Abstract
S-nitrosylation, the selective posttranslational modification of protein cysteine residues to form S-nitrosocysteine, is one of the molecular mechanisms by which nitric oxide influences diverse biological functions. In this study, unique MS-based proteomic approaches precisely pinpointed the site of S-nitrosylation in 328 peptides in 192 proteins endogenously modified in WT mouse liver. Structural analyses revealed that S-nitrosylated cysteine residues were equally distributed in hydrophobic and hydrophilic areas of proteins with an average predicted pKa of 10.01 ± 2.1. S-nitrosylation sites were over-represented in α-helices and under-represented in coils as compared with unmodified cysteine residues in the same proteins (χ2 test, P < 0.02). A quantile–quantile probability plot indicated that the distribution of S-nitrosocysteine residues was skewed toward larger surface accessible areas compared with the unmodified cysteine residues in the same proteins. Seventy percent of the S-nitrosylated cysteine residues were surrounded by negatively or positively charged amino acids within a 6-Å distance. The location of cysteine residues in α-helices and coils in highly accessible surfaces bordered by charged amino acids implies site directed S-nitrosylation mediated by protein–protein or small molecule interactions. Moreover, 13 modified cysteine residues were coordinated with metals and 15 metalloproteins were endogenously modified supporting metal-catalyzed S-nitrosylation mechanisms. Collectively, the endogenous S-nitrosoproteome in the liver has structural features that accommodate multiple mechanisms for selective site-directed S-nitrosylation.
Keywords: cysteine modification, nitric oxide, S-nitrosation, posttranslational modification, proteomics
Cysteine S-nitrosylation is a reversible and apparently selective posttranslational protein modification that regulates protein activity, localization, and stability within a variety of organs and cellular systems (1–6). Despite the considerable biological importance of this posttranslational modification, significant gaps exist regarding its in vivo specificity and origin. The identification of in vivo S-nitrosylated proteins has indicated that not all reduced cysteine residues and not all proteins with reduced cysteine residues are modified, implying a biased selection. Several biological chemistries have been proposed to account for the S-nitrosylation of proteins in vivo (1, 7, 8). Broadly, these include (i) oxidative S-nitrosation by higher oxides of NO, (ii) transnitrosylation by small molecular weight NO carriers such as S-nitrosoglutathione or dinitrosyliron complexes, (iii) catalysis by metalloproteins, and (iv) protein-assisted transnitrosation, as elegantly documented for the S-nitrosylation of caspase-3 by S-nitrosothioredoxin (9, 10). With the exception of the protein-assisted transnitrosylation and metalloprotein catalyzed S-nitrosylation, which we presume necessitates protein–protein interaction, the other proposed mechanisms are rather nondiscriminatory unless the microenvironment of selective cysteine residues in proteins can specifically accommodate these chemical modifications. Therefore, structural interrogation of endogenous S-nitrosylated proteins with site specific identification of the modified cysteine residues could provide valuable insights to appreciate the biological selectivity of this posttranslational modification. Attempts to investigate this very important biological question have not been possible largely because datasets of in vivo modified proteins are not available (1, 11). Previous structural analyses have been attempted using limited data sets or by including all sites of modification identified after exposing tissues or cells to S-nitrosylating agents (11). However, as the authors of these articles have indicated, these sites of modification represent putative sites but not necessarily those modified in vivo (11). We used organomercury reagents that react directly, efficiently, and specifically with S-nitrosocysteine and thus enable the precise identification of S-nitrosocysteine–containing peptides and independently S-nitrosylated proteins to assemble the in vivo S-nitrosocysteine proteome of the mouse liver. By using bioinformatic tools, we then interrogated this enriched endogenous S-nitrosocysteine proteome to define the biochemical, biophysical, and structural environment of the cysteine residues modified by S-nitrosylation, elements that might inform on how specificity of S-nitrosylation is achieved.
Results and Discussion
Complementary MS-Based Proteomics Identify the Endogenous Liver S-Nitrosocysteine Proteome.
The reaction of phenylmercury compounds with S-nitrosocysteine results in the formation of a relatively stable thiol–mercury bond (12). Therefore, we used an organomercury resin (MRC) synthesized by conjugation of ρ-amino-phenylmercuric acetate to N-hydroxysuccinimide–activated Affi-Gel 10 agarose beads and a phenylmercury-polyethyleneglycol-biotin (mPEGb) compound to capture S-nitrosylated proteins and peptides (Fig. S1). The method consists of three steps: (i) blocking of reduced cysteine residues with methyl methanethiosulfonate (MMTS), (ii) capture and release of S-nitrosylated proteins or peptides, and (iii) liquid chromatography/tandem MS analysis. β-Mercaptoethanol or performic acid was used to release captured proteins or peptides, respectively. Mild performic acid was used to selectively and quantitatively release the bound peptides and more importantly oxidize cysteine thiols to sulfonic acid, thereby creating a unique MS signature that permits site-specific identification of the modified cysteine residues (13). Under the workflow used (Fig. S1), 100% of cysteine-containing peptides were detected with the sulfonic acid modification.
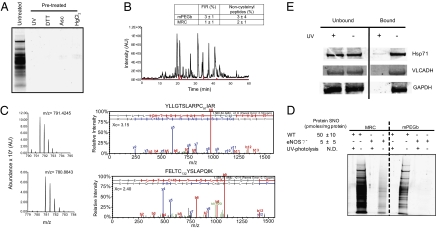
To control for the inclusion of false-positive protein S-nitrosocysteine that may result from incomplete blocking of reduced cysteine residues or nonspecific interactions with the resins during enrichment, pretreatment with UV, DTT, and copper-ascorbate (Cu-Asc) reduction or reaction with mercury chloride were used to displace NO before the reaction with the phenylmercury compounds (Fig. 1A and Fig. S2A). For these experiments, we used mouse liver homogenates with 50 ± 10 pmol protein S-nitrosocysteine per milligram of protein as quantified by reductive chemistries coupled to ozone-based chemiluminescence. Displacement of NO from S-nitrosocysteine residues by pretreatment with UV, DTT, Cu-Asc, or mercury chloride decreased protein S-nitrosocysteine levels by greater than 99% as quantified by chemiluminescence (n = 3) and also eliminated reaction with the phenylmercury compounds (Fig. 1A and Fig. S2A). On average, only 3% of peptides (Fig. 1B) and 5% of proteins (Fig. S2B) were identified as false positives (present in untreated and UV-pretreated samples), demonstrating that the method maintains high specificity at each step. This false positive rate compares favorably with the greater than 90% specificity reported for immobilized metal affinity chromatography used for the enrichment of phosphopeptides from mouse liver (14). Therefore, pretreatment with UV served as negative control throughout based on previous studies that documented the elimination of S-nitrosocysteine without affecting other cysteine modifications (15, 16). Collectively, these experiments showed that the reaction of protein S-nitrosocysteine with the phenylmercury compounds is specific and efficient and achieved selective identification of the modified residue, the ultimate qualifier for the unambiguous assignment of S-nitrosylated proteins through the inclusion of negative controls.
Fig. 1.
Site-specific identification and UV photolysis confirmation of S-nitrosocysteine. (A) Representative short (approximately 2 cm) colloidal blue-stained protein gel of mPEGb protein capture from WT mouse liver that was processed by GeLC-MS/MS analysis. Colloidal blue protein staining of bound fractions from mPEGb protein capture of untreated liver homogenates showed protein enrichment. Pretreatment with UV (for 30 min), DTT (10 mM), Cu-Asc (5 mM Asc/copper 0.1 mM), or HgCl2 (20 mM) prevented this capture, demonstrating specificity for protein S-nitrosocysteine. (B) Representative base peak chromatograms from mPEGb peptide capture demonstrates reduction in ion intensity after UV treatment (red) compared with the untreated sample (black). For clarity, the chromatogram corresponding to UV treatment was plotted with a y-axis offset of 1E4. Inset: Average false-positive identification rate (FIR ± SD) from mPEGb and MRC represented the percentage of peptides that were identified in both the UV-treated and untreated samples across three independent biological replicates. Also reported is the percent of noncysteinyl peptides identified (SI Materials and Methods). (C) Representative MS spectra of doubly charged sulfonic acid-containing tryptic peptides, YLLGTSLARPC97IAR (monoisotopic m/z, 791.4256; Top, Left) and FELTC132YSLAPQIK (monoisotopic m/z, 780.8851; Bottom, Left), from argininosuccinate synthase. MS/MS spectra confirmed the sequence and site of sulfonic acid-containing peptides (C+48) from argininosuccinate synthase identified in mouse liver (SI Materials and Methods). MS/MS spectra passed automatic and manual filter criteria (Fig. S2), and were identified with high SEQUEST cross correlation (Xc) scores at ppm mass error. Cys132 has been previously reported as a target of S-nitrosylation in human argininosuccinate synthase (20), whereas the identification of Cys97 corresponded to a previously unidentified site of S-nitrosocysteine formation. (D) Colloidal blue protein staining of bound fractions from MRC and mPEGb enrichment of S-nitrosylated proteins from WT and eNOS−/− mouse livers. UV photolysis demonstrated specificity of the enrichment. The levels of protein S-nitrosocysteine were quantified by reductive chemistries coupled to chemiluminescent-based detection (41). The amount of S-nitrosylated protein captured by mPEGb from eNOS−/− livers (lane 8) was below the limit of detection by colloidal blue staining. (E) Western blot analysis of bound and unbound fractions from WT mouse liver that were processed by MRC protein capture.
The ability of the method to reveal endogenous S-nitrosoproteomes was assessed in WT mouse liver homogenates. Initially, three different mouse livers were analyzed independently for protein and peptide capture by using both the MRC and mPEGb approach with 3 mg of starting material. From the three biological replicates, sulfonic acid-containing peptides identified by SEQUEST database searches were pooled and those also present in the UV-pretreated samples were removed. Similarly, all proteins identified by protein capture were pooled (those also identified in the UV-pretreated samples were removed). The use of solid-phase and in-solution–based enrichment approaches was largely complementary, as the number of shared protein identifications between protein capture methods was approximately 50%, whereas each method individually contributed about the same number of unique protein identifications (Table S1). As the MRC capture method allows for higher input, site-specific peptide capture by this method was also performed with the use of 30 mg of liver extract originating from three different mice. The inclusion of these additional biological replicates reduced biological variance and improved the depth of the analysis while maintaining less than 3% false identification rate. Overall by matching sulfonic acid-containing peptides with corresponding protein identifications, we precisely pinpointed 328 S-nitrosocysteine–containing peptides in 192 proteins in untreated livers with a high level of confidence (complete list is provided in Table S1 and all of the MS/MS spectra from peptide capture can be viewed at http://www.research.chop.edu/tools/msms/spectra.pdf). The depth of this analysis represents a significant advancement versus present methodologies (17). The majority of the proteins identified in the current study, 186, corresponded to previously unidentified endogenous targets of S-nitrosylation in the mouse liver, whereas six proteins (GAPDH, hemoglobin, β-tubulin, argininosuccinate synthase, alcohol dehydrogenase, and catalase) have been previously identified as endogenously S-nitrosylated in hepatocytes and other organ systems (18–21). Proteins were also distributed across a wide range of molecular weights (13–272 kDa) and cellular localization including membrane-associated proteins, demonstrating the efficacy of the method to identify S-nitrosocysteine independent of protein size and location. We selected very long chain specific acyl-CoA dehydrogenase, heat shock cognate 71 protein, for which endogenous S-nitrosylation was not previously described, and GAPDH, which has been known to be S-nitrosylated and independently confirmed their selective enrichment by Western blot analysis after protein capture (Fig. 1E).
To further probe the biological specificity of our method while demonstrating its utility for comparison of endogenous S-nitrosoproteomes, we analyzed livers from mice lacking endothelial NO synthase (eNOS−/−). Using chemiluminescence-based quantification, a 10-fold decrease in protein S-nitrosocysteine levels of eNOS−/− livers was measured as compared with WT livers. Concomitantly, a reduced reactivity with phenylmercury compounds was observed (Fig. 1D). From the eNOS−/− livers, 36 sulfonic acid-containing peptides in 26 proteins were identified (Table S2), of which 24 were also identified in the WT liver. The data indicate that the majority of the endogenous liver S-nitrosoproteome is dependent on eNOS-generated NO.
S-Nitrosylation Is Implicated in Multiple Metabolic Pathways.
Proteomic experiments generate rich, diverse datasets that benefit from computational analysis to extract biologically relevant and potentially novel information. Consequently, functional and ontological analyses were conducted to assist in identifying cellular, molecular, and biological functions in which S-nitrosylation may play a role in the liver. Sixty-five percent of S-nitrosylated proteins were localized to the cytoplasm and mitochondrion, representing a significant enrichment compared with the mouse genome (P = 4.9e−34 and P = 7.2e−22, respectively; Fig. S3A). A subset of S-nitrosylated proteins were found to be present in cell membranes, whereas the remaining proteins were distributed across nearly all cellular compartments (Fig. S3A). Gene ontology analysis revealed that 99 S-nitrosylated proteins had catalytic activity largely composed of oxidoreductases (39%) and transferases (17%; Fig. S3B). These functions were also found to be significantly overrepresented (P = 1.28e−20 and 3.2e−3, respectively) in the liver S-nitrosoproteome compared with the mouse genome (22). This is not surprising, as the molecular functions of S-nitrosylated proteins were assigned to diverse metabolic processes (i.e., amino acid synthesis, energy synthesis, lipid metabolism) that take place within the liver.
Analysis of the data has also confirmed the presence of multiple S-nitrosylated cysteine residues in nearly 45% (86 of 192) of the liver S-nitrosoproteome. Poly-S-nitrosylation is present in all top-ranking molecular functions, suggesting that multiple sites of S-nitrosylation in vivo may regulate protein activity. This is in accordance with other known posttranslational modifications such as phosphorylation and lysine acetylation in which polyphosphorylation and polyacetylation are considered regulators of protein function and signaling (23, 24).
To place these functional assignments into the context of biochemical and molecular signaling pathways, S-nitrosylated proteins were assembled into biological protein interaction and signaling networks (Ingenuity Systems). The analysis was restricted to investigate liver-related pathways. Sixteen S-nitrosylated proteins were significantly clustered in a network that encompassed liver responses to the hormone leptin (Fig. S3C). Leptin is an adipocyte-secreted hormone that primarily acts on the central nervous system to regulate energy homeostasis. Leptin also regulates liver metabolism, evident by the significant accumulation of lipids (fatty liver) in mice deficient in leptin (ob/ob) (25, 26) or leptin long-form receptor (db/db) (27). The regulation of liver metabolism is attributed to the leptin-dependent repression of liver stearoyl-CoA desaturase-1, the rate limiting step in monosaturated fat biosynthesis (28). In addition, recent data indicate that leptin also regulates liver mitochondrial respiratory chain protein expression, mitochondrial function and structure (29), remarkably similar to the previously recognized regulation of mitochondrial function by NO (30, 31). Interestingly, delivery of S-nitroso-N-acetylcysteine by gavage to ob/ob mice prevented the development of fatty liver (32). Mice deficient in eNOS also experience abnormal fat deposition in the liver (33), which was attributed in part to regulation of mitochondrial fatty acid synthesis and activation of AMP-activated protein kinase (33). Moreover, 14 of the 16 proteins in the leptin network (Fig. S3C) were absent in eNOS−/− liver S-nitrosocysteine proteome analysis, suggesting a potential relationship between eNOS-derived S-nitrosylation and leptin regulation of fatty acid metabolism. Although it requires further experimentation, the data indicate that S-nitrosylation may be a molecular link between the actions of leptin and NO in liver fatty acid biosynthesis and mitochondrial metabolism. Overall, cellular localization and functional analyses revealed that the S-nitrosylated proteins identified in the liver were largely cytosolic and mitochondrial enzymes that function as oxidoreductases and transferases, which are critical for regulating amino acid, energy, and lipid biosynthesis, and may coordinate the regulation of metabolic pathways by leptin and NO.
Biochemical, Biophysical, and Structural Properties of the Modified Cysteine Residues.
This enriched S-nitrosoproteome was interrogated for the structural properties of the modified cysteine residues by using various bioinformatic tools and available crystal structures of proteins. Table 1 provides the basic biochemical and biophysical properties of the modified cysteine residues using reduced unmodified cysteine residues in the same proteins as a comparison group. Kyte-Doolittle hydropathy indices in 13-residue windows were calculated to determine the influence of primary structure of the protein on modified cysteine residues (Fig. 2A). The average hydropathy index value was calculated to be −0.03 ± 0.69 (n = 309) which did not differ significantly when compared with the mean value of the unmodified cysteines within the same proteins (0.10 ± 0.77, n = 1,382).
Table 1.
Biochemical and biophysical properties of S-nitrosylated and unmodified cysteine residues within the same proteins
| Variable | S-nitrosocysteine residues | Unmodified cysteine residues |
| Hydropathy index | 0.03 ± 0.69 (n = 309) | 0.1 ± 0.77 (n = 382) |
| Predicted pKa | 10.0 ± 2.10 (n = 142) | 9.88 ± 2.20 (n = 559) |
| Helices, % | 40* | 29 |
| β-Sheets, % | 28 | 32 |
| Coils, % | 32† | 39 |
| RSA‡ | 71% buried (n = 99) | 77% buried (n = 561) |
| 29% exposed (n = 40) | 23% exposed (n = 171) |
*P < 0.02, †P < 0.01 using unmodified residues as control group.
‡Residue surface accessibility (RSA) for cysteine residues was calculated by the accessible surface area normalized by the accessible surface area of cysteine in the extended tripeptide Ala-Cys-Ala. A value of ≤10% was used as cutoff to denote a buried cysteine.
Fig. 2.
Hydropathy index and pKa values of S-nitrosylated residues. (A) Kernel density plot of hydropathy indices for S-nitrosocysteine (Left) and unmodified cysteine residues (Right). Hydropathy index was calculated for all S-nitrosylated (n = 170 hydrophilic, n = 139 hydrophobic) and unmodified (n = 635 hydrophilic, n = 747 hydrophobic) cysteine residues within a 13 amino acid window, with a negative value indicating hydrophilicity. (B) Predicted pKa value for each S-nitrosylated cysteine was calculated from the experimental structures and the distribution was represented as a histogram.
Using crystal structures (Protein Data Bank) and the Propka 2.0 algorithm (34), the average predicted pKa of 142 cysteine residues S-nitrosylated in vivo was 10.0, which was not significantly different from the average pKa of reduced unmodified cysteine residues in the same proteins (pKa of 9.88). Only 15 modified cysteine residues had predicted pKa values lower than 7.4 (5.66 ± 1.24), indicating that these particular residues may be deprotonated at physiological pH (Fig. 2B). Secondary structure analysis revealed that S-nitrosocysteine residues were present in β-sheets (28%), helices (40%), and coils (32%). Unmodified cysteine residues within the same proteins were localized primarily in coils (39%) and equally distributed across helices and β-sheets (Table 1). Statistical analysis revealed an overrepresentation and underrepresentation of modified cysteines in helices and coils, respectively (P < 0.02).
None of the cysteine residues found to be S-nitrosylated participate in disulfide bonding in their known structures and none has been reported to date to be modified by glutathiolation and alkylating agents (www.uniprot.org). Furthermore, by using predictive algorithms and literature searches, it was found that less than 20% of the cysteine residues were predicted to be sites of oxidation (35). These data and analysis indicate that the majority of the in vivo sites of S-nitrosylation represent a unique population of cysteine residues not chemically modified through other biological processes.
S-Nitrosocysteine Residues Are Equally Distributed in Hydrophobic and Hydrophilic Areas of the Proteins.
The overall slightly negative hydropathy index (Table 1) is not indicative of a trend for S-nitrosylated cysteine residues to localize in hydrophilic regions of the protein. Further inspection of the hydropathy indices of modified cysteine residues revealed that 139 of the 309 cysteine residues reside nearly in hydrophobic regions, whereas 170 of the 309 are located in hydrophilic regions. This observation led us to further examine whether S-nitrosylated cysteines belong to two distinct populations regarding their hydrophobicity/hydrophilicity. Kernel density estimation revealed that hydropathy indices exhibited a roughly normal distribution (Fig. 2A, Left), indicating that they corresponded to a single population of cysteine residues. Unmodified cysteine residues within the same proteins also showed a roughly normal distribution as well (Fig. 2A, Right), indicating that there is no distinction between S-nitrosylated and unmodified cysteine residues within the same proteins to localize in hydrophobic or hydrophilic areas of proteins. Previous studies have indicated that S-nitrosylation is favored in hydrophobic regions of the proteins (36), presumably because of the increased localized concentration of NO-derived oxides, which may provide a suitable microenvironment for the S-nitrosylation of these cysteine residues. The present data imply that, for a subset of proteins, hydrophobicity may serve as a determinant for selective targeting of cysteine residues for S-nitrosylation.
S-Nitrosylation Occurs on Cysteine Residues Adjacent to Flexible Regions Within the Protein.
To further explore the location of S-nitrosylated cysteine residues in protein secondary structure, the frequency of secondary structures for flanking residues (positions −10 to +10) was calculated and compared with the respective frequencies of the unmodified cysteine residues using the χ2 test. A shift in secondary structure mainly from coils to helices was observed over the range of positions from −6 to 0 (P < 0.05), consistent with the presence of the majority of S-nitrosocysteine residues in α-helices (Fig. 3A). Moreover, a 10% increase in the frequency of coils from positions 0 to +3 (P < 0.001) concomitant with a reduction in β-sheet frequency (P < 0.001) indicative of a change in secondary structure was observed C-terminal from the modified cysteine residues. Unmodified cysteine residues within the same proteins were localized primarily in coils and with lower frequency in β-sheets and helices (Fig. S4A). Moreover, the frequency of coils did not change significantly across all flanking residues (−10 to +10), indicating that shifts in secondary structure were only between helices and β-sheets. Collectively, these data demonstrate that modified cysteine residues are predominantly present in secondary structures of proteins which may facilitate site-directed S-nitrosylation by protein-protein interactions.
Fig. 3.
Analysis of primary sequence, distribution in secondary structures, and surface accessibility of S-nitrosylated cysteine residues. (A) The frequency of S-nitrosocysteine (n = 142; black bars) and unmodified cysteine residues (n = 473; white bars) within the same proteins in different secondary structures was calculated using the available crystal structures. (B) Distribution of residues flanking S-nitrosylated cysteines in secondary structures. SNO-cysteines are located at position 0 and the frequency for 10 residues upstream (−10) and 10 residues downstream (+10) was calculated as described earlier. (C) Quantile–quantile plot of relative RSA for S-nitrosylated (x axis) versus unmodified (y axis) cysteine residues. RSA values were calculated from the biological assemblies defined by PISA (42) for available crystal structures. (D) Top three scoring sequence motifs for residues flanking S-nitrosylated cysteine residue. Note the presence of charged as well as aliphatic amino acids able to “accommodate” protein and small-molecule binding. (E) Top two sequence motifs assigned to the residues sensitive to Trx system.
Surface Accessibility of S-Nitrosocysteine Residues.
The relative residue surface accessibility (RSA) for all cysteines (modified and unmodified) within the S-nitrosoproteome was calculated with Naccess 2.1.1 using the radius of a water molecule (1.4 Å2) as a probe (37). Ninety-nine S-nitrosylated residues (71%) were calculated with an RSA of 10% or lower, indicating that those residues were not accessible to the solvent, whereas the remaining 40 modified cysteines (29%) had a relative RSA greater than 10%, meaning they were solvent-accessible. Unmodified cysteines within the same proteins exhibited similar distribution between buried (77%) and solvent-accessible residues (23%). A quantile–quantile probability plot was used to determine if there was enrichment for exposed or buried cysteines within the modified versus the unmodified group of cysteine residues (Fig. 3C). To produce this plot, the RSA values of modified and unmodified cysteine residues were sorted and values of RSA for each percentile were plotted against each other (i.e., RSA of smallest 1% of modified cysteine residues vs. RSA of smallest 1% of unmodified cysteine residues, smallest 2% vs. smallest 2%). The plot demonstrates that S-nitrosocysteine residues have a distribution skewed toward larger surface-accessible areas than unmodified cysteine residues within the same proteins. In addition, 70% of S-nitrosylated cysteine residues within 6 Å were surrounded by negatively or positively charged amino acids that had their side chains pointed away from cysteine thiol groups. Although the presence of charged residues in the vicinity of the modified residues did not impact their predicted pKa, it may facilitate site specific modification by accommodating protein or S-nitrosoglutathione association. This is in agreement with findings of Mitchell et al., who demonstrated that charged residues near cysteine 73 were required for interaction and transnitrosylation of procaspase-3 (38). Accordingly, the presence of cysteine residues in highly exposed areas of proteins and in proximity to charged amino acids suggests a protein or small molecule transnitrosation assisted mechanism of S-nitrosylation.
Metal Catalyzed S-Nitrosylation.
Studies exploring the S-nitrosylation of proteins in cells indicated that more than 50% of the cellular formation of protein S-nitrosocysteine is derived by dinitrosyliron complexes (7). Within the liver S-nitrosoproteome, 13 S-nitrosylated cysteine residues, which are directly involved in the chelation of metal ions, were identified (Table S3). Metal ligation may provide site-directed modification of these residues. Alternatively, dinitrosyliron complexes could be stabilized near cysteine residues by interactions with neighboring acidic residues. As stated, more than 70% of the modified cysteine residues are in close proximity (<6 Å) to acidic residues, which could serve as interacting sites for dinitrosyliron complexes. Moreover, 15 metalloproteins were identified as endogenously S-nitrosylated, suggesting a self-catalyzed mechanism of S-nitrosylation similar to the proposed mechanism for the selective S-nitrosylation of hemoglobin (39). The possibility also exists that these metalloproteins can catalyze S-nitrosylation of interacting proteins, as has been indicated previously (39). This will require protein–protein association and, as discussed later, the majority of the S-nitrosylated proteins, either in primary sequence or within the tertiary structure, contain charged amino acids that can provide interactive interfaces for specific transfer of a NO equivalent from a metalloprotein to cysteine residues.
Linear Motifs.
To further interrogate structural elements that may distinguish modified cysteines from unmodified within the same proteins, flanking amino acids were examined for the presence of linear motifs using the program Motif-X (40). The top scoring motif for modified cysteines (n = 37, 12%) had glycine exclusively at position −1 (P ≤ 0.001) and consisted mostly of hydrophobic residues (Fig. 3D). Remarkably, the second (n = 31, 10%) and third (n = 25, 8%) top scoring motifs had negatively charged amino acids exclusively at positions +3 and −1, respectively (P ≤ 0.001; Fig. 3D). The same analysis for unmodified cysteines within the same proteins revealed top sequence motifs lacking negatively charged amino acids flanking cysteine residues (Fig. S4B), indicating that specific elements of the primary structure are required for a cysteine to be S-nitrosylated in vivo. These motifs could serve as scaffolds for protein and small molecule binding such as thioredoxin (Trx) or S-nitrosoglutathione.
To further explore this possibility we used the Trx ex vivo denitrosylation assay (17) to identify sites of S-nitrosylation that can interact with Trx. Freshly isolated liver homogenates were treated with a Trx system (Trx/Trx-reductase/NADPH). This treatment resulted in a 72% reduction in protein S-nitrosocysteine levels as quantified by chemiluminescence. In comparison with the untreated liver S-nitrosoproteome (Table S1), 72 proteins were no longer identified after Trx treatment (Table S4). Linear motif analysis revealed two motifs in this subset of S-nitrosylated proteins. The highest-scoring motif had exclusively aspartic acid at position −1, whereas the second motif had exclusively threonine at position +5 (Fig. 3E). Notably, both motifs contained charged amino acids at positions before and after the cysteine residue, consistent with the idea that charged amino acids in S-nitrosocysteine–containing motifs may facilitate interaction with Trx.
In conclusion, by using unique, highly specific MS-based proteomic methods, we identified an expanded endogenous S-nitrosoproteome from WT mouse liver. Despite that S-nitrosylated cysteine residues had, in general, similar hydropathy distribution and predicted pKa values as nonmodified cysteine residues in the same proteins, closer interrogation of the surrounding primary and secondary structures revealed distinctions that direct site-specific S-nitrosylation of certain cysteine residues. The structural analysis of these proteins also uncovered structural features that can accommodate multiple mechanisms for S-nitrosylation in vivo. In addition, the data also revealed a putative link among leptin, eNOS, and protein S-nitrosylation in the regulation of liver fatty acid metabolism. Overall, the use of global proteomic methods enabled structural and functional characterization of the in vivo S-nitrosocysteine proteome and the formulation of testable new hypotheses that can be explored in the future using targeted approaches.
Materials and Methods
Chemicals and Reagents.
All HPLC solvents were purchased from Burdick and Jackson, and unless stated all other reagents were purchased from Sigma-Aldrich. Mercury/PEG/biotin compound was designed by the authors and synthesized by SoluLink.
Capture of S-Nitrosylated Proteins Using MRC or mPEGb.
Mercury resin synthesis, MRC solid-phase, and mPEGb compound-based protein and peptide enrichment are described in detail in the SI Materials and Methods and Scheme S1.
Protein Sequence and Structural Analysis.
A detailed description of protein sequence and structural analysis exists in SI Materials and Methods.
Statistical Analyses.
Graphs were constructed and statistical analyses were performed using GraphPad Prism 5 software (GraphPad). Statistical significance was determined by paired or unpaired nonparametric two-tailed t tests using either the Mann-Whitney (unpaired) or Wilcoxon matched-pairs test.
Supplementary Material
Acknowledgments
We thank the Protein Core at the Children's Hospital of Philadelphia Research Institute for their assistance with mass spectrometry, Dr. Santosh S. Venkatesh for assistance with the χ2 test, Dr. David Schwartz (Solulink Biosciences, San Diego, CA) for the synthesis of organomercury-polyethyleneglycol-biotin, and Dr. Qi Fang for support with structural analysis. This work was supported by National Institutes of Health Grants AG13966 and HL054926, National Institute of Environmental Health Sciences Center of Excellence in Environmental Toxicology Grant ES013508 (to H.I), and National Institute of General Medical Sciences Award F31GM085903 (to J.L.G.). H.I. is the Gisela and Dennis Alter Research Professor of Pediatrics.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008036107/-/DCSupplemental.
References
- 1.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: Purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 2.Whalen EJ, et al. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129:511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 3.Hara MR, et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 4.Cho DH, et al. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzo MA, Piston DW. Regulation of beta cell glucokinase by S-nitrosylation and association with nitric oxide synthase. J Cell Biol. 2003;161:243–248. doi: 10.1083/jcb.200301063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo CJ, et al. S-nitrosylation of surfactant protein-D controls inflammatory function. PLoS Biol. 2008;6:e266. doi: 10.1371/journal.pbio.0060266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosworth CA, Toledo JC, Jr, Zmijewski JW, Li Q, Lancaster JR., Jr Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide. Proc Natl Acad Sci USA. 2009;106:4671–4676. doi: 10.1073/pnas.0710416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Hogg N. S-Nitrosothiols: Cellular formation and transport. Free Radic Biol Med. 2005;38:831–838. doi: 10.1016/j.freeradbiomed.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell DA, Marletta MA. Thioredoxin catalyzes the S-nitrosation of the caspase-3 active site cysteine. Nat Chem Biol. 2005;1:154–158. doi: 10.1038/nchembio720. [DOI] [PubMed] [Google Scholar]
- 10.Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marino SM, Gladyshev VN. Structural analysis of cysteine S-nitrosylation: A modified acid-based motif and the emerging role of trans-nitrosylation. J Mol Biol. 2010;395:844–859. doi: 10.1016/j.jmb.2009.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saville B. A scheme for the colorimetric determination of microgram amounts of thiols. Analyst (Lond) 1958;83:670–672. [Google Scholar]
- 13.Pesavento JJ, Garcia BA, Streeky JA, Kelleher NL, Mizzen CA. Mild performic acid oxidation enhances chromatographic and top down mass spectrometric analyses of histones. Mol Cell Proteomics. 2007;6:1510–1526. doi: 10.1074/mcp.M600404-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Villén J, Gygi SP. The SCX/IMAC enrichment approach for global phosphorylation analysis by mass spectrometry. Nat Protoc. 2008;3:1630–1638. doi: 10.1038/nprot.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forrester MT, Foster MW, Stamler JS. Assessment and application of the biotin switch technique for examining protein S-nitrosylation under conditions of pharmacologically induced oxidative stress. J Biol Chem. 2007;282:13977–13983. doi: 10.1074/jbc.M609684200. [DOI] [PubMed] [Google Scholar]
- 16.Derakhshan B, Wille PC, Gross SS. Unbiased identification of cysteine S-nitrosylation sites on proteins. Nat Protoc. 2007;2:1685–1691. doi: 10.1038/nprot.2007.210. [DOI] [PubMed] [Google Scholar]
- 17.Paige JS, Xu G, Stancevic B, Jaffrey SR. Nitrosothiol reactivity profiling identifies S-nitrosylated proteins with unexpected stability. Chem Biol. 2008;15:1307–1316. doi: 10.1016/j.chembiol.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 19.Hao G, Derakhshan B, Shi L, Campagne F, Gross SS. SNOSID, a proteomic method for identification of cysteine S-nitrosylation sites in complex protein mixtures. Proc Natl Acad Sci USA. 2006;103:1012–1017. doi: 10.1073/pnas.0508412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao G, Xie L, Gross SS. Argininosuccinate synthetase is reversibly inactivated by S-nitrosylation in vitro and in vivo. J Biol Chem. 2004;279:36192–36200. doi: 10.1074/jbc.M404866200. [DOI] [PubMed] [Google Scholar]
- 21.López-Sánchez LM, et al. Alteration of S-nitrosothiol homeostasis and targets for protein S-nitrosation in human hepatocytes. Proteomics. 2008;8:4709–4720. doi: 10.1002/pmic.200700313. [DOI] [PubMed] [Google Scholar]
- 22.Al-Shahrour F, Díaz-Uriarte R, Dopazo J. FatiGO: A web tool for finding significant associations of Gene Ontology terms with groups of genes. Bioinformatics. 2004;20:578–580. doi: 10.1093/bioinformatics/btg455. [DOI] [PubMed] [Google Scholar]
- 23.Olsen JV, et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Choudhary C, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 25.Halaas JL, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 26.Pelleymounter MA, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 27.Mohan S, et al. Diabetic eNOS knockout mice develop distinct macro- and microvascular complications. Lab Invest. 2008;88:515–528. doi: 10.1038/labinvest.2008.23. [DOI] [PubMed] [Google Scholar]
- 28.Cohen P, Friedman JM. Leptin and the control of metabolism: Role for stearoyl-CoA desaturase-1 (SCD-1) J Nutr. 2004;134:2455S–2463S. doi: 10.1093/jn/134.9.2455S. [DOI] [PubMed] [Google Scholar]
- 29.Singh A, et al. Leptin-mediated changes in hepatic mitochondrial metabolism, structure, and protein levels. Proc Natl Acad Sci USA. 2009;106:13100–13105. doi: 10.1073/pnas.0903723106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 31.Nisoli E, et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci USA. 2004;101:16507–16512. doi: 10.1073/pnas.0405432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Oliveira CP, et al. Prevention and reversion of nonalcoholic steatohepatitis in OB/OB mice by S-nitroso-N-acetylcysteine treatment. J Am Coll Nutr. 2008;27:299–305. doi: 10.1080/07315724.2008.10719703. [DOI] [PubMed] [Google Scholar]
- 33.Schild L, et al. Impairment of endothelial nitric oxide synthase causes abnormal fat and glycogen deposition in liver. Biochim Biophys Acta. 2008;1782:180–187. doi: 10.1016/j.bbadis.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Robertson AD, Jensen JH. Very fast empirical prediction and rationalization of protein pKa values. Proteins. 2005;61:704–721. doi: 10.1002/prot.20660. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez R, Riddle M, Woo J, Momand J. Prediction of reversibly oxidized protein cysteine thiols using protein structure properties. Protein Sci. 2008;17:473–481. doi: 10.1110/ps.073252408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nedospasov A, Rafikov R, Beda N, Nudler E. An autocatalytic mechanism of protein nitrosylation. Proc Natl Acad Sci USA. 2000;97:13543–13548. doi: 10.1073/pnas.250398197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee B, Richards FM. The interpretation of protein structures: Estimation of static accessibility. J Mol Biol. 1971;55:379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell DA, Morton SU, Fernhoff NB, Marletta MA. Thioredoxin is required for S-nitrosation of procaspase-3 and the inhibition of apoptosis in Jurkat cells. Proc Natl Acad Sci USA. 2007;104:11609–11614. doi: 10.1073/pnas.0704898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weichsel A, et al. Heme-assisted S-nitrosation of a proximal thiolate in a nitric oxide transport protein. Proc Natl Acad Sci USA. 2005;102:594–599. doi: 10.1073/pnas.0406549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz D, Gygi SP. An iterative statistical approach to the identification of protein phosphorylation motifs from large-scale data sets. Nat Biotechnol. 2005;23:1391–1398. doi: 10.1038/nbt1146. [DOI] [PubMed] [Google Scholar]
- 41.Greco TM, et al. Identification of S-nitrosylation motifs by site-specific mapping of the S-nitrosocysteine proteome in human vascular smooth muscle cells. Proc Natl Acad Sci USA. 2006;103:7420–7425. doi: 10.1073/pnas.0600729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.