Abstract
The plausibility of constructing vascularized three-dimensional (3D) kidney tissue from cells was investigated. The kidney develops from mutual inductive interactions between cells of the ureteric bud (UB), derived from the Wolffian duct (WD), and the metanephric mesenchyme (MM). We found that isolated MMs were capable of inducing branching morphogenesis of the WD (an epithelial tube) in recombination cultures; suggesting that the isolated MM retains inductive capacity for WD-derived epithelial tubule cells other than those from the UB. Hanging drop aggregates of embryonic and adult renal epithelial cells from UB and mouse inner medullary collecting duct cell (IMCD) lines, which are ultimately of WD origin, were capable of inducing MM epithelialization and tubulogenesis with apparent connections (UB cells) and collecting duct-like tubules with lumens (IMCD). This supports the view that the collecting system can be constructed from certain epithelial cells (those ultimately of WD origin) when stimulated by MM. Although the functions of the MM could not be replaced by cultured mesenchymal cells, primary MM cells and one MM-derived cell line (BSN) produced factors that stimulate UB branching morphogenesis, whereas another, rat inducible metanephric mesenchyme (RIMM-18), supported WD budding as a feeder layer. This indicates that some MM functions can be recapitulated by cells. Although engineering of a kidney-like tissue from cultured cells alone remains to be achieved, these results suggest the feasibility of such an approach following the normal developmental progression of the UB and MM. Consistent with this notion, implants of kidney-like tissues constructed in vitro from recombinations of the UB and MM survived for over 5 weeks and achieved an apparently host-derived glomerular vasculature. Lastly, we addressed the issue of optimal macro- and micro-patterning of kidney-like tissue, which might be necessary for function of an organ assembled using a tissue engineering approach. To identify suitable conditions, 3D reconstructions of HoxB7–green fluorescent protein mouse rudiments (E12) cultured on a filter or suspended in a collagen gel (type I or type IV) revealed that type IV collagen 3D culture supports the deepest tissue growth (600 ± 8 μm) and the largest kidney volume (0.22 ± 0.02 mm3), and enabled the development of an umbrella-shaped collecting system such as occurs in vivo. Taken together with prior work (Rosines et al., 2007; Steer et al., 2002),1,2 these results support the plausibility of a developmental strategy for constructing and propagating vascularized 3D kidney-like tissues from recombinations of cultured renal progenitor cells and/or primordial tissue.
Introduction
With over 89,000 people in the United States alone awaiting a kidney transplant (as of April 2010), the necessity for alternatives to allogenic renal transplantation is clear.3 Currently, the alternative to transplantation in patients with chronic renal failure is dialysis; this is despite the facts that this does not recapitulate all the functions of the kidney, nor have improvements in the technique eliminated the need for transplantation. A potential solution to the shortage of transplantable kidneys is engineering of kidney-like tissues for therapeutic purposes. This is a daunting task because the human kidney is characterized by over two dozen cell types and a complex three-dimensional (3D) architecture, including structures such as the loop of Henle, the juxtaglomerular apparatus, and a complex microvasculature, critical to its many functions.
Embryonic kidney primordia represent a potential source of transplantable organs.4–6 A strategy has been proposed by us for in vitro propagation of nephrons and generation of many kidney-like tissues from a single primordium by exploiting the capacity of the UB to branch.2 However, an ultimate goal would be to construct kidney or kidney-like tissues with the level of complexity seen in the adult organ from cultured cells, which represent a seemingly unlimited resource and which could potentially be derived from an individual patient using stem cell technology. We recently described a strategy for the constructing of kidney-like tissues through in vitro reconstitution of developmental stages beginning with renal progenitor tissues, ureteric bud (UB; the progenitor tissue from which the renal collecting system is derived), or Wolffian duct (WD; the embryonic tissue from which the UB is derived) along with metanephric mesenchyme (MM; the tissue from which the noncollecting duct portion of the nephron is ultimately derived).1 In this study, we have examined the feasibility of using rodent cultured renal cell lines to model kidney progenitor tissues and evaluated the potential of constructing kidney-like tissues from cultured cells.
Although many questions remain to be answered, the data appear to support the concept that it may be possible to construct renal progenitor tissues from cultured cells. Even though the creation of functional renal tissue with appropriate 3D spatial relations from UB and MM cells alone has yet to be achieved, the feasibility of such an approach for the generation of both progenitor tissues was strongly supported using both cells of WD/UB derivation (UB and inner medullary collecting duct [IMCD] cells) and MM derivation (BSN and rat inducible metanephric mesenchyme [RIMM]-18 cells). In vitro recombinations of progenitor tissues and transplantation into nude mice suggest that the strategy we propose can result in a vascularized kidney with long-term viability. To obtain appropriate macro- and micro-patterning necessary for whole kidney function, we propose a novel 3D culture approach based on a type IV collagen–rich matrix. Together, the results of this study represent important and necessary first steps in the utilization of developmental approaches to de novo in vitro constructing of kidney-like tissues from cultured cells.
Materials and Methods
Materials and reagents
Fibroblast growth factor-1 (FGF1) and glial-cell-derived neurotrophic factor (GDNF) were obtained from R&D Systems (Minneapolis, MN). Mouse anti-E-cadherin antibodies were from BD Biosciences Pharmingen (San Diego, CA) and goat anti-mouse AlexaFluor 594 was from Molecular Probes (Eugene, OR). FITC-conjugated Dolichos biflorus (DB) lectin and rhodamine-conjugated peanut agglutinin (PNA) were from Vector Laboratories (Burlingame, CA). Type I and type IV collagens, and growth-factor-reduced Matrigel were from BD Biosciences (San Jose, CA). Antibiotics, Dulbecco's modified Eagle's medium (DMEM)/F12 1:1 (v:v), and phosphate-buffered saline (PBS) were from Gibco-BRL (Grand Island, NY). Unless otherwise noted, all other reagents were from Sigma (St. Louis, MO).
Embryonic tissue isolation
The urogenital tract was isolated from timed E13 Holtzman rat or E12 mouse embryos and WDs were dissected free of surrounding tissue. The mesonephric tubules and intermediate mesoderm were carefully stripped away leaving only the epithelial tube of the WD.7,8 Metanephric kidneys were isolated and directly used in the kidney culture as described below or further separated in to the UB and MM tissues as described previously.1
Implantation of reconstructed kidney-like tissue
Recombinations of UB and MM were implanted into adult male nude mice according to previously described methods.1 The implanted tissue was inserted into a subcapsular tunnel created on the right kidney, the incision was closed, and the animal was allowed to recover from the anesthesia. After 37 days, the kidneys with implants were removed and processed for histological and immunocytochemical analysis.
WD/MM coculture
A ∼100 μm segment of WD was excised and suspended within the isolated MM from one kidney in a 1 mg/mL type I collagen solution (supplemented with DMEM and buffered by HEPES and NaHCO3 to a pH of ∼7.2). Before the gel was completely solidified, the WD segment was placed in the crevice of the MM left behind from the removal of the UB. The WD/MM tissue was cultured in the presence of a DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT) and 1% antibiotics for 7 or 12 days. All cultures were incubated at 37°C in a humidified 5% CO2 and 100% humidity atmosphere.
Hanging drop cell aggregate/MM coculture
Confluent monolayers of mouse SV40 large-T-antigen-transfected UB cells9 or IMCD cells10 were trypsinized and suspended in DMEM/F12 (supplemented with 10% FBS and 1% antibiotics) at a concentration of 1 × 105 cells/mL. About 20 μL of the cell solution was placed on the bottom of a Petri dish lid with 10 mL of PBS in the Petri dish. The cells were incubated as a hanging drop for 2 days at 37°C in a humidified 5% CO2 atmosphere. The cell aggregates were removed from the hanging drops and placed on a 0.4 μm Transwell filter surrounded by freshly isolated MM with 400 μL DMEM/F12 medium supplemented with 10% FBS and 1% antibiotics placed below the filter and incubated for an additional 7 days.
Generation of the primary MM cell line
MMs isolated from day 13 embryonic kidneys as previously described11–13 were placed directly onto a cell-culture-treated plate and cultured for 5 days in the growth medium supplemented with 50 ng/mL of FGF2 and 10 ng/mL transforming growth factor-α, which support MM survival.9 The MM cells were then trypsinized and placed on new plates and used for the experiments.
MM cell (BSN, RIMM-18 or primary) culture and conditioned medium
To create conditioned medium (CM), BSN,14 RIMM-18,15 MM primary, or NIH/3T316 cells were cultured on plates and allowed to reach confluence. After confluence was reached, the medium was replaced with DMEM/F12 (no antibiotics or FBS) and the cells were incubated for 3 days. After 3 days the CM was removed and concentrated 5 times with a 5000 MW cutoff Millipore (Billerica, MA) filter.
Isolated UB culture
Isolated UBs were suspended in a growth-factor-reduced Matrigel solution (1:1 Matrigel:DMEM/F12), and cultured with CM from the BSN, RIMM-18, MM primary, or 3T3 cell lines supplemented with 10% FBS, 1% antibiotics, 125 ng/mL FGF1, and 125 ng/mL GDNF as described previously.12 The suspended UBs were then cultured for 7 days at which time tips were counted.
3D kidney culture
Metanephric kidneys were isolated and suspended in extracellular matrix (ECM) solutions of type I collagen, type IV collagen, or growth-factor-reduced Matrigel at the noted concentrations. All matrix solutions were supplemented with DMEM and buffered by HEPES and NaHCO3 to a pH of ∼7.2. Kidneys were cultured in the presence of 600 μL DMEM/F12 supplemented with 10% fetal calf serum and 1% antibiotics for 7 days.
Immunofluorescence and lectin staining
After the indicated number of days, kidney cultures were fixed in 4% paraformaldehyde in PBS and processed for immunohistochemical analysis as previously described.6,11,17 The localization of Flk-1, Pod, vW factor, E-cadherin, cytokeratin, and/or PAX-2 (1:500 in blocking solution) was determined in the samples. For PNA staining, after the blocking step, the tissues were washed twice with Neuraminidase buffer (150 mM NaCl and 50 mM sodium acetate, pH 5.5), and incubated with Neuraminidase (1 unit/mL) for 4 h at 37°C, and then with rhodamine-conjugated PNA (50 μg/mL) and FITC-DB (1:500) for 24 h at 4°C. Fluorescently stained samples were imaged the Nikon EZ-C1 confocal system.
3D imaging and morphometric analysis
Kidney cultures from HoxB7-GFP mice were fixed for 30 min with 4% paraformaldehyde in PBS and rinsed thrice for 5 min in PBS. Kidneys were then cleared with Focus Clear (Cedarlane Laboratories, Burlington, NC) for 20 min and mounted on depression slides with Mount Clear (Cedarlane Laboratories). Samples were imaged on the FV300 Olympus 2-photon microscopy system. 3D Reconstructions, isosurfacing, and 3D measurements of fluorescent stacks were performed using Image Pro Plus 3D Constructor 5.1 (Media Cybernetics, Bethesda, MD). Tissue thickness was determined as being the minimum distance between two planes (or Feret minimum). Length to thickness ratio was calculated as the Feret maximum to Feret minimum ratio. Kidney volumes were estimated as the volume of an ellipsoid with the dimensions of half the length, depth, and width of a bounding box around the branching structure. All samples were analyzed with n ≥ 3 and with errors reported as the standard error of the mean.
Results
Over 50 years ago, Grobstein and colleagues demonstrated that the isolated MM retained the ability to undergo morphogenetic changes similar to that seen in vivo when cultured in the presence of an appropriate inducing tissue, including the UB and other heterologous tissues.18–20 Subsequent studies from a number of laboratories have lead to the conclusion that the Wnt family of growth factors are involved in this induction of renal epithelia from the MM.21–33
Because the UB and MM are mutually inductive tissues, initial studies were aimed at determining whether the UB could be replaced by another tubular tissue with the potential to form collecting ducts in coculture with the MM. Because 3D tubular epithelial growth can be achieved with cells of WD origin (UB and IMCD cells)14,34–38 and because it is the progenitor tissue of the UB and thus might be able to respond to potential inductive signals arising from the whole isolated MM, the WD was chosen. Thus, we examined the ability of the E13 embryonic MM to induce the growth and development of a portion of an isolated WD into a branching UB-like structure. The isolated WD, when cultured within the freshly isolated MM (in which the UB has been removed via manual dissection), not only developed a branched collecting duct system but also proved to be capable of inducing the MM to differentiate in a manner similar to that of traditional in vitro metanephric kidney culture (Fig. 1E–G). Although it is conceivable that some basement membrane from the UB remains within the isolated MM, only those MMs in which the entire UB was removed were used for the experiments.
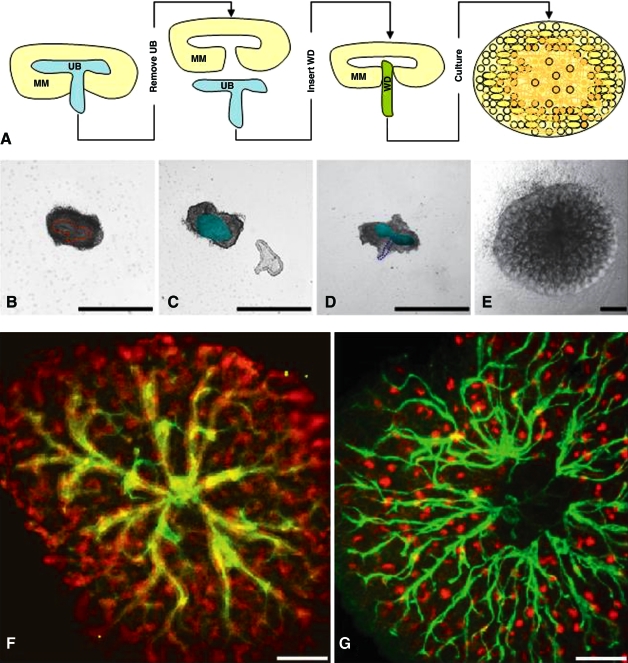
FIG. 1.
WD/MM coculture. (A) Schematic of the procedure followed in engineering kidney tissue from the WD and MM. (B) Embryonic day 13 rat kidney; UB is outlined by dashed red line. (C) Kidney that has been separated into isolated UB and isolated MM; light green oval indicates empty space in MM where the UB was removed. (D) Isolated MM from (C), in which a piece of WD has been used to replace the UB; light green oval indicates empty space in MM where the UB was removed, and dashed blue line demarcates the section of WD. (E) After 7 days, the WD/MM coculture grew similar to traditional in vitro kidney culture. (F) After 7 days, the WD/MM coculture grew similar to traditional in vitro kidney culture (green = Dolichos biflorus lectin, UB-derived tissues; red = E-cadherin, UB- and MM-derived polarized epithelial tissues). (G) After 12 days, peanut agglutinin lectin staining (red) revealed differentiation of glomerular podocytes. Scale bars = B–G 300 μm. UB, ureteric bud; WD, Wolffian duct; MM, metanephric mesenchyme. Color images available online at www.liebertonline.com/ten.
After 7 days of in vitro culture, convoluted, presumably MM-derived epithelial structures expressing E-cadherin were visible, suggesting the formation of nascent nephrons (red-stained structures in Fig. 1F). After 12 days of in vitro culture, in addition to increased growth of the collecting duct system, a large number of developing glomeruli were evident by PNA staining (red dots in Fig. 1G). PNA lectin has been shown to bind with high affinity to the podocyte coat after neuramidase treatment and thus represents a convenient marker of developing glomeruli.39
Taken together, these results indicate that the MM, even after some early morphogenetic events involved in kidney development have occurred (i.e., induction of UB outgrowth, UB penetration of MM, and formation of the T-shaped UB), retains inductive capacity for early morphogenetic events in kidney development (i.e., UB emergence from the WD). Moreover, this raises the possibility that the post-UB outgrowth MM has the potential to serve as an inductive tissue for studies aimed at engineering kidney-like tissues from cultured cells that have the potential to form branching tubular collecting duct-like structures (i.e., UB cells and IMCD cells).14,34,35,38,40,41
Constructing an epithelial tube from cells
We attempted to construct an epithelial tube from a homogenous cell line possessing the potential to act as a UB or WD. Although it is currently unclear what source of cells (mature kidney cells or stem cells that are of embryonic, amniotic, or adult derivations) will ultimately be best suited for this purpose, it is, nevertheless, necessary to develop strategies to construct tissues from cells should they become available. Thus, in these studies, the process of constructing an epithelial tissue from cells was simulated using an immortalized mouse UB cell line9,14 or an IMCD cell line derived from the mouse adult collecting duct.10 Both of these renal epithelial cell lines are capable of undergoing branching tubulogenesis in 3D ECM gels in response to either CM from a MM cell line (BSN cell line)14 or medium containing hepatocyte growth factor and/or an epidermal growth factor receptor ligand,34,35 respectively.
The UB and IMCD cells were induced to form cell aggregates in hanging drop cultures, and, when suspended in ECM gels, these aggregates appeared to grow and develop as a single mass of cells. Interestingly, a large hanging drop aggregate of IMCD cells (Fig. 2a) formed a multicellular tubule with a lumen after 6 days culture, in response to BSN-CM with 125 ng/mL GDNF and 250 ng/mL FGF1 (Fig. 2b) (similar tubular structures with lumens were not seen in 3D ECM cultures of the UB cell hanging drop aggregates). This tubule eventually formed a structure (Fig. 2c, d) remarkably similar, in both size and shape, to the T-shaped UB (Fig. 2e). In addition, IMCD cell hanging drop aggregates after 7 days of culture in the 3T3-CM with 125 ng/mL GDNF were also able to form multiple buds with lumens (Fig. 3a, b) in a similar manner seen with WD budding (Fig. 3d).
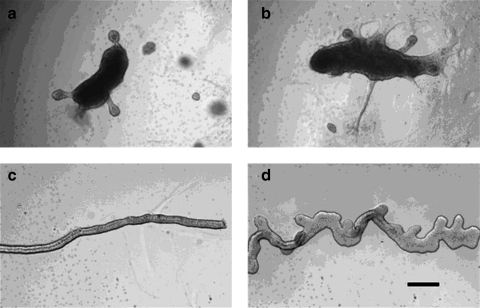
FIG. 2.
IMCD hanging drop cell aggregates. (a) An IMCD aggregate from the hanging drop. (b) After 6 days in the Matrigel suspension culture, the IMCD cell aggregate protruded a tubule with a lumen. (c, d) The tubule continued growing to form a structure resembling a T-shaped UB (compare with e) after 8 days of culture. (e) Phase-contrast photomicrograph of a freshly isolated rat E13 T-shaped UB. Scale bars = 200 μm. IMCD, inner medullary collecting duct.
FIG. 3.
Budding of IMCD cell aggregates. (a, b) IMCD cell aggregates from the handing drop method formed buds with lumens in a manner like WD budding after 7 days in the Matrigel suspension culture. (c) An isolated clean WD. (d) Budding of the clean WD after 5 days culture supplemented with a medium with glial-cell-derived neurotrophic factor and fibroblast growth factor1. Scale bar = 200 μm.
Coculture of the UB-like aggregate (constructed from UB and IMCD cells) with MM
The hanging drop cell aggregates were then combined with freshly isolated E13 rat MM (Fig. 4a). Although the UB cell aggregate did not appear to branch within the isolated MMs (perhaps because a 3D ECM is not included in the standard recombination), multicellular epithelial structures with apparent apico-basolateral polarity were observed (Fig. 4e); however, it was difficult to identify tubules with patent lumens. Nevertheless, not only did UB cell aggregates induce mesenchymal-to-epithelial transition in patches of the MM (Fig. 4b, c), but costaining with E-cadherin (a convenient marker for the transition of MM cells to epithelial cells) and fluorescently labeled DB (a marker of UB-derived tissue42) suggested that the recombination of the MM with preformed UB-like aggregates of UB cells results in the formation of an apparently contiguous tissue segment (Fig. 4d), reminiscent of the recombination of cultured isolated UB with MM.11–13,43 Thus, cultured cells derived from the UB can, in this system, induce a mesenchymal-to-epithelial transition in the isolated MM. The E-cadherin-positive/DB-negative MM-derived tubule appeared continuous with the DB-positive/E-cadherin-positive UB-cell-derived structure. By staining for both E-cadherin and DB, one can readily identify epithelial cells arising from the MM, as these epithelial cells do not bind DB, whereas UB epithelial cells are both E-cadherin and DB positive.
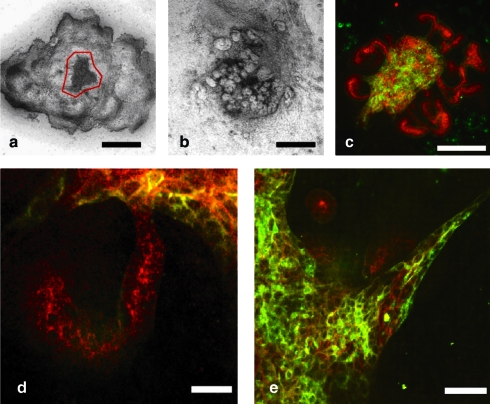
FIG. 4.
UB cell aggregate coculture with MM. (a) Hanging drop aggregate of UB cells (outlined in red) surrounded by numerous freshly isolated MMs. (b) Phase-contrast of coculture after 7 days. (c) Confocal fluorescent photomicrograph of coculture tissue after 7 days of growth in culture (green = Dolichos biflorus lectin, UB-derived tissues; red = E-cadherin, UB- and MM-derived polarized epithelial tissues). (d) Higher magnification examination of the recombined tissue showing that the MM-derived tubule is continuous with the green UB cells. (e) UB-cell-derived multicellular extensions. Scale bars = a–c, 400 μm; d and e, 25 μm. Color images available online at www.liebertonline.com/ten.
In contrast to UB cell aggregates, IMCD cell hanging drop aggregates with T-shaped UB-like structures were found to be capable of self-organizing into tubules with lumens when cocultured with isolated MM (Fig. 5a–c). Although the IMCD cell aggregates did not induce the MM to develop nascent nephrons to the same extent as that seen with UB cell hanging drop aggregates (Fig. 4c, d), formation of some small comma-shaped bodies was evident (Fig. 5e). Taken together, these results suggest that when recombined with MM, UB-like tissue constructed from hanging drop aggregates of renal epithelial cell lines not only can form tubules with lumens in response to the inductive signals from the MM, but also can induce epithelialization and early tubulogenesis in the MM.
FIG. 5.
IMCD cell aggregate coculture with MM. (a) After 7 days, the IMCD cells organized into epithelial tubules; however, MM induction did not appear very widespread. (b–d) Cytokeratin staining (green) demonstrates that the IMCD cell aggregate formed tubular structures with lumens (noted by the asterisk). (e) Occasional comma-shaped bodies (evident by PAX-2 staining, red) were induced by the IMCD cell aggregate. Scale bars = a and b 400 μm; d and e, 50 μm. Color images available online at www.liebertonline.com/ten.
Coculture of isolated UB and WD with cultured MM cells
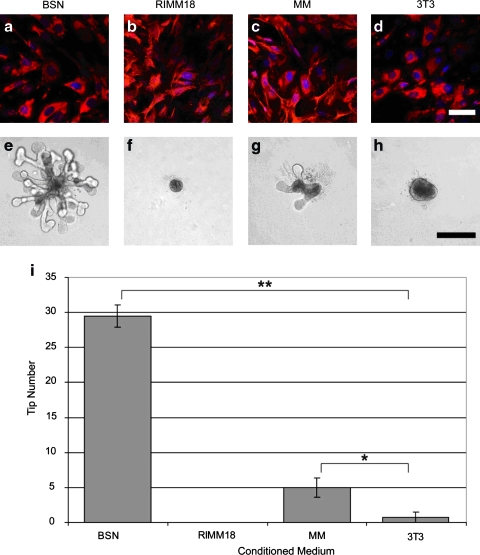
For the purposes of constructing a kidney-like tissue from cells alone (by co-opting the developmental program), one would ideally like to perform equivalent experiments on the MM side. Therefore, we investigated whether any of the currently available cultured MM cells possess the potential to substitute for the native progenitor tissue. Three different MM-derived cell types, the well-characterized BSN cell line,14 a conditionally immortalized metanephric mesenchymal cell line (RIMM-18),15 and a primary rat E13 MM cell culture (verified to be vimentin positive and cytokeratin negative [Fig. 6a–c], along with NIH/3T3 fibroblasts [controls; Fig. 6d]), were tested (Fig. 6e–i). However, cell aggregates from each of the three MM cell types (generated through the use of hanging drop culture or via centrifugation) could not be made to induce UB branching morphogenesis after recombination with freshly isolated UB (data not shown). The cell aggregates also lacked the ability to undergo mesenchymal-to-epithelial transition after recombination with the freshly isolated E13 rat UB (data not shown). This is despite the fact that the medium conditioned by BSN cells12,14 and primary MM cells was capable of inducing isolated UB branching morphogenesis in a 3D ECM (Fig. 6e–i), the BSN-CM being, somewhat surprisingly, even more potent than primary MM-CM itself (Fig. 6i). Although the RIMM-18 and 3T3 conditioned medium did not induce UB branching, the 3T3-CM (known to contain the branch-promoting factor, pleiotrophin,44 and other branching factors) appeared to induce slight globular growth of the isolated UB (Fig. 6h).
FIG. 6.
Three MM-derived cell lines tested for the ability to induce isolated WD budding. (a–c) The BSN, rat inducible metanephric mesenchyme (RIMM)-18, and MM primary cell lines are all MM-derived cell lines that are mostly vimentin positive and cytokeratin negative or low. (d) 3T3 fibroblasts are also vimentin positive, cytokeratin negative cells, but are not MM derived. (e) Conditioned medium (CM) from BSN cells strongly induced isolated UB branching. (g) CM from primary MM cells only slightly induced branching. (f, h) CM from RIMM-18 or 3T3 cells did not induce branching morphogenesis. (i) Plot of tip number versus cell-CM used (analysis of variance, p ≤ 0.00001); *p ≤ 0.05, **p ≤ 0.00005. Scale bars = a–d, 50 μm; e–g, 250 μm. Color images available online at www.liebertonline.com/ten.
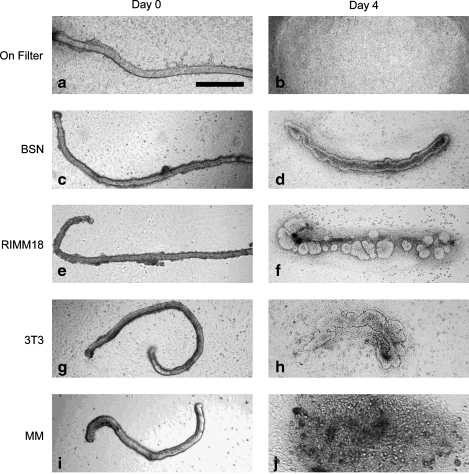
Moreover, budding of the WD occurred only when the isolated WD was cultured in direct contact with RIMM-18 cells (Fig. 7e, f). BSN and 3T3 cells allowed for survival with an apparent increase in the width of the WD, whereas WDs cultured on top of primary MM cells disintegrated (Fig. 7c, d, g, and h). This suggests that the formation of a UB-like structure from WD cell epithelial tube can be made to occur when the tube is in contact with MM-derived cells.
FIG. 7.
Three MM derived cell lines were tested for the ability to support isolated WD budding. (a, b) WD cultured on a filter disintegrated. (c, d) WD cultured on a layer of BSN cells appeared to survive, (e, f) but WD cultured on RIMM-18 cells underwent budding at multiple sites along the WD. (g, h) 3T3 only supported WD survival. (i, j) MM primary cells resulted in WD disintegration. (Scale bar = 500 μm).
Implantation of in vitro engineered kidney-like tissue into nude mice
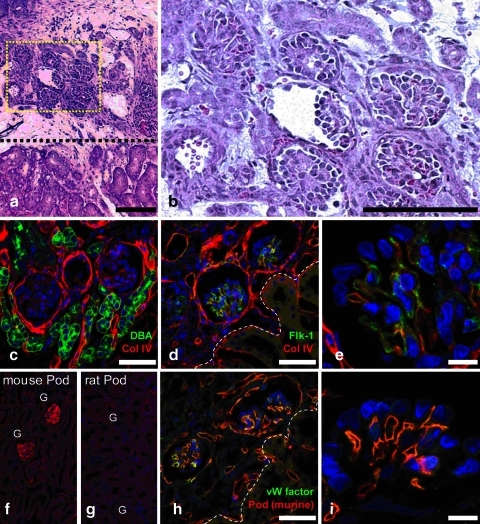
Whole embryonic kidney or kidney-like tissue (constructed in vitro from a recombination of cultured UB and MM) has been previously implanted under the renal capsule of a host animal leading to the development of glomeruli with rudimentary vascular supplies.1,4–6,45 However, growth and vascularization of the kidney-like recombinant implant was severely limited beyond 2 weeks, possibly due to chronic rejection as evidenced by a mononuclear cellular infiltration.1 It is also possible that since the implant does not have an outlet for the filtrate, the tissue degradation is due to the accumulation of filtrate.45 To address this problem, rat recombination cultures (of native UB and native MM) were implanted under the renal capsule of an immunocompromised host nude mouse. Their use as recipients of the implanted kidney-like tissues allowed for extended growth of the implant, up to 5 weeks after implantation. Histological examination of the implantation site revealed the presence of multiple glomerular-like structures within the implanted tissue with little apparent necrosis (Fig. 8a–c). Immunocytochemical and histocytochemical examination revealed that these glomerular-like structures had a vasculature containing an endothelial cell marker, Flk1 (Fig. 8d, e), and were apparently derived from the host animal (Fig. 8f–i). Although it remains to be determined whether or not the implanted kidney-like tissue is capable of producing concentrated urine or demonstrates other mature kidney functions, the results described above do suggest that in vitro constructed kidney-like tissue can continue to differentiate and grow into a more histolomorphologically mature renal tissue in the proper environment.
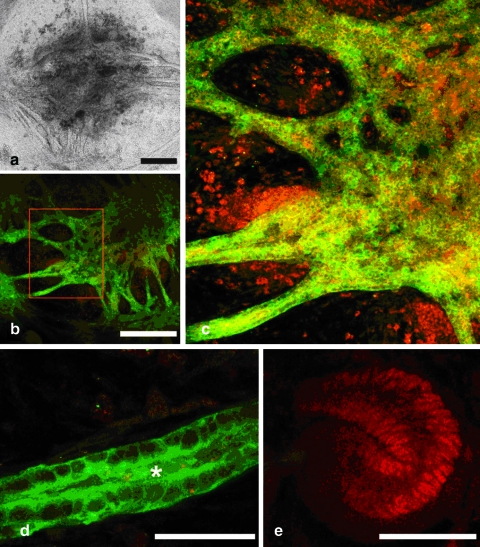
FIG. 8.
Examination of engineered kidney tissue 37 days after implantation under the renal capsule of a kidney in a nude mouse. (a, b). Hematoxylin and eosin stained section through the host kidney and implanted kidney tissue. (c–i) Confocal fluorescent micrographs showing extent of implant vascularization. (c) Dolichos biflorus (green); Collagen IV (red). (d) Flk-1 (green); Collagen IV (red). (e) Higher magnification examination of d. (f) Specific staining for mouse podocalyxin (red). (g) Specific staining for rat podocalyxin (red). (h) von Willebrand factor (green); mouse podocalyxin (red). (i) Higher magnification examination of h. (Scale bar = a, 400 μm; b, 150 μm; c, d, h, 50 μm; e, i, 10 μm). Color images available online at www.liebertonline.com/ten.
Achieving appropriate 3D spatial relationships in vitro
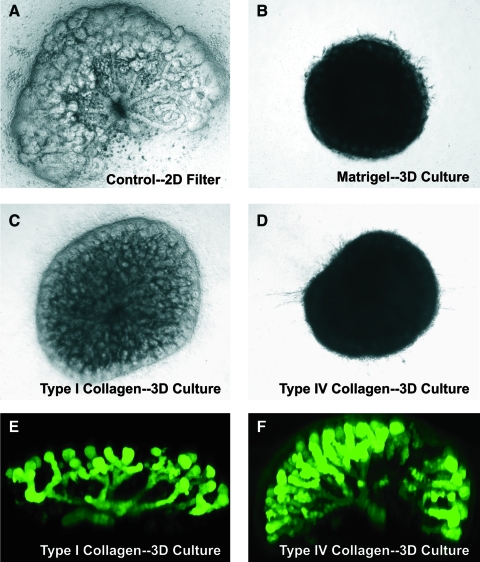
If renal progenitor tissues are to be constructed from cells and these tissues then assembled to reproduce an embryonic kidney-like structure as described in Figure 1, another potentially important step would be to culture the early embryonic kidney-like tissues in a system that allows for recapitulation of 3D spatial relations within the kidney as these relations are necessary for normal kidney function. Metanephric kidney culture has traditionally been performed by culturing the embryonic kidney rudiment in the presence of a normal growth medium on a filter at the air–medium interface. Kidneys cultured in this manner grow in primarily in two-dimensions with little increase in its width (Figs. 9a and 10A). Consequently, despite the fact that much of early kidney development can be simulated in this setting, this culture system does not accurately reproduce the 3D structure important for renal function. Therefore, we investigated whether an E13 rat embryonic kidney could undergo 3D growth and branching morphogenesis when suspended within an ECM gel. Type I collagen, type IV collagen, and Matrigel all supported 3D kidney growth and development (Figs. 9 and 10), whereas artificial matrices of either alginate or puramatrix did not support any appreciable growth (data not shown).
FIG. 9.
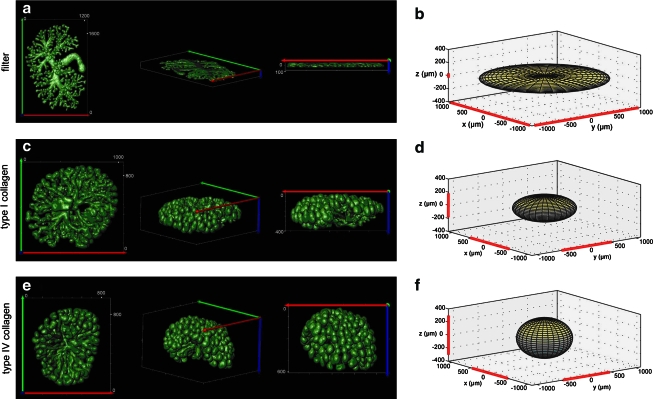
Three-dimensional (3D) projection of the branching UB of E12 HoxB7–green fluorescent protein mouse kidneys cultured for 7 days. (a, b) Kidneys in the traditional filter culture grew flat and along the filter. (c–e) Kidneys cultured in type I collagen or type IV collagen grew much thicker and in a more 3D manner (units, μm). (e, f) Type IV collagen supported the deepest tissue growth, but the least distance from the origin of branching. Color images available online at www.liebertonline.com/ten.
FIG. 10.
(A–D) Phase-contrast photomicrographs of whole embryonic kidneys cultured for 7 days directly on the Transwell filter (A) or in 3D culture suspended in either Matrigel (B, 25%), type I collagen (C, 1 mg/mL), or type IV collagen (0.65 mg/mL). (E, F) Fluorescent photomicrographs of whole embryonic kidneys from the HoxB7–green fluorescent protein transgenic mouse grown for 7 days in 3D culture suspended in either type I collagen (E) or type IV collagen (F). Note especially “umbrella-like” branching of UB in F. Color images available online at www.liebertonline.com/ten.
The increasing opacity of the kidneys imaged with brightfield microscopy (type IV collagen = Matrigel > type I collagen > filter) suggested a thicker, more 3D tissue (Fig. 10). Interestingly, type IV collagen and type I collagen promoted different 3D growth patterns. Kidneys grown in type I collagen only branched upward in the z-direction away from the filter, whereas the branching of kidneys growing in type IV collagen exhibited an umbrella-shape branching pattern characteristic of the in vivo developing kidney (Figs. 9 and 10), suggesting a fundamental difference in how the structural and/or morphogenetic properties of type I collagen and type IV collagen gels affect the orientation of tissue branching. However, to quantify the 3D growth and to observe the extent of 3D branching in the gel suspension culture, E12 kidneys from the HoxB7-GFP mouse were cultured in three culture conditions: traditional filter culture, 1 mg/mL type I collagen, and 0.65 mg/mL type IV collagen. After 7 days of culture, the kidneys were imaged on a 2-photon microscope, 3D reconstructions of the branching structures were performed (Fig. 9a, c, e), morphometric measurements taken, and idealized elliptical models were generated (Fig. 9b, d, f). Culture of the kidney rudiment on a filter in two-dimensions resulted in growth along the filter with little appreciable growth or branching in the z-direction, whereas kidneys grown in 3D gels in either type I collagen or type IV collagen scaffolds exhibited extensive 3D branching growth in the z-direction. Type I collagen cultures exhibited a tissue depth, or Feret minimum (371 ± 7 μm), 4.7 times greater than the filter cultures (79 ± 2 μm), and type IV collagen cultures exhibited a tissue depth (600 ± 8 μm) 7.6 times greater than filter cultures. Total culture volume was larger in the thicker tissues, although this difference is not as significant as the difference in tissue depth because the branches of the kidneys cultured in the collagen gels did not extend as far in the horizontal x–y plane as kidneys grown on a filter.
Discussion
Studies by others have demonstrated that prevascular embryonic kidneys implanted into animal hosts can vascularize and exhibit some excretory function.4–6,45 In a recent study, we demonstrated how one can create implantable tissues in vitro from renal progenitor tissues,1 and now we have advanced the method by addressing the feasibility of using cultured cells to construct the necessary renal progenitor tissues (i.e., UB and MM) and recombining these cells with freshly isolated embryonic kidney tissues. This represents another step toward cell-based strategies for tissue engineering of the kidney. We have also provided evidence for long-term vascularization and viability of a transplanted kidney-like tissue reassembled from progenitor tissues and suggested that more appropriate 3D structural relationships necessary for normal renal function can be achieved in a suitable 3D matrix (type IV collagen in our experiment). In an earlier work, we also provided a method for in vitro propagation of cultured embryonic kidney tissues by exploiting the power of UB branching to generate multiple recombined kidney-like tissues.2,46
The ideal approach would be to create a kidney tissue that would elicit little to no immune response when implanted. As seen in Figure 8, the lack of an immune response allowed implanted kidney-like tissue to grow and differentiate such that extensive glomerular vasculature (apparently of host origin) was obtained. If the goal of constructing transplantable tissues from a source of either autologous cells or other cells is realized, the ability to expand a cell line in vitro and construct an organ from these cells could alleviate the problem of finding sources of tissues or organs. There are many potential sources of cells from which to construct kidney-like tissues, including mature renal cells, cells created by nuclear transplantation,47 embryonic stem cells,48 amniotic stem cells,49 or adult renal stem-like cells,8,58–59 which may be capable of differentiating into various renal progenitor populations and forming tubules.8,60
Once a cell source is identified, the next step would be to organize those cells into the renal progenitor tissues that comprise the embryonic kidney: UB and MM. Here, the process of constructing an epithelial UB-like tissue from cells was simulated using renal epithelial cell lines that are capable of undergoing branching tubulogenesis in 3D ECM gels.14,34–36,38,40,41 A number of studies suggest that branching tubulogenesis of UB and IMCD cell lines is a reasonable, if not good, model of UB growth and branching morphogenesis. Many of the growth factors that regulate in vitro UB cell growth and branching similarly regulate the isolated UB in vitro and, as is increasingly appreciated, in vivo.37,53,57,61,62 For example, two pathways (c-met and epidermal growth factor receptor) initially postulated to be important for UB branching based on data from 3D UB and IMCD cell branching tubulogenesis studies are indeed necessary for normal UB branching.14,34–38,63
Hanging drop aggregates of the UB cell line were found to retain the ability to induce mesenchymal to epithelial transition (MET) and early nephron formation in the isolated MM, as well as apparently joining with the MM to form a contiguous tissue segment. Although this is a very promising finding, the UB cell hanging drop aggregates were unable to organize into a tubular structure with a clearly patent lumen or undergo branching morphogenesis. Unlike the UB cell aggregates, mouse IMCD cell hanging drop aggregates (a more differentiated renal cell type ultimately derived from the WD and UB) were capable of forming tubules with lumens and even T-shaped UB-like structures both in an isolated system (Figs. 2 and 3) and after recombination with MM (Fig. 5). However, while the IMCD cell aggregate was able to induce some MM epithelialization (Fig. 5e), it did not promote the formation of multiple long MM-derived tubules as seen with the UB cell aggregates. Since IMCD cells were isolated from the adult renal collecting duct and are thus ultimately UB-derived, these results, together with those from the UB cells, suggest that there exists a UB (or conceivably WD) cell type that can form tubules similar to the IMCD cell line, and induce MM similar to the UB cell line. It also may be possible that a renal progenitor tissue could be constructed from a mix of UB-like and IMCD-like cells, thereby possessing both abilities. Although connections formed with nascent MM-derived nephrons in our experiments, the capacity for iterative UB-like branching beyond the T-shape was low. Newer technologies such as cell micropatterning may be necessary to achieve the creation of a luminal epithelial structure from cells retaining both the MM-inductive capacity and UB branching capability.64
Although it may seem that the MM tissue would be easier to construct from cells than an epithelial tissue because there appears to be no specific cell orientation or structure, this was not the case, at least for the MM cell lines that were utilized for these experiments. These data suggest that E13 rat MM tissue is not readily modeled as a tissue of homogeneous cells—which is not entirely surprising, as the embryonic MM is likely to be heterogeneous in make-up. The MM gives rise to at least 14 different epithelial cell types found in the nephron; however, the fate of the cells is restricted rapidly after induction.65 Thus, it is possible that the MM cell lines used no longer possess the ability to induce UB growth and branching in the type of in vitro setup used here. In addition, since primary cell aggregates could not act as primary MM tissue, perhaps the particular methods of aggregation or cell suspension did not allow for the cells to act as MM tissue. It also may be possible that only certain sub-selected MM cells were proliferating when cultured on a plate; thus, the primary MM cell line may have lacked all the necessary MM cells in the cell aggregates. Perhaps an earlier mesenchymal tissue potentially containing a less differentiated population of cells would represent a better potential tissue to reconstruct from cells.
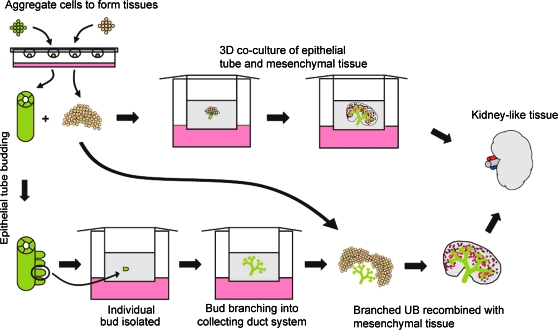
The ability of the embryonic kidney to develop in vitro when the UB was replaced by a WD (Fig. 1a), which can be considered as an epithelial cell tube, suggests that renal progenitor tissues constructed from cells could potentially be assembled into an embryonic kidney-like structure that could be cultured similar to traditional in vitro metanephric kidney culture (Fig. 11). However, traditional kidney rudiment culture occurs as a flat two-dimensional culture (Figs. 9 and 10) and does not accurately recapitulate the 3D growth and structure of in vivo development. Here we demonstrated that a kidney rudiment can recapitulate 3D growth and branching morphogenesis in an in vitro culture system when it is embedded in a 3D matrix. Kidney growth varied depending on the density of the matrix; less dense matrices may have allowed for more growth because there was less material to remodel and break down. This view is supported by the observation that neither alginate nor puramatrix substrates (both of which are nonremodelable artificial matrices) supported significant growth.
FIG. 11.
Schematic of developmental approaches to in vitro engineering of kidney-like tissues. Note that methods for propagation of WD buds and branched UBs are discussed elsewhere.1,2 Color images available online at www.liebertonline.com/ten.
The adult kidney contains three distinct axes of growth, the medio-lateral axis (or cortico-medullary axis), dorso-ventral axis (the shortest dimension), and the rostro-caudal axis (longest dimension). However, it is currently unknown whether the function of the kidney is dependent on the different lengths of those axes or what causes the different axes of growth.66 The kidneys cultured in type IV or type I collagen did not appear to distinguish dorso-ventral and rostro-caudal axes of growth; rather, the kidneys appear round when looking down upon the kidney culture (Figs. 9 and 10). It may be that external factors during in vivo development, such as space constraints or soluble factors from nearby developing tissues, not present in the in vitro systems are responsible for those different axes of growth. Nevertheless, type IV collagen appeared to support the greatest tissue depth and the largest kidney volume and only rudiments cultured in this matrix possessed a 3D curvilinear branching outline (Figs. 9 and 10), or “umbrella shape”, similar to the in vivo developing kidney.67 However, while the total volume of the kidneys cultured in type IV collagen were larger than kidneys cultured in type I collagen or those cultured on a filter (Table 1), the volume of these kidneys only approached the volume of an E14.5 mouse kidney, reported to be 0.25 ± 0.02 mm3.67 Moreover, while the branching pattern of the kidneys in type IV collagen appeared to grossly resemble the in vivo morphology, the branches were significantly shorter than those seen in two-dimensional filter kidney cultures. This maybe a result of the lack of nutrients penetrating through the tissue and reaching the inner branches of the kidney that normally elongate during the development process. This potential explanation is supported by the observation that kidneys cultured on a filter, which grew much further from the origin of branching, were only 79 ± 2 μm thick (Table 1). However, it may be possible for additional growth factors and known branching promoters to be added to the culture and alleviate this problem.
Table 1.
Morphometric Measurements of the Volume and Minimum and Maximum Diameters (FERET) made on Cultured Embryonic Kidneys Grown Either Directly on a Transwell Filter or Embedded within Collagen I or Collagen IV
| |
|
FERET |
||
|---|---|---|---|---|
| Volume, mm3 | Maximum | Minimum | Ratio | |
| Filter | 0.12 ± 0.02 | 2100 ± 250 | 79 ± 2 | 27 ± 4 |
| Collagen I | 0.184 ± 0.008 | 1030 ± 20 | 371 ± 7 | 2.79 ± 0.08 |
| Collagen IV | 0.22 ± 0.02 | 910 ± 40 | 600 ± 8 | 1.51 ± 0.07 |
Although animal-derived matrices may not be suitable for use in human renal therapy because of a potential immune response, these studies reveal that 3D rudiment culture is possible when suspended in a compatible matrix. With the advent of matrix metalloproteinase (MMP)-sensitive artificial matrices, it may be possible to culture progenitor tissues in an artificial scaffold that allows for 3D growth and is biocompatible. Additionally, hyaluronic acid, which is often used in tissue engineering for creating artificial scaffolds, has been demonstrated to enhance in vitro kidney growth and development.17 Thus, a hyaluronic-acid-based MMP-sensitive scaffold may represent a potential artificial scaffold that could simultaneously support and enhance 3D kidney growth and development.
The goal of this type of research is to be able to construct from cells a kidney or kidney-like tissue that can be implanted as a therapy for those with decreased renal function and without causing rejection. The results described here support the hypothesis that it is ultimately possible to construct in vitro engineered kidney tissues from cells alone following the developmental strategy described here.
Acknowledgments
This work was supported by NIH Grants DK057286, DK065831, and HL035018 (to S.K.N.). E.R. was supported by the NSF graduate research fellowship. GFP-expressing transgenic mice were the generous gift from Dr. Frank Costantini. We would like to thank the UCSD Neuroscience microscopy shared facility and NINDS Grant NS047101 for providing us access to their 2-photon imaging facilities.
Disclosure Statement
No competing financial interests exist.
References
- 1.Rosines E. Sampogna R.V. Johkura K. Vaughn D.A. Choi Y. Sakurai H. Shah M.M. Nigam S.K. Staged in vitro reconstitution and implantation of engineered rat kidney tissue. Proc Natl Acad Sci USA. 2007;104:20938. doi: 10.1073/pnas.0710428105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steer D.L. Bush K.T. Meyer T.N. Schwesinger C. Nigam S.K. A strategy for in vitro propagation of rat nephrons. Kidney Int. 2002;62:1958. doi: 10.1046/j.1523-1755.2002.00694.x. [DOI] [PubMed] [Google Scholar]
- 3.OPTN. Annual report of the U.S. Organ procurement and transplantation network (OPTN) and the scientific registry of transplant recipients: Current U.S. Waiting list based on OPTN data as of April 2, 2010. (Dept. of Health and Human Services.) 2010.
- 4.Hammerman M.R. Xenotransplantation of pancreatic and kidney primordia—where do we stand? Transpl Immunol. 2009;21:93. doi: 10.1016/j.trim.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers S.A. Hammerman M.R. Transplantation of rat metanephroi into mice. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1865. doi: 10.1152/ajpregu.2001.280.6.R1865. [DOI] [PubMed] [Google Scholar]
- 6.Rogers S.A. Liapis H. Hammerman M.R. Transplantation of metanephroi across the major histocompatibility complex in rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R132. doi: 10.1152/ajpregu.2001.280.1.R132. [DOI] [PubMed] [Google Scholar]
- 7.Maeshima A. Sakurai H. Nigam S.K. Adult kidney tubular cell population showing phenotypic plasticity, tubulogenic capacity, and integration capability into developing kidney. J Am Soc Nephrol. 2006;17:188. doi: 10.1681/ASN.2005040370. [DOI] [PubMed] [Google Scholar]
- 8.Maeshima A. Sakurai H. Choi Y. Kitamura S. Vaughn D.A. Tee J.B. Nigam S.K. Glial cell-derived neurotrophic factor independent ureteric bud outgrowth from the wolffian duct. J Am Soc Nephrol. 2007;18:3147. doi: 10.1681/ASN.2007060642. [DOI] [PubMed] [Google Scholar]
- 9.Barasch J. Pressler L. Connor J. Malik A. A ureteric bud cell line induces nephrogenesis in two steps by two distinct signals. Am J Physiol. 1996;271:F50. doi: 10.1152/ajprenal.1996.271.1.F50. [DOI] [PubMed] [Google Scholar]
- 10.Rauchman M.I. Nigam S.K. Delpire E. Gullans S.R. An osmotically tolerant inner medullary collecting duct cell line from an sv40 transgenic mouse. Am J Physiol. 1993;265:F416. doi: 10.1152/ajprenal.1993.265.3.F416. [DOI] [PubMed] [Google Scholar]
- 11.Meyer T.N. Schwesinger C. Bush K.T. Stuart R.O. Rose D.W. Shah M.M. Vaughn D.A. Steer D.L. Nigam S.K. Spatiotemporal regulation of morphogenetic molecules during in vitro branching of the isolated ureteric bud: toward a model of branching through budding in the developing kidney. Dev Biol. 2004;275:44. doi: 10.1016/j.ydbio.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Qiao J. Sakurai H. Nigam S.K. Branching morphogenesis independent of mesenchymal-epithelial contact in the developing kidney. Proc Natl Acad Sci USA. 1999;96:7330. doi: 10.1073/pnas.96.13.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah M.M. Tee J.B. Meyer T. Meyer-Schwesinger C. Choi Y. Sweeney D.E. Gallegos T.F. Johkura K. Rosines E. Kouznetsova V. Rose D.W. Bush K.T. Sakurai H. Nigam S.K. The instructive role of metanephric mesenchyme in ureteric bud patterning, sculpting, and maturation and its potential ability to buffer ureteric bud branching defects. Am J Physiol Renal Physiol. 2009;297:F1330. doi: 10.1152/ajprenal.00125.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakurai H. Barros E.J. Tsukamoto T. Barasch J. Nigam S.K. An in vitro tubulogenesis system using cell lines derived from the embryonic kidney shows dependence on multiple soluble growth factors. Proc Natl Acad Sci USA. 1997;94:6279. doi: 10.1073/pnas.94.12.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levashova Z.B. Plisov S.Y. Perantoni A.O. Conditionally immortalized cell line of inducible metanephric mesenchyme. Kidney Int. 2003;63:2075. doi: 10.1046/j.1523-1755.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 16.Jainchill J.L. Aaronson S.A. Todaro G.J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969;4:549. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosines E. Schmidt H.J. Nigam S.K. The effect of hyaluronic acid size and concentration on branching morphogenesis and tubule differentiation in developing kidney culture systems: potential applications to engineering of renal tissues. Biomaterials. 2007;28:4806. doi: 10.1016/j.biomaterials.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grobstein C. Trans-filter induction of tubules in mouse metanephrogenic mesenchyme. Exp Cell Res. 1956;10:424. doi: 10.1016/0014-4827(56)90016-7. [DOI] [PubMed] [Google Scholar]
- 19.Grobstein C. Some transmission characteristics of the tubule-inducing influence on mouse metanephrogenic mesenchyme. Exp Cell Res. 1957;13:575. doi: 10.1016/0014-4827(57)90087-3. [DOI] [PubMed] [Google Scholar]
- 20.Grobstein C. Dalton A.J. Kidney tubule induction in mouse metanephrogenic mesenchyme without cytoplasmic contact. J Exp Zool. 1957;135:57. doi: 10.1002/jez.1401350106. [DOI] [PubMed] [Google Scholar]
- 21.Barasch J. Yang J. Ware C.B. Taga T. Yoshida K. Erdjument-Bromage H. Tempst P. Parravicini E. Malach S. Aranoff T. Oliver J.A. Mesenchymal to epithelial conversion in rat metanephros is induced by lif. Cell. 1999;99:377. doi: 10.1016/s0092-8674(00)81524-x. [DOI] [PubMed] [Google Scholar]
- 22.Carroll T.J. McMahon A.P. Secreted molecules in metanephric induction. J Am Soc Nephrol. 2000;11(Suppl 16):S116. [PubMed] [Google Scholar]
- 23.Herzlinger D. Qiao J. Cohen D. Ramakrishna N. Brown A.M. Induction of kidney epithelial morphogenesis by cells expressing wnt-1. Dev Biol. 1994;166:815. doi: 10.1006/dbio.1994.1360. [DOI] [PubMed] [Google Scholar]
- 24.Karihaloo A. Nickel C. Cantley L.G. Signals which build a tubule. Nephron Exp Nephrol. 2005;100:e40. doi: 10.1159/000084111. [DOI] [PubMed] [Google Scholar]
- 25.Karner C.M. Chirumamilla R. Aoki S. Igarashi P. Wallingford J.B. Carroll T.J. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41:793. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kispert A. Vainio S. McMahon A.P. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 1998;125:4225. doi: 10.1242/dev.125.21.4225. [DOI] [PubMed] [Google Scholar]
- 27.Plisov S.Y. Yoshino K. Dove L.F. Higinbotham K.G. Rubin J.S. Perantoni A.O. Tgf beta 2, lif and fgf2 cooperate to induce nephrogenesis. Development. 2001;128:1045. doi: 10.1242/dev.128.7.1045. [DOI] [PubMed] [Google Scholar]
- 28.Sariola H. Nephron induction. Nephrol Dial Transplant. 2002;17(Suppl 9):88. doi: 10.1093/ndt/17.suppl_9.88. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt-Ott K.M. Barasch J. Wnt/beta-catenin signaling in nephron progenitors and their epithelial progeny. Kidney Int. 2008;74:1004. doi: 10.1038/ki.2008.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stark K. Vainio S. Vassileva G. McMahon A.P. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by wnt-4. Nature. 1994;372:679. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- 31.Vainio S.J. Nephrogenesis regulated by wnt signaling. J Nephrol. 2003;16:279. [PubMed] [Google Scholar]
- 32.Vainio S.J. Itaranta P.V. Perasaari J.P. Uusitalo M.S. Wnts as kidney tubule inducing factors. Int J Dev Biol. 1999;43:419. [PubMed] [Google Scholar]
- 33.Vainio S.J. Uusitalo M.S. A road to kidney tubules via the wnt pathway. Pediatr Nephrol. 2000;15:151. doi: 10.1007/s004670000404. [DOI] [PubMed] [Google Scholar]
- 34.Barros E.J. Santos O.F. Matsumoto K. Nakamura T. Nigam S.K. Differential tubulogenic and branching morphogenetic activities of growth factors: implications for epithelial tissue development. Proc Natl Acad Sci USA. 1995;92:4412. doi: 10.1073/pnas.92.10.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cantley L.G. Barros E.J. Gandhi M. Rauchman M. Nigam S.K. Regulation of mitogenesis, motogenesis, and tubulogenesis by hepatocyte growth factor in renal collecting duct cells. Am J Physiol. 1994;267:F271. doi: 10.1152/ajprenal.1994.267.2.F271. [DOI] [PubMed] [Google Scholar]
- 36.Derman M.P. Cunha M.J. Barros E.J. Nigam S.K. Cantley L.G. Hgf-mediated chemotaxis and tubulogenesis require activation of the phosphatidylinositol 3-kinase. Am J Physiol. 1995;268:F1211. doi: 10.1152/ajprenal.1995.268.6.F1211. [DOI] [PubMed] [Google Scholar]
- 37.Sakurai H. Nigam S.K. Transforming growth factor-beta selectively inhibits branching morphogenesis but not tubulogenesis. Am J Physiol. 1997;272:F139. doi: 10.1152/ajprenal.1997.272.1.F139. [DOI] [PubMed] [Google Scholar]
- 38.Sakurai H. Tsukamoto T. Kjelsberg C.A. Cantley L.G. Nigam S.K. Egf receptor ligands are a large fraction of in vitro branching morphogens secreted by embryonic kidney. Am J Physiol. 1997;273:F463. doi: 10.1152/ajprenal.1997.273.3.F463. [DOI] [PubMed] [Google Scholar]
- 39.Ojeda J.L. Ros M.A. Icardo J.M. Lectin-binding sites during postnatal differentiation of normal and cystic rabbit renal corpuscles. Anat Embryol (Berl) 1993;187:539. doi: 10.1007/BF00214432. [DOI] [PubMed] [Google Scholar]
- 40.Pohl M. Sakurai H. Stuart R.O. Nigam S.K. Role of hyaluronan and cd44 in in vitro branching morphogenesis of ureteric bud cells. Dev Biol. 2000;224:312. doi: 10.1006/dbio.2000.9783. [DOI] [PubMed] [Google Scholar]
- 41.Zent R. Bush K.T. Pohl M.L. Quaranta V. Koshikawa N. Wang Z. Kreidberg J.A. Sakurai H. Stuart R.O. Nigam S.K. Involvement of laminin binding integrins and laminin-5 in branching morphogenesis of the ureteric bud during kidney development. Dev Biol. 2001;238:289. doi: 10.1006/dbio.2001.0391. [DOI] [PubMed] [Google Scholar]
- 42.Laitinen L. Virtanen I. Saxen L. Changes in the glycosylation pattern during embryonic development of mouse kidney as revealed with lectin conjugates. J Histochem Cytochem. 1987;35:55. doi: 10.1177/35.1.3794309. [DOI] [PubMed] [Google Scholar]
- 43.Meyer T.N. Schwesinger C. Sampogna R.V. Vaughn D.A. Stuart R.O. Steer D.L. Bush K.T. Nigam S.K. Rho kinase acts at separate steps in ureteric bud and metanephric mesenchyme morphogenesis during kidney development. Differentiation. 2006;74:638. doi: 10.1111/j.1432-0436.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- 44.Sakurai H. Bush K.T. Nigam S.K. Identification of pleiotrophin as a mesenchymal factor involved in ureteric bud branching morphogenesis. Development. 2001;128:3283. doi: 10.1242/dev.128.17.3283. [DOI] [PubMed] [Google Scholar]
- 45.Rogers S.A. Lowell J.A. Hammerman N.A. Hammerman M.R. Transplantation of developing metanephroi into adult rats. Kidney Int. 1998;54:27. doi: 10.1046/j.1523-1755.1998.00971.x. [DOI] [PubMed] [Google Scholar]
- 46.Steer D.L. Nigam S.K. Developmental approaches to kidney tissue engineering. Am J Physiol Renal Physiol. 2004;286:F1. doi: 10.1152/ajprenal.00167.2003. [DOI] [PubMed] [Google Scholar]
- 47.Lanza R.P. Chung H.Y. Yoo J.J. Wettstein P.J. Blackwell C. Borson N. Hofmeister E. Schuch G. Soker S. Moraes C.T. West M.D. Atala A. Generation of histocompatible tissues using nuclear transplantation. Nat Biotechnol. 2002;20:689. doi: 10.1038/nbt703. [DOI] [PubMed] [Google Scholar]
- 48.Kim D. Dressler G.R. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol. 2005;16:3527. doi: 10.1681/ASN.2005050544. [DOI] [PubMed] [Google Scholar]
- 49.De Coppi P. Bartsch G., Jr. Siddiqui M.M. Xu T. Santos C.C. Perin L. Mostoslavsky G. Serre A.C. Snyder E.Y. Yoo J.J. Furth M.E. Soker S. Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 50.Bussolati B. Bruno S. Grange C. Buttiglieri S. Deregibus M.C. Cantino D. Camussi G. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166:545. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dekel B. Burakova T. Arditti F.D. Reich-Zeliger S. Milstein O. Aviel-Ronen S. Rechavi G. Friedman N. Kaminski N. Passwell J.H. Reisner Y. Human and porcine early kidney precursors as a new source for transplantation. Nat Med. 2003;9:53. doi: 10.1038/nm812. [DOI] [PubMed] [Google Scholar]
- 52.Dekel B. Zangi L. Shezen E. Reich-Zeliger S. Eventov-Friedman S. Katchman H. Jacob-Hirsch J. Amariglio N. Rechavi G. Margalit R. Reisner Y. Isolation and characterization of nontubular sca-1 + lin- multipotent stem/progenitor cells from adult mouse kidney. J Am Soc Nephrol. 2006;17:3300. doi: 10.1681/ASN.2005020195. [DOI] [PubMed] [Google Scholar]
- 53.Gupta S. Verfaillie C. Chmielewski D. Kren S. Eidman K. Connaire J. Heremans Y. Lund T. Blackstad M. Jiang Y. Luttun A. Rosenberg M.E. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol. 2006;17:3028. doi: 10.1681/ASN.2006030275. [DOI] [PubMed] [Google Scholar]
- 54.Hishikawa K. Marumo T. Miura S. Nakanishi A. Matsuzaki Y. Shibata K. Ichiyanagi T. Kohike H. Komori T. Takahashi I. Takase O. Imai N. Yoshikawa M. Inowa T. Hayashi M. Nakaki T. Nakauchi H. Okano H. Fujita T. Musculin/myor is expressed in kidney side population cells and can regulate their function. J Cell Biol. 2005;169:921. doi: 10.1083/jcb.200412167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kale S. Karihaloo A. Clark P.R. Kashgarian M. Krause D.S. Cantley L.G. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003;112:42. doi: 10.1172/JCI17856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitamura S. Yamasaki Y. Kinomura M. Sugaya T. Sugiyama H. Maeshima Y. Makino H. Establishment and characterization of renal progenitor like cells from s3 segment of nephron in rat adult kidney. FASEB J. 2005;19:1789. doi: 10.1096/fj.05-3942com. [DOI] [PubMed] [Google Scholar]
- 57.Maeshima A. Yamashita S. Nojima Y. Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol. 2003;14:3138. doi: 10.1097/01.asn.0000098685.43700.28. [DOI] [PubMed] [Google Scholar]
- 58.Oliver J.A. Barasch J. Yang J. Herzlinger D. Al-Awqati Q. Metanephric mesenchyme contains embryonic renal stem cells. Am J Physiol Renal Physiol. 2002;283:F799. doi: 10.1152/ajprenal.00375.2001. [DOI] [PubMed] [Google Scholar]
- 59.Sagrinati C. Netti G.S. Mazzinghi B. Lazzeri E. Liotta F. Frosali F. Ronconi E. Meini C. Gacci M. Squecco R. Carini M. Gesualdo L. Francini F. Maggi E. Annunziato F. Lasagni L. Serio M. Romagnani S. Romagnani P. Isolation and characterization of multipotent progenitor cells from the bowman's capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 60.Dressler G.R. Advances in early kidney specification, development and patterning. Development. 2009;136:3863. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bush K.T. Sakurai H. Steer D.L. Leonard M.O. Sampogna R.V. Meyer T.N. Schwesinger C. Qiao J. Nigam S.K. Tgf-beta superfamily members modulate growth, branching, shaping, and patterning of the ureteric bud. Dev Biol. 2004;266:285. doi: 10.1016/j.ydbio.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 62.Qiao J. Bush K.T. Steer D.L. Stuart R.O. Sakurai H. Wachsman W. Nigam S.K. Multiple fibroblast growth factors support growth of the ureteric bud but have different effects on branching morphogenesis. Mech Dev. 2001;109:123. doi: 10.1016/s0925-4773(01)00592-5. [DOI] [PubMed] [Google Scholar]
- 63.Ishibe S. Karihaloo A. Ma H. Zhang J. Marlier A. Mitobe M. Togawa A. Schmitt R. Czyczk J. Kashgarian M. Geller D.S. Thorgeirsson S.S. Cantley L.G. Met and the epidermal growth factor receptor act cooperatively to regulate final nephron number and maintain collecting duct morphology. Development. 2009;136:337. doi: 10.1242/dev.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nelson C.M. Vanduijn M.M. Inman J.L. Fletcher D.A. Bissell M.J. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herzlinger D. Koseki C. Mikawa T. al-Awqati Q. Metanephric mesenchyme contains multipotent stem cells whose fate is restricted after induction. Development. 1992;114:565. doi: 10.1242/dev.114.3.565. [DOI] [PubMed] [Google Scholar]
- 66.Al-Awqati Q. Goldberg M.R. Architectural patterns in branching morphogenesis in the kidney. Kidney Int. 1998;54:1832. doi: 10.1046/j.1523-1755.1998.00196.x. [DOI] [PubMed] [Google Scholar]
- 67.Cebrian C. Borodo K. Charles N. Herzlinger D.A. Morphometric index of the developing murine kidney. Dev Dyn. 2004;231:601. doi: 10.1002/dvdy.20143. [DOI] [PubMed] [Google Scholar]