Abstract
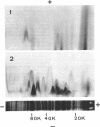
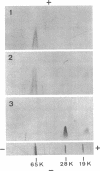
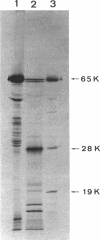
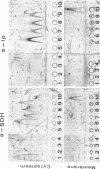
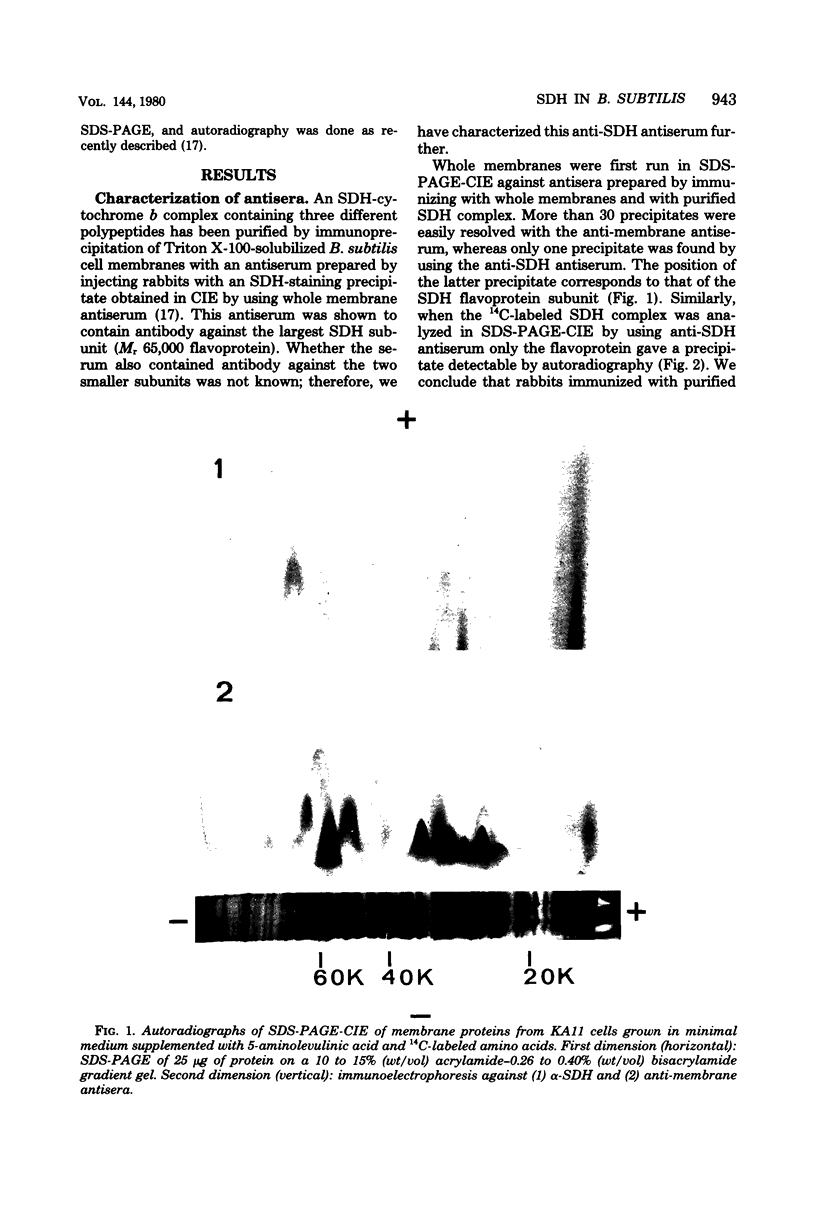
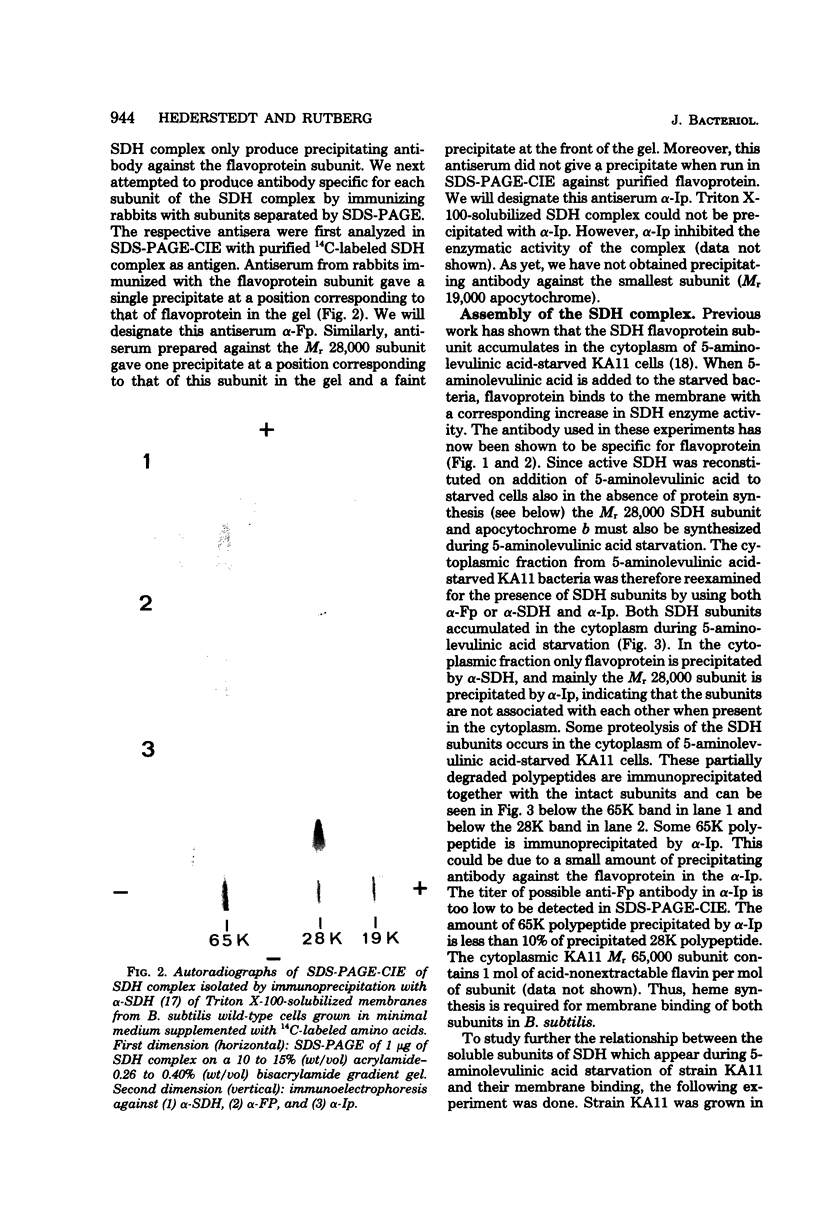
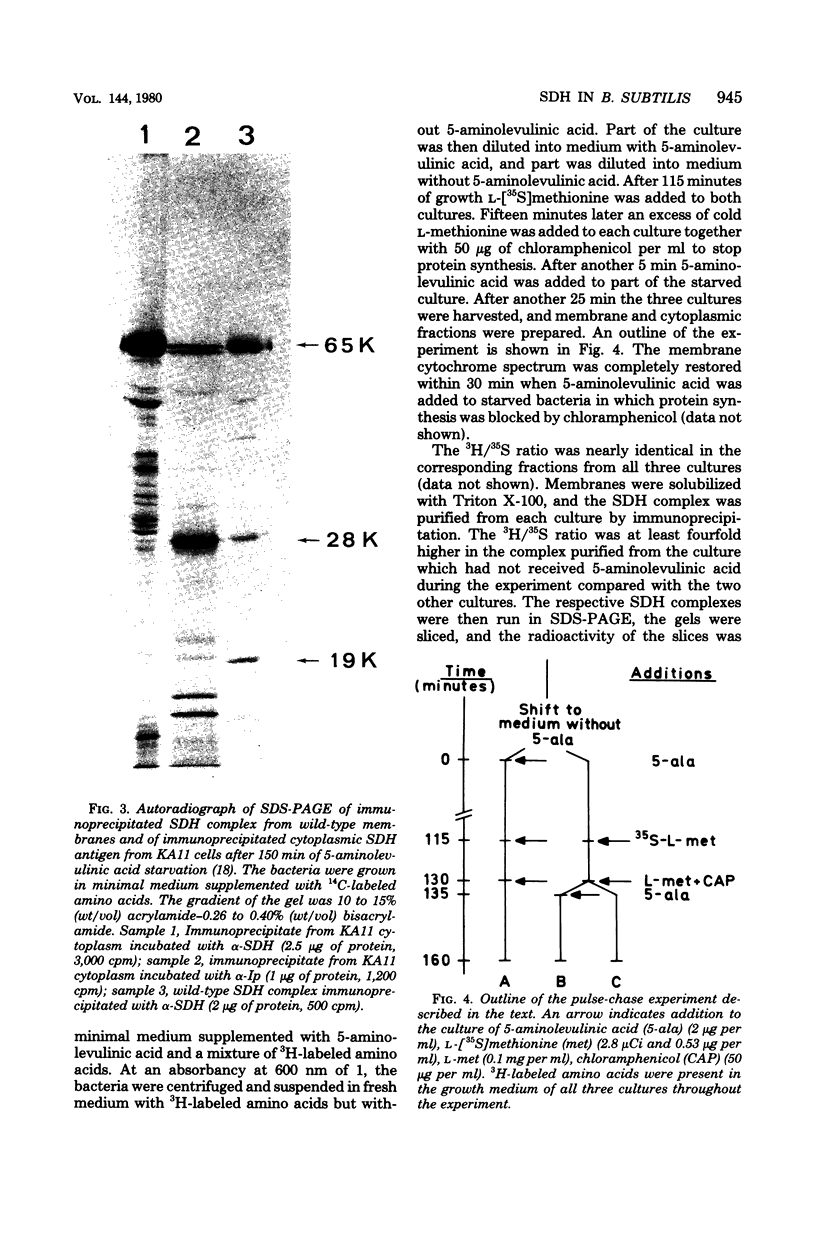
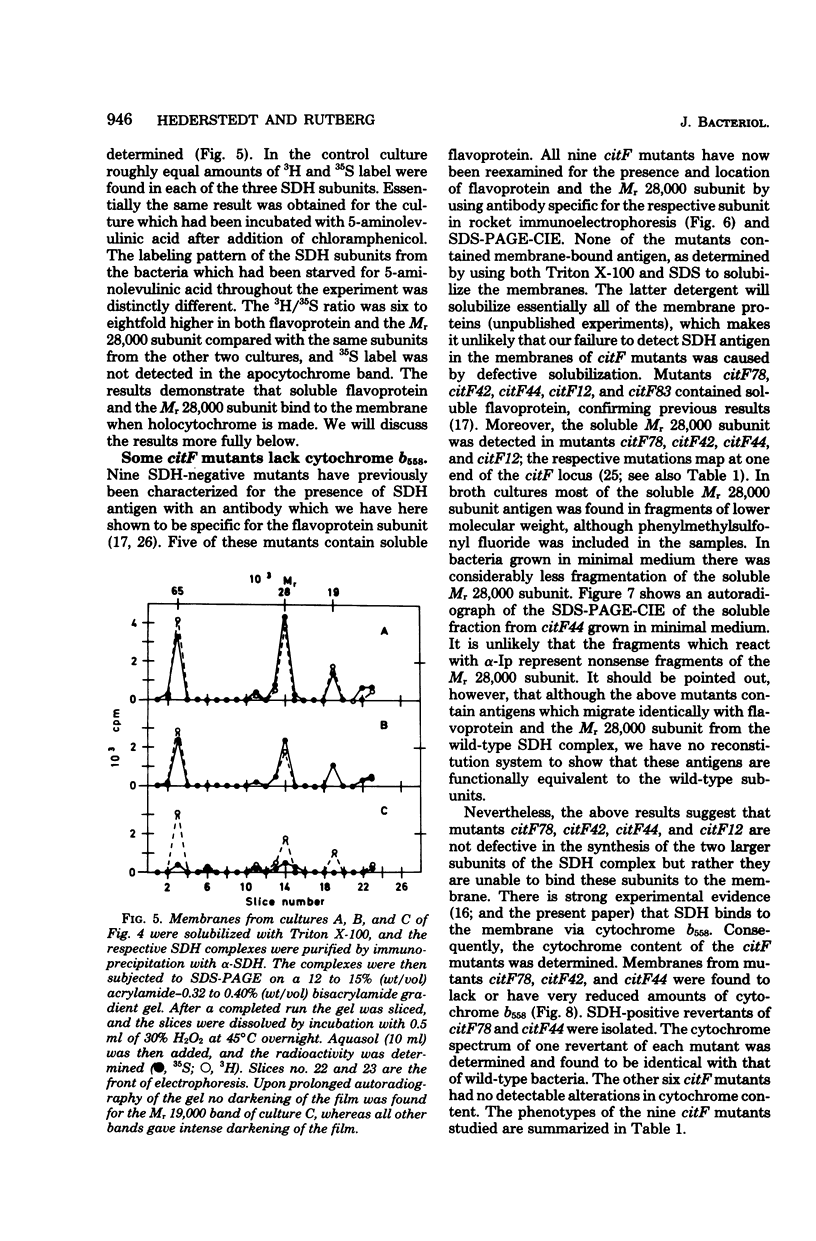
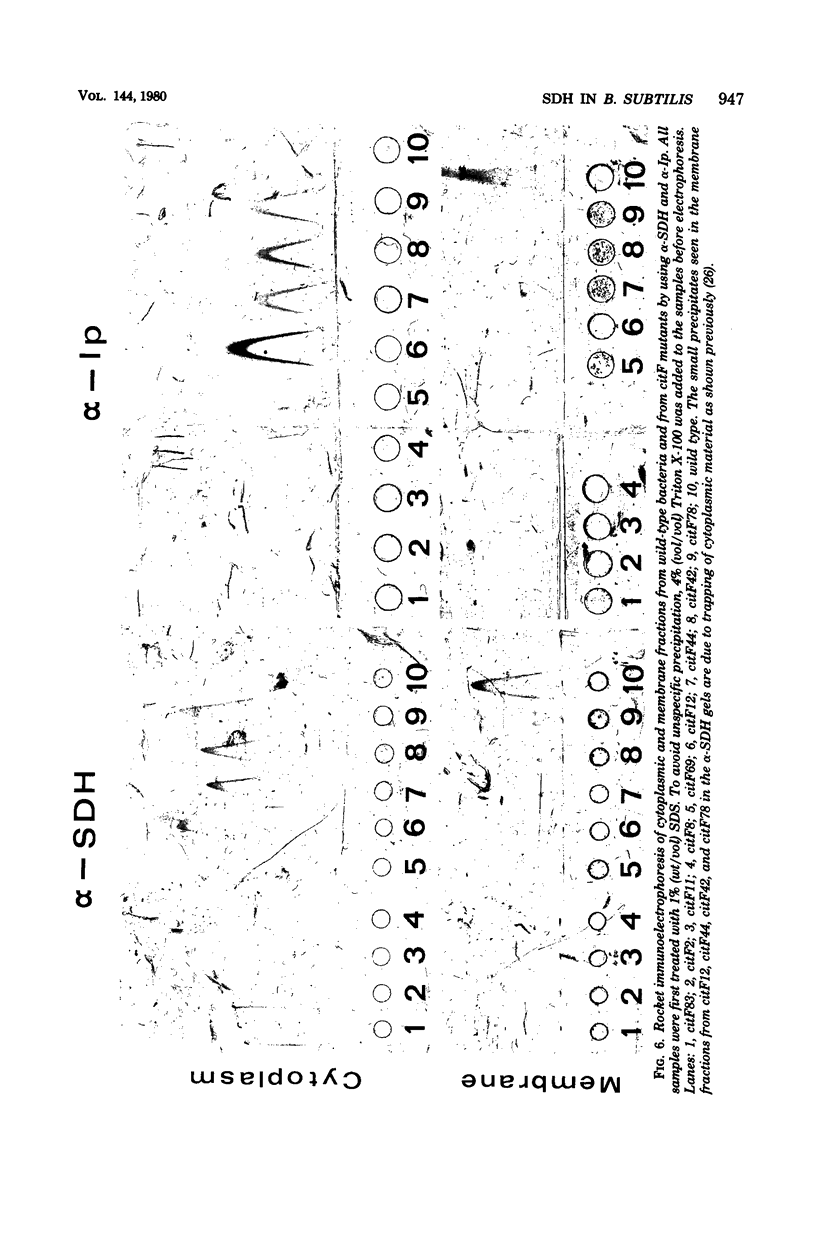
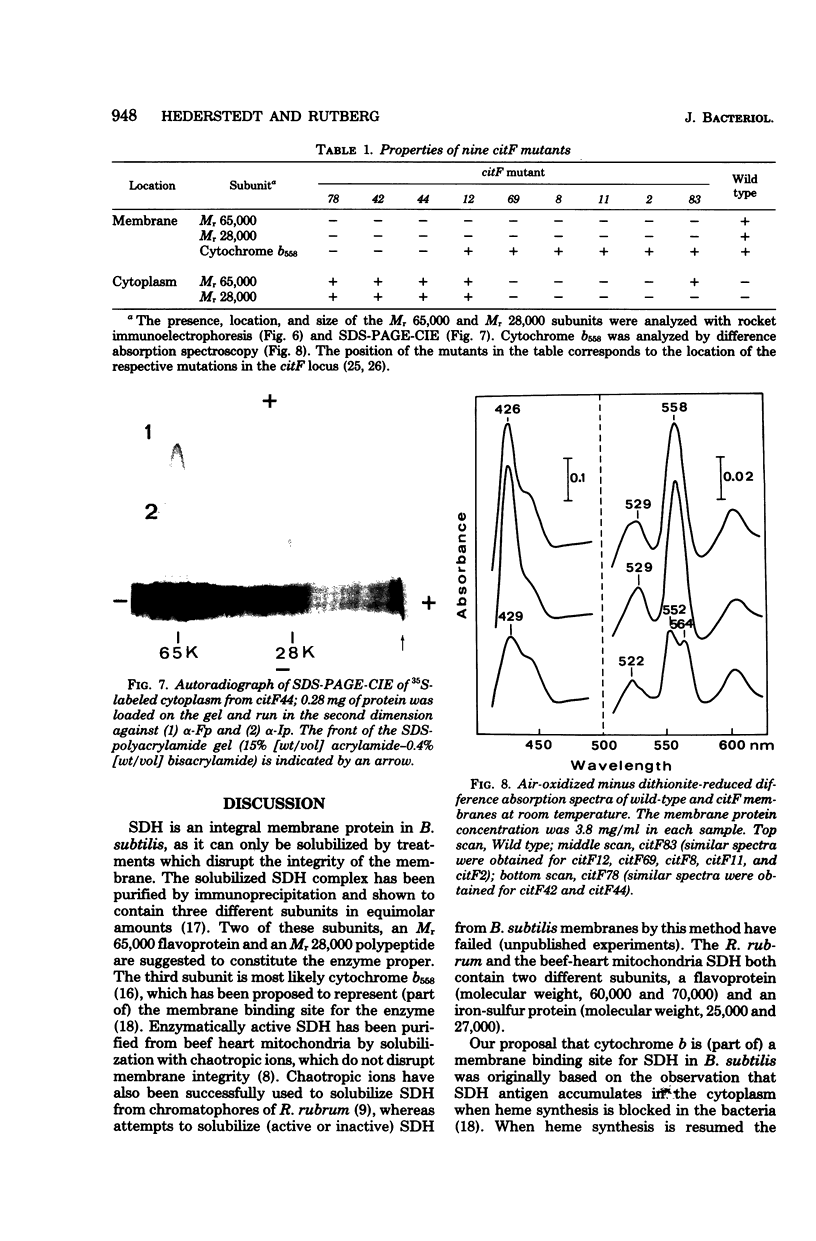
Antibodies specific for the Mr 65,000 (flavoprotein) and the Mr 28,000 subunits of the succinic dehydrogenase (SDH) of Bacillus subtilis were obtained. By using these antibodies it was shown that both subunits accumulated in the cytoplasm during 5-aminolevulinic acid starvation of a 5-aminolevulinic acid auxotroph. In the cytoplasm the subunits were not associated since they precipitated essentially independently of each other with subunit-specific antibody. In sodium dodecyl sulfate-polyacrylamide gel electrophoresis the cytoplasmic subunits migrated identically with the corresponding subunits from the purified membrane-bound SDH complex. Cytoplasmic subunits were pulse-labeled with L-[35S]methionine during 5-aminolevulinic acid starvation. The labeled subunits bound to the membrane when heme synthesis was resumed and also when protein synthesis was blocked by chloramphenicol before readdition of 5-aminolevulinic acid. The experiments thus demonstrated a precursor relationship between cytoplasmic subunits and the subunits of the membrane-bound SDH complex. All SDH-negative mutants isolated so far carry mutations in the citF locus. None of the mutants was found to have either the Mr 65,000 or the Mr 28,000 SDH subunits in the membrane. Four citF mutants, however, contained both subunits in the cytoplasm. Three of these mutants lacked spectrally detectable cytochrome b558. The respective mutations mapped at one end of the citF locus. These results strongly support our previous suggestion that cytochrome b558 is (part of) a membrane binding site for SDH in B. subtilis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baginsky M. L., Hatefi Y. Reconstitution of succinate-coenzyme Q reductase (complex II) and succinate oxidase activities by a highly purified, reactivated succinate dehydrogenase. J Biol Chem. 1969 Oct 10;244(19):5313–5319. [PubMed] [Google Scholar]
- Bjerrum O. J. Immunochemical investigation of membrane proteins. A methodological survey with emphasis placed on immunoprecipitation in gels. Biochim Biophys Acta. 1977 Aug 9;472(2):135–195. doi: 10.1016/0304-4157(77)90016-8. [DOI] [PubMed] [Google Scholar]
- Bruni A., Racker E. Resolution and reconstitution of the mitochondrial electron transport system. I. Reconstitution of the succinate-ubiquinone reductase. J Biol Chem. 1968 Mar 10;243(5):962–971. [PubMed] [Google Scholar]
- Capaldi R. A., Sweetland J., Merli A. Polypeptides in the succinate-coenzyme Q reductase segment of the respiratory chain. Biochemistry. 1977 Dec 27;16(26):5707–5710. doi: 10.1021/bi00645a009. [DOI] [PubMed] [Google Scholar]
- Carls R. A., Hanson R. S. Isolation and characterization of tricarboxylic acid cycle mutants of Bacillus subtilis. J Bacteriol. 1971 Jun;106(3):848–855. doi: 10.1128/jb.106.3.848-855.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Blomberg F. Immunochemical studies of thylakoid membrane polypeptides from spinach and Chlamydomonas reinhardtii. A modified procedure for crossed immunoelectrophoresis of dodecyl sulfate.protein complexes. J Biol Chem. 1979 Jan 10;254(1):215–223. [PubMed] [Google Scholar]
- Cole S. T., Guest J. R. Production of a soluble form of fumarate reductase by multiple gene duplication in Escherichia coli K12. Eur J Biochem. 1979 Dec;102(1):65–71. doi: 10.1111/j.1432-1033.1979.tb06263.x. [DOI] [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y. Succinate dehydrogenase. I. Purification, molecular properties, and substructure. Biochemistry. 1971 Jun 22;10(13):2509–2516. doi: 10.1021/bi00789a014. [DOI] [PubMed] [Google Scholar]
- Dickie P., Weiner J. H. Purification and characterization of membrane-bound fumarate reductase from anaerobically grown Escherichia coli. Can J Biochem. 1979 Jun;57(6):813–821. doi: 10.1139/o79-101. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm H. H., Decker K. FAD is covalently attached to peptidyl-tRNA during cell-free synthesis of 6-hydroxy-D-nicotine oxidase. Eur J Biochem. 1978 Dec;92(2):449–454. doi: 10.1111/j.1432-1033.1978.tb12766.x. [DOI] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Hederstedt L. Cytochrome b reducible by succinate in an isolated succinate dehydrogenase-cytochrome b complex from Bacillus subtilis membranes. J Bacteriol. 1980 Dec;144(3):933–940. doi: 10.1128/jb.144.3.933-940.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstedt L., Holmgren E., Rutberg L. Characterization of a succinate dehydrogenase complex solubilized from the cytoplasmic membrane of Bacillus subtilis with the nonionic detergent Triton X-100. J Bacteriol. 1979 May;138(2):370–376. doi: 10.1128/jb.138.2.370-376.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren E., Hederstedt L., Rutberg L. Role of heme in synthesis and membrane binding of succinic dehydrogenase in Bacillus subtilis. J Bacteriol. 1979 May;138(2):377–382. doi: 10.1128/jb.138.2.377-382.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James G. T. Inactivation of the protease inhibitor phenylmethylsulfonyl fluoride in buffers. Anal Biochem. 1978 Jun 1;86(2):574–579. doi: 10.1016/0003-2697(78)90784-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacGregor C. H. Anaerobic cytochrome b1 in Escherichia coli: association with and regulation of nitrate reductase. J Bacteriol. 1975 Mar;121(3):1111–1116. doi: 10.1128/jb.121.3.1111-1116.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H. Biosynthesis of membrane-bound nitrate reductase in Escherichia coli: evidence for a soluble precursor. J Bacteriol. 1976 Apr;126(1):122–131. doi: 10.1128/jb.126.1.122-131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. II. Integration of membrane proteins in the absence of lipid synthesis. J Mol Biol. 1970 Apr 28;49(2):433–439. doi: 10.1016/0022-2836(70)90255-x. [DOI] [PubMed] [Google Scholar]
- Ohné M., Rutberg B., Hoch J. A. Genetic and biochemical characterization of mutants of Bacillus subtilis defective in succinate dehydrogenase. J Bacteriol. 1973 Sep;115(3):738–745. doi: 10.1128/jb.115.3.738-745.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg B., Hederstedt L., Holmgren E., Rutberg L. Characterization of succinic dehydrogenase mutants of Bacillus subtilis by crossed immunoelectrophoresis. J Bacteriol. 1978 Oct;136(1):304–311. doi: 10.1128/jb.136.1.304-311.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G. Biogenesis of yeast mitochondria: synthesis of cytochrome c oxidase and cytochrome c. Methods Enzymol. 1979;56:40–50. doi: 10.1016/0076-6879(79)56007-8. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyhach R. J., Hawrot E., Satre M., Kennedy E. P. Increased synthesis of phosphatidylserine decarboxylase in a strain of Escherichia coli bearing a hybrid plasmid. Altered association of enzyme with the membrane. J Biol Chem. 1979 Feb 10;254(3):627–633. [PubMed] [Google Scholar]
- WILSON D. F., KING T. E. THE DETERMINATION OF ACID-NONEXTRACTABLE FLAVIN IN MITOCHONDRIAL PREPARATIONS FROM HEART MUSCLE. J Biol Chem. 1964 Aug;239:2683–2690. [PubMed] [Google Scholar]
- Weiss H., Kolb H. J. Isolation of mitochondrial succinate: ubiquinone reductase, cytochrome c reductase and cytochrome c oxidase from Neurospora crassa using nonionic detergent. Eur J Biochem. 1979 Aug 15;99(1):139–149. doi: 10.1111/j.1432-1033.1979.tb13240.x. [DOI] [PubMed] [Google Scholar]
- Weiss H., Wingfield P. Enzymology of ubiquinone-utilizing electron transfer complexes in nonionic detergent. Eur J Biochem. 1979 Aug 15;99(1):151–160. doi: 10.1111/j.1432-1033.1979.tb13241.x. [DOI] [PubMed] [Google Scholar]
- Young I. G., Jaworowski A., Poulis M. I. Amplification of the respiratory NADH dehydrogenase of Escherichia coli by gene cloning. Gene. 1978 Sep;4(1):25–36. doi: 10.1016/0378-1119(78)90012-4. [DOI] [PubMed] [Google Scholar]