Abstract
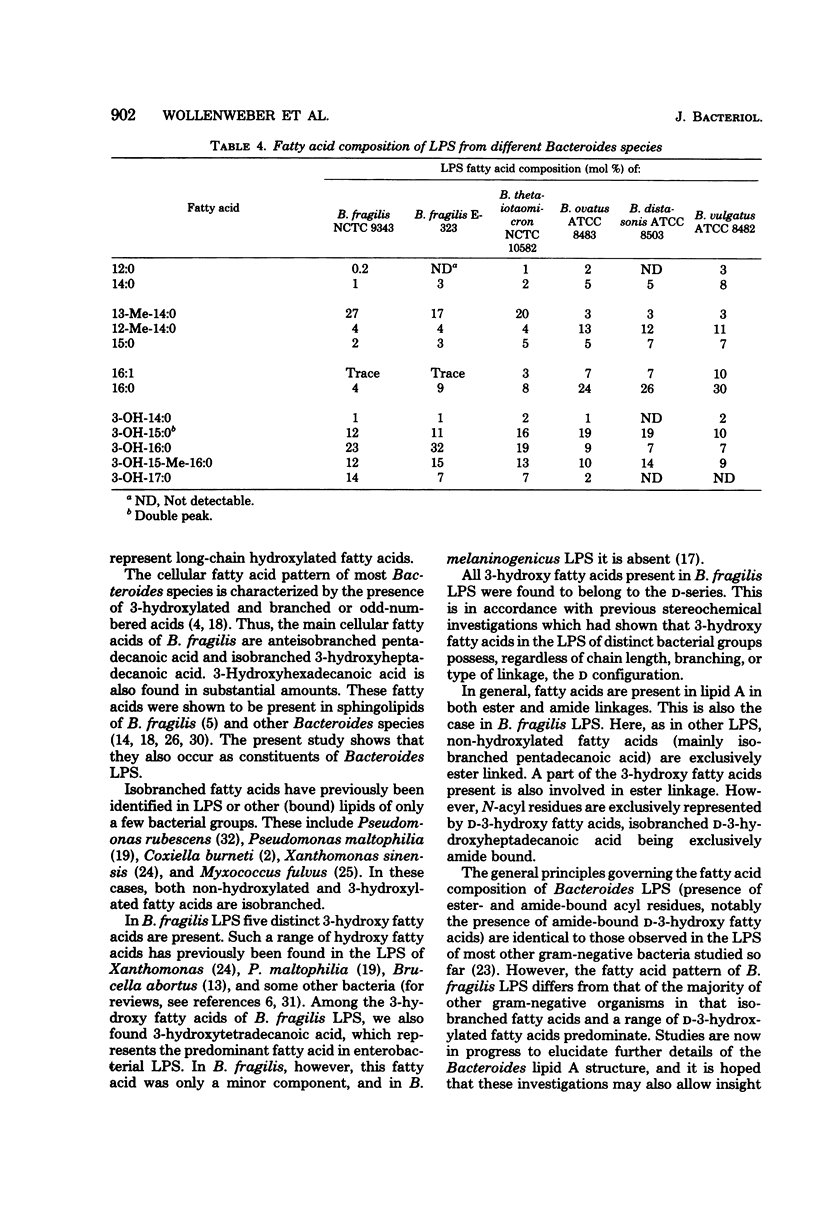
The main fatty acids present in lipopolysaccharides from Bacteroides fragilis NCTC 9343 were identified as 13-methyl-tetradecanoic, D-3-hydroxypentadecanoic, D-3-hydroxyhexadecanoic, D-3-hydroxy-15-methyl-hexadecanoic, and D-3-hydroxyheptadecanoic acids. Of these, 13-methyl-tetradecanoic acid is exclusively ester bound, and 3-hydroxy-15-methyl-hexadecanoic acid is exclusively involved in amide linkage. The other 3-hydroxy fatty acids are both ester and amide bound. All 3-hydroxy fatty acids possess the D configuration, and the 3-hydroxyl group of ester-linked 3-hydroxy fatty acids is not substituted. Lipopolysaccharides of related Bacteroides species (B. thetaiotaomicron, B. ovatus, B. distasonis, and B. vulgatus) showed a fatty acid spectrum with both similar and distinct features compared to that of B. fragilis lipopolysaccharides.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bzdega J., Meisel-Mikolajczyk F., Kubica J. Fatty acids in the endotoxins of various Bacteroides fragilis subspecies. Bull Acad Pol Sci Biol. 1977;25(1):21–25. [PubMed] [Google Scholar]
- Chan M. L., McChesney J., Paretsky D. Further characterization of a lipopolysaccharide from Coxiella burneti. Infect Immun. 1976 Jun;13(6):1721–1727. doi: 10.1128/iai.13.6.1721-1727.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche C. D. Zur Struktur der Lipoide des Genus Bacteroides. Zentralbl Bakteriol Orig A. 1974;228(1):100–106. [PubMed] [Google Scholar]
- Haeffner N., Chaby R., Szabó L. Identification of 2-methyl-3-hydroxydecanoic and 2-methyl-3-hydroxytetradecanoic acids in the 'lipid X' fraction of the Bordetella pertussis endotoxin. Eur J Biochem. 1977 Aug 1;77(3):535–544. doi: 10.1111/j.1432-1033.1977.tb11696.x. [DOI] [PubMed] [Google Scholar]
- Hofstad T. Chemical characteristics of Bacteroides melaninogenicus endotoxin. Arch Oral Biol. 1968 Sep;13(9):1149–1155. doi: 10.1016/0003-9969(68)90067-8. [DOI] [PubMed] [Google Scholar]
- Hofstad T., Kristoffersen T. Chemical characteristics of endotoxin from Bacteroides fragilis NCTC 9343. J Gen Microbiol. 1970 Apr;61(1):15–19. doi: 10.1099/00221287-61-1-15. [DOI] [PubMed] [Google Scholar]
- Hofstad T. O-antigenic specificity of lipopolysaccharides from Bacteroides fragilis ss. fragilis. Acta Pathol Microbiol Scand B. 1975 Oct;83(5):477–481. doi: 10.1111/j.1699-0463.1975.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Kasper D. L. Chemical and biological characterization of the lipopolysaccharide of Bacteroides fragilis subspecies fragilis. J Infect Dis. 1976 Jul;134(1):59–66. doi: 10.1093/infdis/134.1.59. [DOI] [PubMed] [Google Scholar]
- Kreutzer D. L., Buller C. S., Robertson D. C. Chemical characterization and biological properties of lipopolysaccharides isolated from smooth and rough strains of Brucella abortus. Infect Immun. 1979 Mar;23(3):811–818. doi: 10.1128/iai.23.3.811-818.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunsman J. E. Characterization of the lipids of six strains of Bacteroides ruminicola. J Bacteriol. 1973 Mar;113(3):1121–1126. doi: 10.1128/jb.113.3.1121-1126.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A., Holme T. Evaluation of some extraction methods for the preparation of bacterial lipopolysaccharides for structural analysis. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(5):751–759. doi: 10.1111/j.1699-0463.1972.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A., Weintraub A., Nord C. E. The humoral antibody response to Bacteroides fragilis infections in humans. Scand J Infect Dis Suppl. 1979;(19):46–51. [PubMed] [Google Scholar]
- Mansheim B. J., Onderdonk A. B., Kasper D. L. Immunochemical and biologic studies of the lipopolysaccharide of Bacteroides melaninogenicus subspecies asaccharolyticus. J Immunol. 1978 Jan;120(1):72–78. [PubMed] [Google Scholar]
- Moss C. W., Samuels S. B., Liddle J., McKinney R. M. Occurrence of branched-cahin hydroxy fatty acids in Pseudomonas maltophilia. J Bacteriol. 1973 Jun;114(3):1018–1024. doi: 10.1128/jb.114.3.1018-1024.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny A. Molecular aspects of endotoxic reactions. Bacteriol Rev. 1969 Mar;33(1):72–98. doi: 10.1128/br.33.1.72-98.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T. Absolute configuration of 3-hydroxy fatty acids present in lipopolysaccharides from various bacterial groups. Eur J Biochem. 1976 May 1;64(2):423–428. doi: 10.1111/j.1432-1033.1976.tb10318.x. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Gottert H., Lüderitz O., Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972 Jul 13;28(2):166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Lüderitz O., Volk W. A. Nature, type of linkage, and absolute configuration of (hydroxy) fatty acids in lipopolysaccharides from Xanthomonas sinensis and related strains. J Bacteriol. 1975 Jun;122(3):1180–1188. doi: 10.1128/jb.122.3.1180-1188.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfelder G., Lüderitz O., Westphal O. Composition of lipopolysaccharides from Myxococcus fulvus and other fruiting and non-fruiting myxobacteria. Eur J Biochem. 1974 May 15;44(2):411–420. doi: 10.1111/j.1432-1033.1974.tb03499.x. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Dittmar K., Wilmes R. Sphingolipid metabolism in Bacteroideaceae. Hoppe Seylers Z Physiol Chem. 1975 Jun;356(6):715–725. doi: 10.1515/bchm2.1975.356.s1.715. [DOI] [PubMed] [Google Scholar]
- Sveen K., Hofstad T., Milner K. C. Lethality for mice and chick embryos, pyrogenicity in rabbits and ability to gelate lysate from amoebocytes of Limulus polyphemus by lipopolysaccharides from Bacteroides, Fusobacterium and Veillonella. Acta Pathol Microbiol Scand B. 1977 Dec;85B(6):388–396. doi: 10.1111/j.1699-0463.1977.tb01994.x. [DOI] [PubMed] [Google Scholar]
- Sveen K. The capacity of lipopolysaccharides from bacteroides, fusobacterium and veillonella to produce skin inflammation and the local and generalized Shwartzman reaction in rabbits. J Periodontal Res. 1977 Sep;12(5):340–350. doi: 10.1111/j.1600-0765.1977.tb01525.x. [DOI] [PubMed] [Google Scholar]
- White D. C., Tucker A. N., Sweeley C. C. Characterization of the iso-branched sphinganines from the ceramide phospholipids of Bacteroides melaninogenicus. Biochim Biophys Acta. 1969 Dec 17;187(4):527–532. doi: 10.1016/0005-2760(69)90050-2. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. G., Galbraith L., Lightfoot G. A. Cell walls, lipids, and lipopolysaccharides of Pseudomonas species. Eur J Biochem. 1973 Feb 15;33(1):158–174. doi: 10.1111/j.1432-1033.1973.tb02666.x. [DOI] [PubMed] [Google Scholar]


