish type (nephrin) | 0.36 |
| BCOR | BCL6 corepressor | 0.31 |
| PTGDS | Prostaglandin D2 synthase 21 kd (brain) | 0.30 |
| SYNPO | Synaptopodin | 0.30 |
| CLDN5 | Claudin 5 (transmembrane protein deleted in velocardiofacial syndrome) | 0.29 |
| PLA2R1 | Phospholipase A2 receptor 1, 180 kd | 0.27 |
| MAGI2 | Membrane-associated guanylate kinase, WW and PDZ domain containing 2 | 0.24 |
| INF2 | Inverted formin, FH2 and WH2 domain containing | 0.21 |
Microarray Validation with Quantitative Real-Time RT-PCR
Ten gene transcripts were chosen for independent validation of differential expression by microarray. The fold and direction of expression was confirmed by TaqMan quantitative real-time RT-PCR (Figure 4) using the same RNA samples that had been applied to the microarray with the addition of four Normal and three COLL processed in the same manner and at the same time. The overexpression of ABLIM1 and CDH2 did not reach statistical significance after Bonferroni correction, a stringent method for multiple comparisons correction, but did show a trend concordant with the array data (P < 0.1), nor did PAX2 reach statistical significance after correction for multiple comparisons (P = 0.15).
Figure 4.
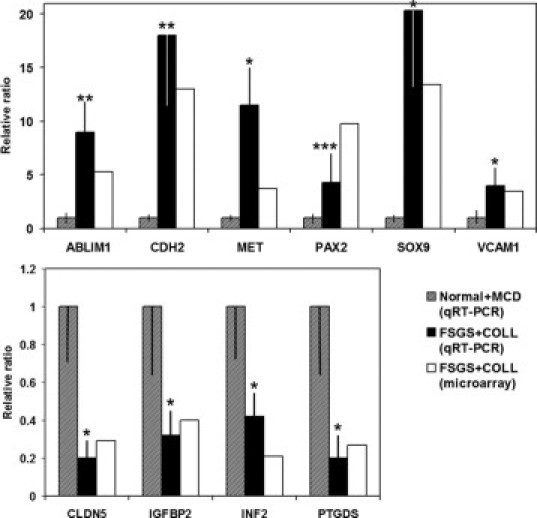
Validation of FSGS + COLL versus Normal + MCD gene expression profiling using TaqMan quantitative real-time RT-PCR with 10 genes, normalized to GAPDH expression. *P < 0.05; **P < 0.1; ***P = 0.15, after Bonferroni correction for multiple comparisons.
Immunohistochemistry for SOX9
SOX9 encodes a TF and is the most prominently up-regulated gene in FSGS + COLL after COL1A1. To localize the expression of SOX9 in the glomerulus, we performed immunohistochemistry on normal kidney. Figure 5 demonstrates that in the glomerulus, SOX9 is primarily located in the podocyte (arrows), which are collected by laser capture, and parietal cells (arrowheads), which are often destroyed by the cutting laser and not collected. This suggests the differential expression of glomerular SOX9 is in the podocyte, however we cannot rule out expression of SOX9 in mesangial or glomerular endothelial cells.
Figure 5.
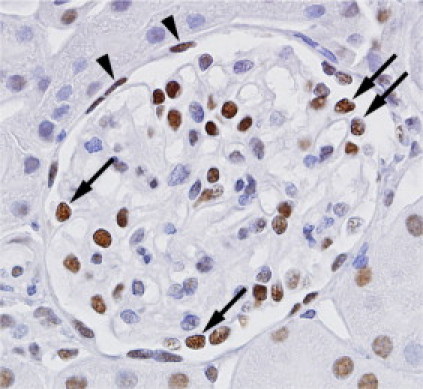
Glomerular SOX9 protein expression. Immunohistochemistry for SOX9 reveals strong nuclear staining in podocytes (arrows) and parietal cells (arrowheads). Most of the nuclei within the center of the glomerular tuft (mesangial and endothelial cells) are negative or weakly positive, although some demonstrate strong staining. The nuclei of surrounding tubular cells show variable staining. Glomerular laser-capture does not collect the parietal cells or surrounding tubular cells.
Gene Ontology Analysis
To gain insight into the function of genes differentially regulated in FSGS + COLL versus Normal + MCD, we clustered genes based on Gene Ontology (Biological Processes) categories (Table 5). Compared with Normal + MCD, differentially regulated genes in the FSGS + COLL group contained overrepresented categories of development, differentiation and morphogenesis, cell motility and migration, cytoskeleton organization, and signal transduction. Examination of TFs differentially expressed in the injured glomerulus should provide insight into important transcriptional regulatory mechanisms. Table 5 lists the differentially regulated TFs by Gene Ontology category. Thirty-three TFs were differentially regulated in the FSGS + COLL group. Of these, 18 are included in Table 5 and 15 are associated with developmental processes, 12 of which are up-regulated, highlighting a role for reactivation of developmental programs in the pathogenesis of FSGS.
Table 5.
Top Gene Ontology (Biological Process) Categories in FSGS + COLL versus Normal + MCD
| Gene ontology term | Observed genes | Z-score | Transcription factors up-regulated | Transcription factors down-regulated |
|---|---|---|---|---|
| Developmental process | 72 | 4.85 | BHLHB2, HNF1B, HOXB5, HOXC10, MAFF, MITF, PAX2, PAX8, SOX9, TGIF1 | DACH1, MAFB |
| Anatomical structure development | 57 | 6.32 | HNF1B, HOXB5, MAFF, NFE2L1, PAX2, PAX8, SOX9 | MAFB, NFE2L1 |
| Anatomical structure morphogenesis | 27 | 4.21 | HOXB5, MAFF, NFE2L1, PAX2, PAX8 | MAFB, NFE2L1 |
| Cellular developmental process | 34 | 4.23 | HOXB5, MAFF, MITF, PAX2, PAX8, SOX9 | MAFB |
| Cell differentiation | 31 | 4.02 | SOX9, PAX2, PAX8, MITF, MAFF, HOXB5 | MAFB |
| Cell development | 16 | 4.14 | SOX9, PAX2 | |
| Skeletal muscle fiber development | 4 | 4.00 | ||
| System development | 47 | 5.29 | FOSL2, HNF1B, HOXB5, HOXC10, MAFF, MITF, PAX2, PAX8, SOX9, TGIF1 | DACH1, MAFB, NFE2L1 |
| Organ development | 39 | 5.71 | HNF1B, MAFF, SOX9 | MAFB |
| Multicellular organismal process | 73 | 4.83 | BHLHB2, HNF1B, HOXB5, HOXC10, MAFF, MITF, PAX2, PAX8, SOX9, TGIF1 | DACH1, MAFB |
| Multicellular organismal development | 59 | 5.35 | HNF1B, MAFF, PAX2, SOX9 | MAFB |
| Cell motility | 15 | 4.26 | ||
| Cell migration | 14 | 5.46 | ||
| Neuron migration | 4 | 4.71 | ||
| Cytoskeleton organization and biogenesis | 21 | 5.42 | ||
| Actin cytoskeleton organization and biogenesis | 11 | 4.68 | ||
| Cell adhesion | 31 | 6.87 | SOX9 | |
| Cell-cell adhesion | 14 | 5.81 | SOX9 | |
| Cellular component organization and biogenesis | 44 | 4.07 | BCOR, RSF1 | |
| Positive regulation of cellular component organization and biogenesis | 5 | 4.16 | ||
| Regulation of signal transduction | 23 | 5.75 | ||
| Regulation of G-protein coupled receptor protein signaling pathway | 4 | 4.62 | ||
| Negative regulation of biological process | 33 | 4.08 | ||
| Positive regulation of biological process | 33 | 4.26 | CREB5, HNF1B, HOXC10, NCOA2, PAX8, SOX9 | MAFB, RSF1 |
| Positive regulation of protein metabolic process | 6 | 4.16 | ||
| Regulation of kinase activity | 11 | 4.11 | ||
| Response to steroid hormone stimulus | 4 | 4.32 |
Class comparison for MCD versus Normal and COLL versus FSGS
In the unsupervised clustering dendrogram using Significance Analysis of Microarrays for analysis of Affymetrix probesets (Figure 3), MCD did not segregate from Normal, thus indicating that differentially expressed genes were few and could not be detected by the method we used for unsupervised clustering and combined class comparison. For single group class comparison (MCD versus Normal and COLL versus FSGS) we used a modified single probe-based microarray data analysis method more robustly defining functional categories in studies with limited sample size.16 Table 6 displays the list of differentially expressed genes obtained from class comparison of MCD with Normal. Analysis of this list by Gene Ontology reveals two categories, amino acid and derivative metabolic processes (BHMT, DDC, GATM, and XPNEP2) and cell adhesion (CDH11, MPZL2, OPCML, and TRO). For validation of these genes, we used glomerular gene expression data from Berthier et al.17 In that study, the gene expression profiles of glomeruli microdissected from biopsies of patients with MCD and from living kidney donors are reported. To obtain a list of differentially regulated genes from an independent cohort of patients, we compared the glomerular gene expression of MCD versus that of living kidney donors. Ten genes (50%) that were significantly differentially regulated (FDR <0.05) in the independent cohort, and shared the same direction of fold change, were also found in our study (Table 6, far right column), serving as validation by an independent cohort.
Table 6.
Differentially Expressed Genes in MCD versus Normal
| Gene symbol | Description | Fold change | FDR | Independent validation: fold change |
|---|---|---|---|---|
| ANKRD32 | Ankyrin repeat domain 32 | 10.33 | 0.09 | |
| LTBR | Lymphotoxin β receptor | 3.75 | 0.21 | 1.71 |
| CDH11 | Cadherin 11, type 2, ob-cadherin | 3.48 | 0.44 | 1.43 |
| C17orf80 | Chromosome 17 open reading frame 80 | 3.27 | 0.48 | |
| MPZL2 | Epithelial v-like antigen 1 | 2.81 | 0.48 | |
| TPST2 | Tyrosylprotein sulfotransferase 2 | 2.35 | 0.48 | |
| GJA4 | Gap-junction protein, α4 | 2.28 | 0.00 | 1.94 |
| CPLX1 | Complexin 1 | 0.38 | 0.15 | |
| WSCD2 | kiaa0789 gene product | 0.33 | 0.10 | |
| TRO | Trophinin | 0.28 | 0.41 | 1.42 |
| KLHL26 | Kelch-like 26 (Drosophila) | 0.27 | 0.00 | |
| XPNPEP2 | x-Prolyl aminopeptidase 2, membrane-bound | 0.23 | 0.00 | 0.49 |
| C19orf77 | Chromosome 19 open reading frame 77 | 0.22 | 0.38 | |
| GATM | Glycine amidinotransferase | 0.18 | 0.27 | 0.55 |
| FMO1 | Flavin containing monooxygenase 1 | 0.16 | 0.00 | 0.55 |
| SLC5A2 | Solute carrier family 5, member 2 | 0.15 | 0.17 | 0.58 |
| GDA | Guanine deaminase | 0.13 | 0.00 | |
| OPCML | Opioid-binding protein/cell adhesion molecule-like | 0.12 | 0.00 | 0.58 |
| BHMT | Betaine-homocysteine methyltransferase | 0.11 | 0.00 | 0.51 |
| DDC | Dopa decarboxylase | 0.11 | 0.00 | 0.55 |
The COLL and FSGS groups did not segregate apart in the unsupervised clustering dendrogram (Figure 3). Table 7 displays the differentially expressed genes generated with the same method described above by comparing COLL versus FSGS. A major Gene Ontology category highly represented in this gene set is regulation of DNA-dependent transcription represented by genes that encode either for TFs (IKZF2, MAEL, JUND, NFATC4, and retinoic acid receptor α) or genes that encode for proteins that interact with additional proteins to suppress other transcription factors (RBAK).
Table 7.
Differentially Expressed Genes in COLL versus FSGS
| Gene symbol | Description | Fold change | FDR |
|---|---|---|---|
| USP38 | Ubiquitin-specific peptidase 38 | 8.4 | 0 |
| KCNJ2 | Potassium inwardly-rectifying channel, J2 | 7.9 | 0.43 |
| NANOS1 | Nanos homolog 1 | 6.9 | 0.36 |
| RBAK | RB-associated KRAB zinc finger | 6.1 | 0 |
| IKZF2 | IKAROS family zinc finger 2 | 5.8 | 0.43 |
| KCNG1 | Potassium voltage-gated channel, G1 | 4.7 | 0 |
| MAEL | Maelstrom homolog (Drosophila) | 4.5 | 0.39 |
| WDR51A | WD repeat domain 51A | 4.4 | 0 |
| FBX045 | F-box protein 45 | 4.3 | 0.39 |
| SF3B2 | Splicing factor 3b, subunit 2, 145 kDa | 3.6 | 0.43 |
| RBAK | rb-associated krab repressor | 2.8 | 0.46 |
| ODF3B | Outer dense fiber of sperm tails 3B | 2.2 | 0.39 |
| CNGA1 | Cyclic nucleotide gated channel α1 | 2.2 | 0.39 |
| NFATC4 | Nuclear factor of activated T cells, 4 | 2.1 | 0.44 |
| RARA | Retinoic acid receptor, α | 2.0 | 0.2 |
| SEMA3F | Semaphorin 3F | 2.0 | 0.43 |
| RSRC2 | Similar to splicing factor, arginine/serine-rich 4 | 2.0 | 0.43 |
| MICALL2 | Mical-like 2 | 1.9 | 0.39 |
| JUND | Jun d proto-oncogene | 1.9 | 0.43 |
| SERPINB9 | Serpin peptidase inhibitor, clade B, member 9 | 0.3 | 0.25 |
| TBC1D20 | tbc1 domain family, member 20 | 0.3 | 0.25 |
Promoter Modeling
TFs bind to specific DNA sequences motifs or TF binding sites (TFBSs) to exert control of gene expression. The analysis of gene promoters for organizational features of TFBSs is important to characterize complex gene regulatory networks. The ordered complex of two or more TFBSs that are conserved in distance and orientation is designated a promoter module.18 Several promoter modules were found to be overrepresented in the genes differentially regulated in FSGS + COLL (Table 8). Three promoter modules (EGRF_SP1F_01, SP1F_KLFS_01, and SP1F_SP1F_05) were found in many genes differentially regulated in FSGS + COLL.
Table 8.
Promoter Modules Enriched in FSGS + COLL
| Promoter modules | # Observed genes | Z-score |
|---|---|---|
| EGRF_SP1F_01 | 195 | 4.80 |
| SP1F_KLFS_01 | 129 | 4.11 |
| SP1F_SP1F_05 | 91 | 4.18 |
| SP1F_MZF1_01 | 10 | 4.30 |
| IRFF_NFKB_03 | 5 | 4.17 |
| SP1F_SP1F_01 | 4 | 5.23 |
| RXRF_HAML_ 02 | 3 | 4.02 |
| STAF_STAF_01 | 1 | 4.41 |
| SMAD_SMAD_MITF_02 | 1 | 4.42 |
| GATA_GATA_NFKB_NFKB_01 | 1 | 7.88 |
Correlation Between Gene Expression and FSGS + COLL Lesions
We sought to correlate gene expression with morphometric data from each FSGS + COLL case to gain insight into the differentially regulated genes that may be important in the pathogenesis of FSGS. Figure 6 graphically displays the three genes from the FSGS + COLL versus Normal + MCD gene list whose expression was significantly (FDR < 0.05) correlated with the percentage of glomeruli with FSGS lesions in each biopsy (Supplemental Table 1 at http://ajp.amjpathol.org). Allograft inflammatory factor 1 (AIF1) was negatively correlated (r2 = 0.54) with lesions of sclerosis among all FSGS + COLL biopsies. Desmoplakin (DSP) and coiled-coil domain containing 91 (CCDC91) were found to be positively (r2 = 0.83) and negatively (r2 = 0.83) correlated with classic FSGS only. This analysis suggests an important role for these three genes in the pathogenesis of segmental glomerulosclerosis either through a gain or loss of function.
Figure 6.
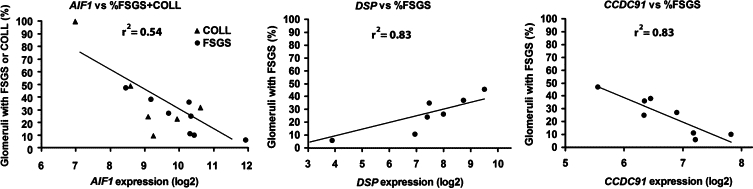
Correlation of gene expression with segmental sclerosis. Among genes differentially regulated in the FSGS + COLL group, the expression of three were found to significantly correlate (P < 0.05) with the percentage of collapsing and classic FSGS lesions (AIF1) or the percentage of classic FSGS lesions alone (DSP and CCDC91).
Discussion
We have performed detailed gene-expression profiling of isolated glomeruli from patients with biopsy-proven idiopathic classic FSGS, collapsing FSGS, MCD, and normal controls. Our work provides proof-of-principle that successful gene expression profiling can be achieved even with an exceedingly small quantity of sample from archived FFPE material. Until recently, frozen samples have been required for microarray analysis after laser-capture of glomeruli.9–12 The use of FFPE material for gene expression profiling has the advantage of a large readily available archive with many years of clinical follow-up. For example, we were able to use samples from FFPE blocks as old as 5 years, but successful profiling has been accomplished in blocks collected as long as 24 years prior.19 Furthermore, in our study, a substantial number of transcripts were differentially regulated in FSGS + COLL versus Normal + MCD, allowing novel insights into the molecular pathogenesis of glomerulosclerosis and glomerular degeneration.
Injury to the podocyte has a central role in the pathogenesis of FSGS. We find differential expression of several transcripts whose proteins are important to the structure and function of the podocyte slit diaphragm. Nephrin and podocin expression, which decrease in disease conditions associated with proteinuria,20,21 are reduced in FSGS + COLL (down 3-fold and nearly 1.9-fold, respectively). Synaptopodin, a podocyte-specific actin-associated protein, is also reduced 3.3-fold. Additional differentially regulated genes known to be part of the slit diaphragm junctional complex, but not podocyte specific, include FAT1 (down 2-fold), GRB2 (up 2.1-fold), MAGI-2 (down 4.2-fold), and TJP1 (down 1.8-fold, also known as ZO1). All but one of these genes is down-regulated, raising the possibility that, in a disease process where podocyte loss is a feature, the gene expression changes observed are due to fewer podocytes per glomerular cross-section captured in the FSGS + COLL group. While we cannot exclude podocyte loss as a contributing factor, we believe it is not a major explanation for these differences in gene expression because the glomeruli with FSGS lesions constituted no more than one-third of the captured glomeruli, the sclerosing lesions are segmental, and almost half of the cases contained lesions of collapsing FSGS, with more podocytes than the normal complement.
A distinctive molecular feature of collapsing FSGS is the dysregulated podocyte phenotype.6 We observe a recapitulation of this phenotype in the differentially regulated gene list. Expression of the cell cycle inhibitors p27 and p57 is reduced (1.7-fold and 2.3-fold, respectively). The expression of PAX2, a marker for the immature podocyte, is considerably increased (9.8-fold), and several markers for the mature podocyte are decreased such as CALLA (2.7-fold, also known as MME or CD10), CR1 (2.2-fold), podocalyxin (2.3-fold), and synaptopodin (3.3-fold). The expression of WT1, another podocyte maturity marker, was down-regulated 1.6-fold with a FDR of 0.15, but this did not pass the FDR filter and was not included in our gene list.
Several genes potentially expressed by parietal epithelial cells, such as CD24, CLDN1, KRT 18, and KRT 19 were up-regulated in FSGS + COLL versus Normal + MCD. These findings support the newly emerging evidence that parietal epithelial cells may serve as a reservoir of podocyte progenitor cells.22,23 Recruitment of parietal epithelial cells may repopulate glomerular capillaries denuded of injured podocytes in COLL, a process that has been shown to occur in some forms of human COLL.24
An examination of the differentially regulated genes in FSGS + COLL using Gene Ontology demonstrates an overrepresentation of developmental categories, implicating an important role for reactivation of developmental transcriptional pathways in the injured glomerulus. Indeed most differentially regulated TFs in Table 4 are associated with development and most are up-regulated. PAX2 is expressed in metanephric mesenchyme and ureteric bud of the developing kidney. Its expression declines with development and is absent in the mature podocyte, though persistent PAX2 expression is seen in some renal diseases.25 Interestingly, transgenic podocyte-specific PAX2 protein expression in the mouse results in early glomerular collapse and its induction in adulthood leads to severe glomerular disease.26 SOX9 has a well characterized role in testicular development and chondrocyte differentiation and its induction in vitro is associated with up-regulation of genes key to the pathology of fibrosis, such as COL2A2, COMP1, and COL1.27 In cultured mesangial cells, SOX9 directly up-regulated COL4A2 expression and was dramatically up-regulated with Col4a2 in whole kidney in a mouse model of nephritis.28 Thus SOX9 may play an important role in the activation of fibrosis in FSGS. MAFB, also known as Kreisler, is one of the few down-regulated TFs we find in FSGS + COLL. It is known for its role in hindbrain patterning, but the lack of MAFB in newborn mice results in proteinuria, podocyte foot process effacement, and the reduction of nephrin and podocin expression.29
The comparison of FSGS + COLL versus Normal + MCD groups offers insight into a multitude of interesting transcripts and pathways differentially regulated in human glomeruli. In recent years, multiple gene mutations have been identified that lead to inherited forms of FSGS5 through podocyte dysfunction. The expression of three of these genes, NPHS2, INF2, and PLCE1, are down-regulated in FSGS + COLL versus Normal + MCD. NPHS2 (podocin) mutations have been described in steroid-resistant nephrotic syndrome.30 Mutations in the gene for INF2, a member of the formin family of proteins that accelerate actin filament assembly, are inherited in an autosomal-dominant fashion in multiple families with inherited FSGS.31 And finally PLCE1, which encodes an enzyme not associated with the podocyte cytoskeleton, was found to be mutated in a rare form of familial FSGS or diffuse mesangial sclerosis32 Thus our analytic approach may yield additional candidates for genetic analysis in FSGS.
Literature mining and analysis identified IL-8 (up 10-fold), NFKBIA (down 1.8-fold), and Osteopontin (SPP-1; up 4.5-fold) as key interconnected nodes in a transcriptional network (Supplemental Figure 1 at http://ajp.amjpathol.org). IL-8 is a chemokine and a major mediator of the inflammatory response. Peripheral blood mononuclear cells overexpress IL-8 in patients with MCD33 and blocking antibodies against IL-8 reverse proteinuria and foot process effacement in a model of acute immune-complex glomerulonephritis.34 Podocytes are able to produce IL-8 and express its receptor CXCR1, suggesting activation in an autocrine manner.35 NFKBIA, also known as inhibitory nuclear factor κB (NF-κB)α, is an inhibitor of the NF-κB pathway. An increase of NF-κB:inhibitory NF-κB expression ratios has been documented in pediatric transplant patients with recurrent FSGS,36 suggesting a pathogenetic role for NF-κB activation. Finally, osteopontin is a cytokine broadly up-regulated in various conditions including inflammation37 and cancer.38 It is increased in the podocytes of several mouse models of renal injury and activates the NF-κB pathway in cultured podocytes.39 Osteopontin is also significantly increased in the urine of children with steroid-sensitive nephrotic syndrome, most of whom had FSGS.39
Unsupervised clustering analysis revealed little to no difference in the molecular signatures of glomeruli from MCD compared with Normal, and COLL compared with FSGS. There are several possible explanations for this observation. In MCD, glomeruli display little or no histological abnormalities by light microscopy and require ultrastructural evaluation to discern the foot process effacement that differentiates MCD from Normal. Our data are consistent with a smaller gene set regulated in MCD versus Normal than was found for Normal + MCD versus FSGS + COLL. Of note, a more sensitive method was able to identify regulated transcripts in MCD. In addition, compared with FRZN samples, many of the individual gene expression levels are reduced with RNA extracted from FFPE material, which can be seen in the graphs on the right side of Figure 2 (note the asymmetrical shift of the gene expression data points to the left of each graph where the expression levels for many individual genes are lower for FFPE than FRZN). Despite these limitations, it was possible to identify differentially expressed genes in MCD versus Normal glomeruli in which cell adhesion and amino acid and derivative metabolic processes were enriched. Disruption of podocyte adhesion, whether to the glomerular basement membrane or neighboring podocytes, has been shown to result in loss of maintenance of the glomerular filtration barrier and morphological changes in the podocyte including foot process effacement.40 Changes in amino acid metabolism would be expected to disrupt many cellular processes and it has been shown that the formation of podocyte processes depends on a constant supply of lipids and proteins.41 However the role of these differentially regulated genes remains to be determined.
The unsupervised analysis did not reveal a robust distinction in gene expression between COLL and FSGS. This might be a consequence of the fact that approximately one-third of the glomeruli from FSGS + COLL had segmental sclerosis or collapsing lesions, and the rest of the glomeruli were identical, that is normal by light microscopy and with foot process effacement by ultrastructural analysis. And of the COLL cases, half had a mixture of classic and collapsing lesions of FSGS. Thus, there was not a substantial difference in the morphology of collected glomeruli in FSGS and COLL biopsies, as one might expect, for cases diagnostically classified as FSGS variants. Accordingly, only a limited group of differentially expressed genes was identified comparing COLL to FSGS. The list was enriched for genes involved in DNA-dependent transcription and included several TFs, such as retinoic acid receptor α (RARA). Retinoic acid has anti-apoptotic effects42 and has been shown to inhibit matrix production43 in cultured mesangial cells that are, in part, retinoic acid receptor α dependent. In podocytes, retinoic acid inhibits HIV-1-induced podocyte proliferation through the cAMP pathway and causes G1 arrest and restoration of podocyte expression of differentiation markers.44 This suggests a transcriptional pathway that inhibits apoptosis and sclerosis and regulates proliferation in collapsing lesions compared with classical segmental sclerosis. Future experiments should focus on laser capturing only glomeruli with specific morphology, which we now show to be technically feasible.
Analysis of the promoter regions in the differentially expressed genes in FSGS + COLL versus Normal + MCD reveals a significant overrepresentation of several promoter modules. The EGRF_SP1F_01, SP1F_KLFS_01, and SP1F_SP1F_05 promoter modules are found in many genes from our list indicating shared transcriptional pathways for these regulated genes. The EGRF binding site contains a domain for early growth response (EGR) family nuclear proteins, including EGR1 and EGR2, and similar C2H2-type zinc-finger proteins.45 SP1F and KLFS contain binding domains for the stimulating protein family and Kruppel-like zinc finger family TFs. Members of these TF families were not differentially regulated in FSGS + COLL with the filtering criteria used. However, expanding the list of regulated genes to include those at a FDR ≤ 0.15 revealed EGR1 to be up-regulated 2.6 fold. Functional analyses of these promoter modules may yield new insights into shared transcriptional pathways.
Using morphometric data, we compared the percentage of glomeruli affected by classic or collapsing lesions of FSGS with differentially regulated genes (FSGS + COLL versus Normal + MCD) for each case and found three genes with statistically significant correlations. Only one gene, AIF1, was shared by both and was significantly negatively correlated with sclerosis. AIF1 is a calcium-binding protein encoded within the human leukocyte antigen class III genomic region and is implicated in the inflammatory process. Tsubata et al46 found that AIF1 is diffusely expressed in the cytoplasm of podocytes and suggested that AIF1 colocalizes with actin. Furthermore, AIF1 is associated with cell migration through actin polymerization and Rac1 activation in vascular smooth muscle cells.47 Thus it may play a role in actin cytoskeleton stability in FSGS and COLL. DSP and CCD91 (also known as p56) expression was correlated with percent focal sclerosis in FSGS cases, but not COLL. DSP is an intermediate-filament binding protein essential for desmosomal intercellular adhesion function. It is expressed in all epithelial cells of the developing tubule and renal capsule, including both parietal and visceral epithelia. However in the mature glomerulus, its protein expression is restricted to the lateral membranes of parietal epithelial cells.48 Positive correlation of DSP expression with FSGS may indicate a return to developmental transcriptional programs in the diseased glomerulus or involvement of parietal epithelial cells. Finally CCDC91 expression was found to be significantly negatively correlated with FSGS. CCDC91 is a trans-Golgi network accessory protein important in the sorting of cathepsin D to lysosomes.49 Its function in the glomerulus is unknown.
Our study demonstrates the feasibility of gene expression profiling using minute quantities of FFPE material, once thought to be too poor in quality for microarray analysis, enriched for the tissue of interest with laser-capture microdissection. We present the utility of this approach first through direct comparison of global gene expression of frozen versus fixed glomeruli from the same source. In addition, we can observe an overlap of differentially regulated genes from ours and similar studies. Bennett et al12 profiled isolated glomeruli from four FSGS patients and compared the gene expression to three controls. Multiple differentially regulated genes (23 total) are shared with our study including substantial upregulation of osteopontin, SOX9, RGS4, MET, thrombospondin-2 and cathepsin C (see Supplemental Table 3 for full list of shared genes at http://ajp.amjpathol.org).12 Bioinformatics tools now exist, such as Gene Expression Online (http://www.ncbi.nlm.nih.gov/geo/) and the Molecular Signatures Database (http://www.broad.mit.edu/gsea/msigdb/), where one can compare a gene set to a large collection of user uploaded gene sets from previous experiments. Uploading our set of differentially regulated genes to Molecular Signatures Database reveals a significant overlap (42 genes) with Baelde et al,10 in which isolated glomeruli from diabetic patients were compared with normal, which implicates shared transcriptional regulation of glomerular injury in diabetes and FSGS (see Supplemental Table 4 for full list of shared genes at http://ajp.amjpathol.org10). Our gene set also significantly overlaps with Cui et al,50 in which isolated glomeruli from Pod1 knockout mouse embryos were profiled and compared with normal glomeruli. Since Pod1 is a transcription factor key to ureteric bud branching and glomerular differentiation, we interpret this as additional evidence for the importance of reactivation of developmental transcriptional pathways in glomerular injury. The application of microarray technology to the archives of FFPE renal biopsies will expand our ability to elucidate the molecular complexities of renal disease.
Footnotes
Supported by National Kidney Foundation (J.B.H. and C.C.B.) and National Institute of Diabetes and Digestive and Kidney Diseases grants P30DK081943 and U54DA021519 (M.K.). This work was supported in part by a Research Fellowship grant from the National Kidney Foundation.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Web Extra Material
Clinical, Pathological, and Array QC findings by Specimen
Differentially regulated genes (FSGS + COLL vs Normal + MCD)
Differentially expressed genes shared with partial gene list from Bennett et. al.12, human FSGS
Differentially expressed genes shared with Baelde et. al. (10), human diabetic glomerulosclerosis
References
- 1.D'Agati VD, Fogo AB, Bruijn JA, Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004;43:368–382. doi: 10.1053/j.ajkd.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 2.Korbet SM. Primary focal segmental glomerulosclerosis. J Am Soc Nephrol. 1998;9:1333–1340. doi: 10.1681/ASN.V971333. [DOI] [PubMed] [Google Scholar]
- 3.Stokes MB, Valeri AM, Markowitz GS, D'Agati VD. Cellular focal segmental glomerulosclerosis: clinical and pathologic features. Kidney Int. 2006;70:1783–1792. doi: 10.1038/sj.ki.5001903. [DOI] [PubMed] [Google Scholar]
- 4.Thomas DB, Franceschini N, Hogan SL, Ten Holder S, Jennette CE, Falk RJ, Jennette JC. Clinical and pathologic characteristics of focal segmental glomerulosclerosis pathologic variants. Kidney Int. 2006;69:920–926. doi: 10.1038/sj.ki.5000160. [DOI] [PubMed] [Google Scholar]
- 5.D'Agati VD. The spectrum of focal segmental glomerulosclerosis: new insights. Curr Opin Nephrol Hypertens. 2008;17:271–281. doi: 10.1097/MNH.0b013e3282f94a96. [DOI] [PubMed] [Google Scholar]
- 6.Barisoni L, Kriz W, Mundel P, D'Agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- 7.Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int. 1998;54:687–697. doi: 10.1046/j.1523-1755.1998.00044.x. [DOI] [PubMed] [Google Scholar]
- 8.D'Agati VD. Podocyte injury in focal segmental glomerulosclerosis: lessons from animal models (a play in five acts) Kidney Int. 2008;73:399–406. doi: 10.1038/sj.ki.5002655. [DOI] [PubMed] [Google Scholar]
- 9.Peterson KS, Huang JF, Zhu J, D'Agati V, Liu X, Miller N, Erlander MG, Jackson MR, Winchester RJ. Characterization of heterogeneity in the molecular pathogenesis of lupus nephritis from transcriptional profiles of laser-captured glomeruli. J Clin Invest. 2004;113:1722–1733. doi: 10.1172/JCI19139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baelde HJ, Eikmans M, Doran PP, Lappin DW, de Heer E, Bruijn JA. Gene expression profiling in glomeruli from human kidneys with diabetic nephropathy. Am J Kidney Dis. 2004;43:636–650. doi: 10.1053/j.ajkd.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y, Liu Z, Xiang Z, Zeng C, Chen Z, Ma X, Li L. Obesity-related glomerulopathy: insights from gene expression profiles of the glomeruli derived from renal biopsy samples. Endocrinology. 2006;147:44–50. doi: 10.1210/en.2005-0641. [DOI] [PubMed] [Google Scholar]
- 12.Bennett MR, Czech KA, Arend LJ, Witte DP, Devarajan P, Potter SS. Laser capture microdissection-microarray analysis of focal segmental glomerulosclerosis glomeruli. Nephron Exp Nephrol. 2007;107:e30–e40. doi: 10.1159/000106775. [DOI] [PubMed] [Google Scholar]
- 13.Masuda N, Ohnishi T, Kawamoto S, Monden M, Okubo K. Analysis of chemical modification of RNA from formalin-fixed samples and optimization of molecular biology applications for such samples. Nucleic Acids Res. 1999;27:4436–4443. doi: 10.1093/nar/27.22.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coudry RA, Meireles SI, Stoyanova R, Cooper HS, Carpino A, Wang X, Engstrom PF, Clapper ML. Successful application of microarray technology to microdissected formalin-fixed, paraffin-embedded tissue. J Mol Diagn. 2007;9:70–79. doi: 10.2353/jmoldx.2007.060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen CD, Lindenmeyer MT, Eichinger F, Hahn A, Seifert M, Moll AG, Schmid H, Kiss E, Grone E, Grone HJ, Kretzler M, Werner T, Nelson PJ. Improved elucidation of biological processes linked to diabetic nephropathy by single probe-based microarray data analysis. PLoS One. 2008;3:e2937. doi: 10.1371/journal.pone.0002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berthier CC, Zhang H, Schin M, Henger A, Nelson RG, Yee B, Boucherot A, Neusser MA, Cohen CD, Carter-Su C, Argetsinger LS, Rastaldi MP, Brosius FC, Kretzler M. Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes. 2009;58:469–477. doi: 10.2337/db08-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dohr S, Klingenhoff A, Maier H, Hrabe de Angelis M, Werner T, Schneider R. Linking disease-associated genes to regulatory networks via promoter organization. Nucleic Acids Res. 2005;33:864–872. doi: 10.1093/nar/gki230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J, Reich M, Chan JA, Glickman JN, Ikeda K, Hashimoto M, Watanabe G, Daidone MG, Roayaie S, Schwartz M, Thung S, Salvesen HB, Gabriel S, Mazzaferro V, Bruix J, Friedman SL, Kumada H, Llovet JM, Golub TR. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doublier S, Salvidio G, Lupia E, Ruotsalainen V, Verzola D, Deferrari G, Camussi G. Nephrin expression is reduced in human diabetic nephropathy: evidence for a distinct role for glycated albumin and angiotensin II. Diabetes. 2003;52:1023–1030. doi: 10.2337/diabetes.52.4.1023. [DOI] [PubMed] [Google Scholar]
- 21.Horinouchi I, Nakazato H, Kawano T, Iyama K, Furuse A, Arizono K, Machida J, Sakamoto T, Endo F, Hattori S. In situ evaluation of podocin in normal and glomerular diseases. Kidney Int. 2003;64:2092–2099. doi: 10.1046/j.1523-1755.2003.00303.x. [DOI] [PubMed] [Google Scholar]
- 22.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol. 2009;20:333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20:322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smeets B, Dijkman HB, Wetzels JF, Steenbergen EJ. Lessons from studies on focal segmental glomerulosclerosis: an important role for parietal epithelial cells? J Pathol. 2006;210:263–272. doi: 10.1002/path.2051. [DOI] [PubMed] [Google Scholar]
- 25.Dressler GR, Woolf AS. Pax2 in development and renal disease. Int J Dev Biol. 1999;43:463–468. [PubMed] [Google Scholar]
- 26.Wagner KD, Wagner N, Guo JK, Elger M, Dallman MJ, Bugeon L, Schedl A. An inducible mouse model for PAX2-dependent glomerular disease: insights into a complex pathogenesis. Curr Biol. 2006;16:793–800. doi: 10.1016/j.cub.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 27.Hanley KP, Oakley F, Sugden S, Wilson DI, Mann DA, Hanley NA. Ectopic SOX9 mediates extracellular matrix deposition characteristic of organ fibrosis. J Biol Chem. 2008;283:14063–14071. doi: 10.1074/jbc.M707390200. [DOI] [PubMed] [Google Scholar]
- 28.Sumi E, Iehara N, Akiyama H, Matsubara T, Mima A, Kanamori H, Fukatsu A, Salant DJ, Kita T, Arai H, Doi T. SRY-related HMG box 9 regulates the expression of Col4a2 through transactivating its enhancer element in mesangial cells. Am J Pathol. 2007;170:1854–1864. doi: 10.2353/ajpath.2007.060899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadl V, Jin F, Yu J, Cui S, Holmyard D, Quaggin S, Barsh G, Cordes S. The mouse Kreisler (Krml1/MafB) segmentation gene is required for differentiation of glomerular visceral epithelial cells. Dev Biol. 2002;249:16–29. doi: 10.1006/dbio.2002.0751. [DOI] [PubMed] [Google Scholar]
- 30.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 31.Brown EJ, Schlondorff JS, Becker DJ, Tsukaguchi H, Uscinski AL, Higgs HN, Henderson JM, Pollak MR: Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet 42:72–76 [DOI] [PMC free article] [PubMed]
- 32.Hinkes B, Wiggins RC, Gbadegesin R, Vlangos CN, Seelow D, Nurnberg G, Garg P, Verma R, Chaib H, Hoskins BE, Ashraf S, Becker C, Hennies HC, Goyal M, Wharram BL, Schachter AD, Mudumana S, Drummond I, Kerjaschki D, Waldherr R, Dietrich A, Ozaltin F, Bakkaloglu A, Cleper R, Basel-Vanagaite L, Pohl M, Griebel M, Tsygin AN, Soylu A, Muller D, Sorli CS, Bunney TD, Katan M, Liu J, Attanasio M, O'Toole JF, Hasselbacher K, Mucha B, Otto EA, Airik R, Kispert A, Kelley GG, Smrcka AV, Gudermann T, Holzman LB, Nurnberg P, Hildebrandt F. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat Genet. 2006;38:1397–1405. doi: 10.1038/ng1918. [DOI] [PubMed] [Google Scholar]
- 33.Garin EH, Blanchard DK, Matsushima K, Djeu JY. IL-8 production by peripheral blood mononuclear cells in nephrotic patients. Kidney Int. 1994;45:1311–1317. doi: 10.1038/ki.1994.171. [DOI] [PubMed] [Google Scholar]
- 34.Wada T, Tomosugi N, Naito T, Yokoyama H, Kobayashi K, Harada A, Mukaida N, Matsushima K. Prevention of proteinuria by the administration of anti-interleukin 8 antibody in experimental acute immune complex-induced glomerulonephritis. J Exp Med. 1994;180:1135–1140. doi: 10.1084/jem.180.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huber TB, Reinhardt HC, Exner M, Burger JA, Kerjaschki D, Saleem MA, Pavenstadt H. Expression of functional CCR and CXCR chemokine receptors in podocytes. J Immunol. 2002;168:6244–6252. doi: 10.4049/jimmunol.168.12.6244. [DOI] [PubMed] [Google Scholar]
- 36.Schachter AD, Strehlau J, Zurakowski D, Vasconcellos L, Kim YS, Zheng XX, Gunshin Y, Overstreet SL, Benfield MR, Tejani A, Harmon WE, Herrin JT, Strom TB. Increased nuclear factor-κB and angiotensinogen gene expression in posttransplant recurrent focal segmental glomerulosclerosis. Transplantation. 2000;70:1107–1110. doi: 10.1097/00007890-200010150-00021. [DOI] [PubMed] [Google Scholar]
- 37.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 38.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79–87. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Lorenzen J, Shah R, Biser A, Staicu SA, Niranjan T, Garcia AM, Gruenwald A, Thomas DB, Shatat IF, Supe K, Woroniecki RP, Susztak K. The role of osteopontin in the development of albuminuria. J Am Soc Nephrol. 2008;19:884–890. doi: 10.1681/ASN.2007040486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 41.Simons M, Saffrich R, Reiser J, Mundel P. Directed membrane transport is involved in process formation in cultured podocytes. J Am Soc Nephrol. 1999;10:1633–1639. doi: 10.1681/ASN.V1081633. [DOI] [PubMed] [Google Scholar]
- 42.Xu Q, Konta T, Kitamura M. Retinoic acid regulation of mesangial cell apoptosis. Exp Nephrol. 2002;10:171–175. doi: 10.1159/000058343. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Lu L, Tao BB, Zhu YC. All-trans retinoic acid inhibits the increases in fibronectin and PAI-1 induced by TGF-β1 and Ang II in rat mesangial cells. Acta Pharmacol Sin. 2008;29:1035–1041. doi: 10.1111/j.1745-7254.2008.00849.x. [DOI] [PubMed] [Google Scholar]
- 44.He JC, Lu TC, Fleet M, Sunamoto M, Husain M, Fang W, Neves S, Chen Y, Shankland S, Iyengar R, Klotman PE. Retinoic acid inhibits HIV-1-induced podocyte proliferation through the cAMP pathway. J Am Soc Nephrol. 2007;18:93–102. doi: 10.1681/ASN.2006070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sukhatme VP. The Egr family of nuclear signal transducers. Am J Kidney Dis. 1991;17:615–618. doi: 10.1016/s0272-6386(12)80333-7. [DOI] [PubMed] [Google Scholar]
- 46.Tsubata Y, Sakatsume M, Ogawa A, Alchi B, Kaneko Y, Kuroda T, Kawachi H, Narita I, Yamamoto T, Gejyo F. Expression of allograft inflammatory factor-1 in kidneys: a novel molecular component of podocyte. Kidney Int. 2006;70:1948–1954. doi: 10.1038/sj.ki.5001941. [DOI] [PubMed] [Google Scholar]
- 47.Autieri MV, Kelemen SE, Wendt KW. AIF-1 is an actin-polymerizing and Rac1-activating protein that promotes vascular smooth muscle cell migration. Circ Res. 2003;92:1107–1114. doi: 10.1161/01.RES.0000074000.03562.CC. [DOI] [PubMed] [Google Scholar]
- 48.Garrod DR, Fleming S. Early expression of desmosomal components during kidney tubule morphogenesis in human and murine embryos. Development. 1990;108:313–321. doi: 10.1242/dev.108.2.313. [DOI] [PubMed] [Google Scholar]
- 49.Mardones GA, Burgos PV, Brooks DA, Parkinson-Lawrence E, Mattera R, Bonifacino JS. The trans-Golgi network accessory protein p56 promotes long-range movement of GGA/clathrin-containing transport carriers and lysosomal enzyme sorting. Mol Biol Cell. 2007;18:3486–3501. doi: 10.1091/mbc.E07-02-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui S, Li C, Ema M, Weinstein J, Quaggin SE. Rapid isolation of glomeruli coupled with gene expression profiling identifies downstream targets in Pod1 knockout mice. J Am Soc Nephrol. 2005;16:3247–3255. doi: 10.1681/ASN.2005030278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical, Pathological, and Array QC findings by Specimen
Differentially regulated genes (FSGS + COLL vs Normal + MCD)
Differentially expressed genes shared with partial gene list from Bennett et. al.12, human FSGS
Differentially expressed genes shared with Baelde et. al. (10), human diabetic glomerulosclerosis


