Abstract
Oxidative stress occurs when generation of reactive oxygen species (ROS) overwhelms antioxidant defenses. Oxidative stress has been associated with male infertility. The transcription factor Nuclear Factor-Erythroid 2-Related Factor 2 (NRF2) regulates basal and inducible transcription of genes encoding enzymes important for protection against ROS. We hypothesized that deletion of the Nrf2 gene causes testicular and epididymal oxidative stress, which disrupts spermatogenesis. Our results show that male Nrf2−/− mice have decreased fertility compared to wild type and heterozygous littermates, due to accumulating seminiferous tubule damage with increasing age. Testicular sperm head counts, epididymal sperm counts, and epididymal sperm motility in 2 month old Nrf2−/− males did not differ from wild type littermates; however, by age 6 months, Nrf2−/− males had 44% lower testicular sperm head counts, 65% lower epididymal sperm counts, and 66% lower epididymal sperm motility than wild type males. Two to 4 month old Nrf2−/− males had elevated levels of testicular and epididymal lipid peroxidation and testicular germ cell apoptosis, and decreased levels of antioxidants compared to wild type males. These results provide evidence that oxidative stress has deleterious effects on the testis and epididymis and demonstrate a critical role for the transcription factor NRF2 in preventing oxidative disruption of spermatogenesis.
Keywords: oxidative stress, spermatogenesis, NRF2, testis, reproduction
Introduction
Reactive oxygen species (ROS) are formed by the sequential addition of electrons to molecular oxygen, forming superoxide anion radical, hydrogen peroxide, and hydroxyl radical. These ROS can damage important cellular components by reacting with lipids, proteins, RNA, and DNA. ROS are generated as byproducts of normal cellular processes such as oxidative phosphorylation and, in steroidogenic tissues like the testis, steroid synthesis [1]. In addition, some cells possess oxidases that enable them to generate ROS. Spermatozoa are thought to contain a membrane-bound NADPH oxidase that generates superoxide anion radical [2–4]. Low levels of ROS are necessary for sperm capacitation, the process by which sperm become capable of fertilizing an oocyte, and for the acrosome reaction, which enables the sperm to penetrate the zona pellucida of the oocyte and fuse with its plasma membrane [5]. To prevent the adverse effects of high levels of ROS, the male reproductive tract possesses antioxidant defenses, including vitamins C and E, the antioxidant tripeptide glutathione (GSH), and ROS scavenging enzymes, such as superoxide dismutases (SOD), glutathione peroxidases (GPX), and catalase [2, 4].
Oxidative stress in spermatozoa is associated with male infertility, due to impairment of sperm motility and, at higher levels, decreased sperm viability [2, 5]. Spermatozoa are particularly susceptible to oxidative damage because their cell membranes contain large amounts of polyunsaturated fatty acids, which can be oxidized in the presence of ROS [2, 5, 6]. The decline in male fertility with aging is associated with increasing oxidative damage and decreased antioxidant capacity in the male reproductive system. Activity or expression of the antioxidant enzymes SOD and GPX and levels of the antioxidant tripeptide glutathione (GSH) have been reported to decrease with aging in the Leydig cells of the rat testis [7] and in rat spermatozoa [8]. Levels of superoxide anion radical and of lipid peroxidation were also elevated in Leydig cells and spermatozoa of aged male rats compared to young male rats [8–10].
The Cap-N-Collar basic leucine zipper (CNC-bZip) transcription factor NRF2 regulates transcription of genes that have antioxidant response elements (AREs) in their promoters [11, 12]. NRF2 must form a heterodimer with other bZip transcription factors, the small Mafs, to bind to AREs [12–14]. AREs are found in the promoters of many Phase II biotransformation and antioxidant enzyme genes [13], and mice that lack Nrf2 have reduced constitutive and/or inducible expression of these genes. For example, Nrf2 knockout mice have decreased expression of glutamate cysteine ligase catalytic (Gclc) and modifier (Gclm) subunits, which together form the rate-limiting enzyme in the synthesis of the antioxidant GSH, as well as of the second enzyme of GSH synthesis, glutathione synthetase [15]. Expression of NAD(P)H:quinone oxidoreductase, various glutathione-S-transferases, UDP-glucuronosyltransferase, microsomal epoxide hydrolase, superoxide dismutase 2, and others are also reduced in these animals [15–19]. Nrf2 knockout mice are more susceptible to chemical toxicity, including cancer induction by the polycyclic aromatic hydrocarbon benz[a]pyrene [19], ovarian toxicity by vinylcyclohexene diepoxide [20], and liver toxicity by acetaminophen [21], and to autoimmune diseases [22, 23] than wild type mice. However, the effects of Nrf2 knockout on male reproduction have not been studied. We hypothesized that male Nrf2 knockout mice would have an accelerated age-related decline in reproductive function due to decreased testicular and epididymal antioxidant capacity and resulting oxidative stress.
Materials and Methods
Animals
Nrf2 knockout mice were generated by disrupting the Nrf2 gene by homologous recombination in embryonic stem cells, using a targeting vector that results in deletion of part of exon 4 and all of exon 5, replacing them with a LacZ reporter gene [24]. Founder mice were backcrossed 8 times onto a C57BL/6NCrl genetic background. Experimental mice were generated by mating heterozygotes. Genotyping of tail or toe snip DNA by PCR was carried out as described [24]. The mice were housed in polycarbonate cages with wood chip bedding in an American Association for the Accreditation of Laboratory Animal Care-accredited facility on a 14:10h light-dark cycle with temperature maintained at 20.6–24°C. The mice had free access to deionized water and laboratory rodent chow (soy-free Harlan Teklad 2019, except during the continuous breeding assay, Purina 2500, a soy-containing diet, was used). Experimental males were group-housed with their male littermates after weaning (up to 5 mice per cage) unless they were used for the breeding study, in which case they were housed with a single female. The experimental protocols were carried out in accordance with the Guide for the Care and Use of Laboratory Animals [25] and were approved by the Institutional Animal Care and Use Committee at the University of California Irvine.
Fertility Assessment
We investigated the effect of lack of one or both alleles of Nrf2 on male fertility using a continuous breeding assay [26]. Beginning at two months of age wild type, Nrf2+/− or Nrf2−/− male mice were paired with wild type 10 week old female mice for 20 weeks. Cages were checked daily for litters, which were removed on the day of birth. Live and dead pups were counted and the live pups were weighed.
Effect of Vitamin E Supplemention on Age-Related Changes in Spermatogenesis
Nrf2−/− and Nrf2+/+ male mice were fed casein-based vitamin E deficient diets (Harlan Teklad TD.07168) supplemented with either the minimum daily requirement for Vitamin E of 32 mg/kg diet (as DL-alpha tocopheryl acetate, 500 IU/g) or 320 mg/kg diet from weaning at the age of 21 days until 4 months of age. Mice were weighed weekly, and the amount of feed consumed was recorded weekly. At the time of euthanasia, one testis and epididymis were processed for sperm counts, sperm motility, and sperm morphology. The other testis and epididymis were fixed for histology.
Testicular, epididymal, and renal histology
Testes and epididymides were fixed in Bouin’s fixative at 4°C overnight, followed by washing four times in 50% ethanol, and storage in 70% ethanol until processing for routine histology, sectioning at 5 μM thickness, and staining with Periodic Acid Schiff and hematoxylin. Kidneys were fixed in 10% neutral buffered formalin for 24h, then stored in 70% ethanol until processing for routine histology and staining with hematoxylin and eosin. Histological sections from each animal were evaluated by a veterinary pathologist (G.L.) without knowledge of genotype. For quantification of seminiferous tubule defects, images of three testicular cross-sections were captured per animal. The total number of seminiferous tubule cross-sections was counted and each cross-section was scored for vacuolization and for rounded germ cells. The percentages of tubules with vacuolization and rounded germ cells were calculated and used for data presentation and statistical analyses.
Sperm analyses
Testicular sperm head counts, cauda epididymal sperm counts, and cauda epididymal sperm motility and morphology assessments were performed according to published methods [27–31]. The testis and epididymis were dissected, cleaned of adherent fat and weighed. The cauda epididymis was dissected from the corpus epididymis and vas deferens, weighed, and then rapidly minced in 500 μl prewarmed phosphate buffered saline (PBS) at 37°C. A 10 μl aliquot was removed and pipetted onto a prewarmed microscope slide for sperm motility assessment. Motile and non-motile sperm were counted in ten 400× microscope fields per sample as rapidly as possible after death of the animal. For the vitamin E supplementation study only, a 10 μl aliquot was removed, spread onto a slide, allowed to dry, fixed in 5% acetic acid in ethanol, and stained with 5% eosin Y for sperm morphology. The remaining minced cauda epididymis was incubated for 15 min at 37°C, allowing sperm to swim into the buffer. An equal volume of 4% paraformaldehyde in PBS was then added to fix the sperm, and epididymal sperm counts were enumerated using a hemacytometer. Testes were frozen on dry ice and stored at −80°C. For testicular sperm head counts, testes were thawed on ice, each testis was homogenized in 8ml of 0.9% sodium chloride, 0.05% Triton X-100 for 2 min at high speed on ice using an Omni Tissue Homogenizer and allowed to settle for at least 1 min. Sperm heads in the homogenate were counted using a hemacytometer.
Testosterone assays
Blood was collected by cardiac puncture after carbon dioxide euthanasia. Serum was separated by centrifugation after clotting at room temperature, and was stored at −20°C. The Coat-A-Count Total Testosterone radioimmunoassay kit from Siemens Medical Solutions Diagnostics (formerly DPC, Los Angeles, CA) was used to measure testosterone concentrations. All samples were run in duplicate in a single assay.
ELISA of double-stranded DNA (dsDNA) antibodies
Antibodies to mouse dsDNA were measured in serum using the Alpha Diagnostics (San Antonio, TX) Mouse Anti-dsDNA Total Ig Elisa Kit (Catalog #5110) according to the manufacturer’s instructions.
Terminal deoxynucleotidyl transferase-mediated dUTP-nick end-labeling (TUNEL)
Nrf2−/− and Nrf2+/+ males were deeply anesthetized with ketamine and xylazine, perfused with PBS, followed by 4% paraformaldehyde in PBS. Testes were removed and postfixed in 4% paraformaldehyde overnight, cryopreserved in 15% sucrose in PBS, and embedded in OCT for storage at −80°C until cryosectioning. Paraformaldehyde-fixed cryosections (10 μM thickness) were used for in situ detection of DNA fragmentation by the TUNEL method using the In Situ Cell Death Detection Kit POD (Roche Diagnostics, Indianapolis, IN) as previously described [32, 33]. TUNEL positive apoptotic cells were counted in every seminiferous tubule cross-section in two testicular sections per animal by an observer blind to genotype (N=4–6 mice per genotype and age).
Immunohistochemistry
Paraformaldehyde-fixed cryosections from the same mice as for TUNEL were used for immunodetection of oxidative DNA damage with 8-hydroxy-2′-deoxyguanosine (8-OHdG) antibody (1:250 dilution; #4354-MC-050, Trevigen) and lipid peroxidation with 4-hydroxynonenal (4HNE) antibody (1:500 dilution; #ALX-210-767, Alexis Biochemicals) using the MOM Immunodetection Kit (8-OHdG) or the ABC Immunodetection Kit (4HNE) (Vector Laboratories, Burlingame, CA). For 8-OHdG, slides were treated with Proteinase K (10 μg/ml) in PBS for 15 min at 37°C, washed, treated with RNase A (100 μg/ml) in 150 mM NaCl, 15 mM sodium citrate for 1 h at 37°C, washed and treated with 2N HCl for 5 min. After washing in PBS, an Avidin/Biotin blocking step was performed using the Avidin/Biotin Blocking Kit (Vector Laboratories). Slides were then blocked for 1h using MOM Mouse Ig Blocking Reagent. After washing again, slides were incubated with primary antibody in MOM diluent overnight at 4°C, washed, incubated with biotinylated anti-mouse Ig for 30 min, washed, blocked with 3% hydrogen peroxide in PBS, washed, incubated with Vectastain ABC reagent for 30 min, washed, incubated with diaminobenzidine subtrate in peroxide buffer for 6 min, washed, and counterstained with hematoxylin. For 4HNE, immunostaining was performed similarly, except that the 3% hydrogen peroxide in PBS blocking step was performed first; antigen retrieval was then performed by incubating slides in 10 mM citrate buffer for 10 min at 95°C, followed by Avidin/Biotin blocking, incubation with blocking serum, incubation with primary antibody in 1% goat serum in PBS, and incubation with biotinylated anti-rabbit Ig. Negative controls included slides incubated with non-immune mouse or rabbit IgG in place of primary antibody. Cells with 8-OHdG or 4HNE immunostaining within seminiferous tubules were counted as for TUNEL-positive cells.
Lipid peroxidation assay
Two products of lipid hydroperoxide decomposition, malondialdehyde (MDA) and 4-hydroxyalkenals (HAE), were measured spectrophotometrically using the LPO-586 Kit (OxisResearch), which utilizes the reaction of these compounds dissolved in methanesulfonic acid with a chromogenic agent, N-methyl-2-phenylindole at 45°C. [34]. Young adult, 3 month old male Nrf2−/− mice and wild type littermates were killed by cervical dislocation, and testes and epididymides were homogenized on ice in PBS with 5 mM butylated hydroxytoluene (BHT) to prevent oxidation (4 μl per mg tissue). After centrifugation at 3,000×g for 10 min at 4°C, the supernatants were stored at −80°C for up to one month prior to performing the assay. A standard curve of known concentrations of 1,1,3,3-tetramethoxypropane, which is hydrolyzed to MDA during the incubation at 45°C, was run simultaneously. Absorbance was measured at 586nm using a VersaMax Microplate Tunable Spectrophotometer (Molecular Devices, Sunnyvale, CA) and sample concentrations were extrapolated from the standard curve. Results are expressed as nmol MDA plus HAE per mg tissue or per mg protein.
Glutathione assays
For glutathione assays, testes and epididymides from 8 to 9 week old male mice were homogenized in 20 mM Tris, 1 mM EDTA, 250 mM sucrose, 2 mM L-serine, 20 mM boric acid (TES-SB). After removal of aliquots for protein assay and enzymatic activity assays, supernatants were acidified with one quarter volume 5% sulfosalicylic acid for glutathione assays [35]. Total and oxidized glutathione were measured in testicular and epididymal homogenates using a modification of an enzymatic recycling assay developed by Griffith [35–37]. For measurement of GSSG, reduced GSH was first removed from the sample by conjugation with 2-vinylpyridine, followed by chloroform extraction. For both GSH and GSSG assays, triplicates of samples or standards were combined with 33 μl metal free water and incubated for 10 min at 30°C. The samples were then mixed with 140 μl of 0.3 mM NADPH, 20 μl of 6 mM DTNB (5,5′-dithiobis(2-nitrobenzoic acid), and 2 μl of 50 units/mL GSH reductase. The rate of thiobis(2-nitrobenzoic acid) (TNB) formation from DTNB is proportional to the total GSH concentration in each sample. TNB formation was monitored by measuring the absorbance at 412 nm for 5 min every 10 sec. The concentrations of total GSH or GSSG in the samples were calculated from a standard curve generated from the slopes of the standards. The concentration of reduced GSH was calculated as [total GSH] − 2× [GSSG].
Enzymatic activity assays
The cytosolic glutathione peroxidase enzymatic activity in testicular and epididymal homogenates was measured by monitoring the oxidation of NADPH spectrophotometrically with hydrogen peroxide as the substrate for glutathione peroxidase [38]. Samples were incubated for 10 min with an excess of glutathione reductase (0.25 U/ml) and 1 mM GSH in 100 mM potassium phosphate, 1 mM EDTA, 1 mM sodium azide, pH 7.2, for 10 min. NADPH was added to a final concentration of 0.15 mM, and the peroxidase independent oxidation of NADPH was monitored by reading the absorbance at 340 nm every 10s for 3 min. After the addition of 0.15 mM final concentration of hydrogen peroxide, the absorbance was monitored every 10s for 5 min. Negative control wells received buffer without hydrogen peroxide. The assay was carried out at 37°C. The peroxide-independent absorbance slope was subtracted from the peroxide dependent absorbance slope, and the change in NADPH concentration per min was calculated from the difference using the extinction coefficient at 370 nm of 0.00622 μM−1cm−1 [38] and the pathlength of the solution. This was used to calculate the activity in Units per liter. A unit is the amount of GPX that results in the oxidation of 1 μmol of NADPH in 1 min at a given starting concentration of glutathione. Results were expressed as mU/mg protein or mU/mg tissue.
The glutathione reductase enzymatic activity was measured as the rate of oxidation of NADPH as GSSG is reduced by glutathione reductase [39, 40]. Homogenates were pipetted in triplicate into a 96-well plate. Reaction mix of NADPH in 100 mM potassium phosphate, 1 mM EDTA buffer was then added to each well resulting in a final concentration of 0.153 mM NADPH. The reaction was initiated by the addition of GSSG in potassium phosphate, EDTA buffer to a final concentration of 1mM GSSG. Negative control wells received buffer without GSSG. Absorbance at 340 nm was monitored and the change in NADPH concentration per minute was calculated as for the glutathione peroxidase assay. One unit of glutathione reductase activity is the amount of enzyme that catalyzes the oxidation of 1 μmol of NADPH in 1 min. Results were expressed as mU/mg protein or mU/mg tissue.
The glutathione-S-transferase (GST) enzymatic activity was measured using 1-chloro-2,4-dinitrobenzene (CDNB) as the substrate [41]. Samples were pipetted in triplicate into a 96-well plate with 100 mM sodium phosphate buffer, pH 6.5, and GSH at a final concentration of 1 mM. Negative control wells received buffer and GSH without sample. CDNB was then added to a final concentration of 1 mM and absorbance at 340 nm was monitored every 10 s at 37°C for 3 min. The activity was calculated from the slope of the absorbance versus time curves, the pathlength of the solution, and the extinction coefficient of CDNB (9.6 mM−1cm−1). One unit of GST activity is the amount of enzyme that conjugates 1 μmol of the substrate in one minute. Results were expressed as mU/mg protein or mU/mg tissue.
Quantitative Real-Time Reverse Transcriptase PCR
Total RNA was extracted from testes or epididymides using the Qiagen RNeasy Kit (Qiagen, Valenica, CA) according to the manufacturer’s instructions. Fifty nanograms of RNA were reverse-transcribed and subjected to PCR using gene-specific forward (F) and reverse (R) primers (purchased from Invitrogen) and the Qiagen Quantitect SYBR Green RT-PCR reagent (Qiagen, Valenica, CA) in 10 μl reaction volumes in the Roche LightCycler. Standard curves derived from serial dilutions of mouse liver, testis or epididymal RNA were used to determine concentrations of mRNAs normalized to 18s using the Roche LightCycler 4.0 software. Forward (F) and reverse (R) primer sequences (5′ to 3′) for Gclc (F:ATGTGGACACCCGATGCAGTATT; R: TGTCTTGCTTGTAGTCAGGATGGTTT) and Gclm (F: GCCACCAGATTTGACTGCCTTT; R: CAGGGATGCTTTCTTGAAGAGCTT) were previously designed for the amplification of mouse and rat mRNA [35]. Gstm1 (F: AGCACCCTGGCCTTCTGCACT; R:TTCGCAGAAACGGGCTGTGAG), Gstm2 (F: TACACCATGGGGGACGCTCCT, R:TGGCCAACTGTATGCGGGTGT), Gsta3 (F:GAGATCGACGGGATGAAACTGGTG, R:GCGCTTTCAGGAGAGGGAAGTTGT), Gstp (F: TTTGGGGGCTTTATGGGAAAAACCA, R: ACATAGGCAGAGAG-CAGGGGGAAG), and 18s (F:TCGGCGTCCCCCAACTTCTTA, R:GGTAGTAGCGAC-GGGCGGTGT) primers were previously designed for amplification of mouse RNA [42]. Validated sequences for mouse Gstm5 (F: TCATCCAAGTCTATGGTTCTGGG, R: CCACAGATGTACCGTTTCTCCT), Gpx1 (F: AGTCCACCGTGTATGCCTTCT, R: GAGACGCGCGACATTCTCAATGA), Gpx2 (F:GCCTCAAGTATGTCCGACATG, R:GGAGAACGGGTCATCATAAGGG), Gpx3 (F:CCTTTTAAGCAGTATGCAGGCA, R:CAAGCCAAATGGCCCAAGTT), Gpx4 (F: GCCTGGATAAGTACAGGGGTT, R: CATGCAGATCGACTAGCTGAG), Gpx5 (F: TCTAGCCAGCTATGTGCAGAC, R: TCCTTCCCATTAAGAGACAGAGC), Hmox1 (F:AAGCCGAGAATGCTGAGTTCA, R:GCCGTGTAGATATGGTACAAGGA), and Sod2 (F:CAGACCTGCCTTACGACTATGG, R:CTCGGTGGCTTGAGATTGTT) primers were obtained from Primer Bank (http://pga.mgh.harvard.edu/primerbank/).
Statistical Analyses
Analysis of variance (ANOVA) was used to test for differences among groups. The effects of Nrf2 genotype at various ages were analyzed by independent samples t-test. Levene’s test was used to test for homogeneity of variances. If variances were not homogeneous non-parametric Kruskal Wallis and Mann Whitney tests were used. Results in tables and figures are expressed as means plus or minus standard error of the mean (SEM).
Results
Male Nrf2−/− mice have decreased fertility
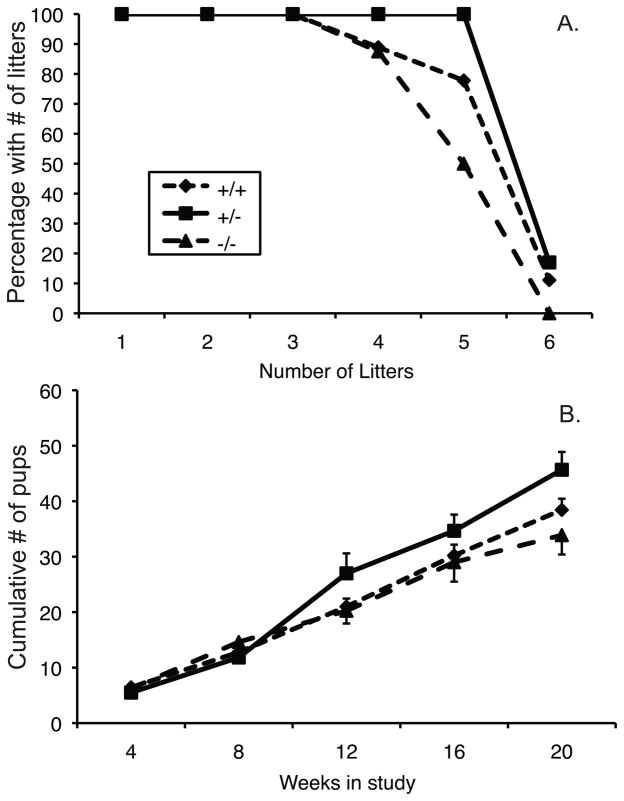
Nrf2 knockout males sired fewer litters and had fewer offspring than wild type or heterozygous males during a 20 week continuous breeding study (Figure 1A,B). The interaction effect between genotype and cumulative number of offspring over time was statistically significant (p=0.046, by repeated measures ANOVA; N=6–9/group). The latter is consistent with an age-dependent decline in the fertility of the Nrf2−/− males compared to the wild type or heterozygous males. Numbers of dead pups on the day of birth and neonatal pup weights did not differ by paternal genotype (data not shown).
Figure 1.
Effect of knockout of Nrf2 on male fertility. Males of the indicated Nrf2 genotypes were paired with wild type females for a 20 week continuous breeding study. A) Percentage of males of each genotype that sired the number of litters indicated on the x-axis during the 20 week study. B) The mean ± SEM cumulative number of offspring sired by males of each genotype during 4 week intervals of the 20 week breeding study. Effect of genotype × time interaction, p=0.046 by ANOVA. N = 8, 9, and 6 for Nrf2−/−, Nrf2+/+, and Nrf2+/−, respectively.
Male Nrf2−/− mice have age-related seminiferous tubule pathology
Compared to those of Nrf2+/+ males, testes of Nrf2−/− males weighed 44% less at 35 days of age (P=0.050, by t-test), 5% less at 2 months of age (P=0.052), 34% less at 3 months of age (P<0.001), 36% less at 4 months of age (P<0.001), and 41% less at 6 months of age (p<0.001; Table 1). Body weights were slightly lower in Nrf2−/− male mice than in their wild type littermates at most ages, but the effect of genotype was not statistically significant. Moreover, adjusting testis weights for body weights did not alter the conclusion that Nrf2−/− males had smaller testes than wild type littermates (Table 1). We also did not observe any increased mortality or clinical signs of illness, such as facial edema, tail lesions, or decreased grooming, in Nrf2−/− males followed to up to 7 months of age, in contrast to a previous report in Nrf2−/− mice on a different genetic background [23].
Table 1.
Testicular weights (mg)
| Genotype | Age (days) | N | Single testis weight (mean±SEM) | Single testis weight (% body weight) |
|---|---|---|---|---|
| Nrf2−/− | 35 | 7 | 30.0±5.7 | 0.205±0.030 |
| Nrf2+/+ | 35 | 7 | a45.4±4.2 | a0.281±0.019 |
| Age (mos) | Paired testis weight (mean±SEM) | Paired testis weight (% body weight) | ||
| Nrf2−/− | 2 | 17 | 179.0±4.3 | 0.764±0.024 |
| Nrf2+/+ | 2 | 12 | a189.1±2.6 | 0.798±0.018 |
| Nrf2−/− | 3 | 6 | 122.9±2.9 | 0.434±0.020 |
| Nrf2+/+ | 3 | 6 | b185.0±9.9 | b0.666±0.046 |
| Nrf2−/− | 4 | 14 | 112.2±8.3 | 0.376±0.026 |
| Nrf2+/+ | 4 | 14 | b176.8±4.2 | b0.569±0.019 |
| Nrf2−/− | 6 | 11 | 103.7±13.5 | 0.307±0.045 |
| Nrf2+/+ | 6 | 5 | b175.4±11.7 | c0.479±0.044 |
| Nrf2−/− | 7 | 2 | 87.7±11.3 | 0.341±0.040 |
| Nrf2+/+ | 7 | 4 | b198.4±7.7 | b0.725±0.023 |
P=0.05, Nrf2+/+ versus Nrf2−/− of same age by t-test
P<0.001, Nrf2+/+ versus Nrf2−/− of same age by t-test
P<0.05, Nrf2+/+ versus Nrf2−/− of same age by t-test
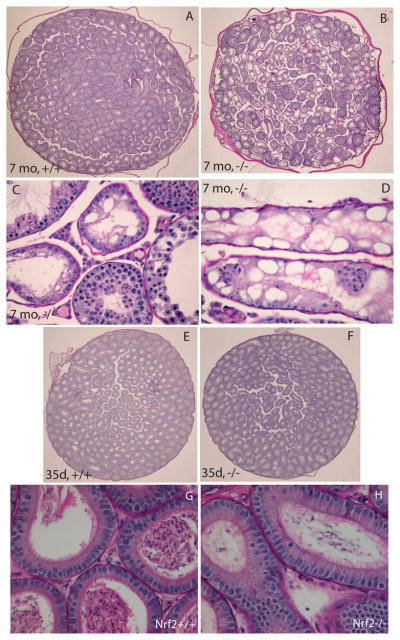
Histological examination of the testes of 7 month old males showed moderate to severe vacuolization of seminiferous tubules in 5 of 8 knockout male mice and no or mild vacuolization in the 9 wild type controls (Figure 2A–D). The degenerative changes consisted of severe intracytoplasmic vacuolization, beginning in the seminiferous tubular epithelium more centrally within the tubule and progressing to the periphery, sparing the Sertoli cells. The most severely affected males had numerous seminiferous tubules that completely lacked germ cells, containing only Sertoli cells (Figure 2C,D). Less affected tubules had spermatid heads near the basement membrane and meiotic stages interspersed with haploid germ cell stages. No histological evidence of inflammation was observed in testicular sections from 35 day old, 4 month old, or 7 month old Nrf2−/− males. Morphometric analysis revealed statistically significantly higher percentages of vacuolated seminiferous tubules per testicular cross-section in Nrf2−/− males than in wild type males (p=0.037, by t-test; Table 2). The distribution of males with abnormal seminiferous tubules also differed significantly by genotype, with 5 of 8 Nrf2−/− males and 0 of 9 Nrf2+/+ males having more than 10% vacuolated seminiferous tubules per cross-section (P=0.009, by Fisher’s exact test). The percentage of vacuolated seminiferous tubules was negatively correlated with the cumulative number of pups sired in the breeding study (Supplemental Figure S1).
Figure 2.
Testes of Nrf2−/− males are histologically normal before puberty, but develop severe seminiferous tubule vacuolization by 7 months of age. A) Representative image of testicular cross-section from 7 mo old Nrf2+/+ male showing normal spermatogenesis. Original magnification 10×. B) Representative image of testicular cross-section from severely affected 7 mo old Nrf2−/− male with numerous vacuolated seminiferous tubules. Original magnification 10×. C,D) Representative severely affected seminiferous tubules from two 7 month old Nrf2−/− males. Original magnifications, 200×. E) Representative image of testicular cross-section from 35 day old Nrf2+/+ male showing normal spermatogenesis. Original magnification 10×. F) Representative image of testicular cross-section from 35 day old Nrf2−/− male showing lack of vacuolated seminiferous tubules. Original magnification 10×. G) Representative image of caput epididymis from 7 mo old Nrf2+/+ male showing normal epididymal epithelium and normal numbers of epididymal sperm. Original magnification 200×. H) Representative image of epididymis from severely affected 7 mo old Nrf2−/− male shows normal epididymal epithelium and few epididymal sperm within the epididymal lumen. Original magnification 200×.
Table 2.
Testicular histology morphometric analysis
| Genotype, age | % vacuolated seminiferous tubules/cross-section | % seminiferous tubules with rounded germ cells | ||
|---|---|---|---|---|
| mean +/− SEM | range | mean +/− SEM | range | |
| Nrf2−/−, 35d | 2.3±1.7 | 0–12.2 | 8.9±4.6 | 0–33.9 |
| Nrf2+/+, 35d | 0.9±0.3 | 0–1.8 | 1.3±0.5 | 0.1–3.8 |
| Nrf2−/−, 7mo | 27.1±9.3 | 0.2–58.2 | 2.4±0.6 | 0.5–6.3 |
| Nrf2+/+, 7mo | a3.1±0.7 | 1.7–8.2 | 2.5±0.6 | 0.2–6.0 |
significantly different from Nrf2−/− of same age, P=0.037 by t-test
Far fewer abnormalities were observed in the testes of 35 day old males (Table 2 and Figure 2E,F). Only 1 of 7 Nrf2−/− males and 0 of 7 Nrf2+/+ males had greater than 10% vacuolated seminiferous tubules per cross-section at 35 days of age (difference not statistically significant by Fisher’s exact test). The mean percentage of seminiferous tubules with rounded germ cells that were losing their intercellular attachments was higher in the 35 day old Nrf2−/− males than in Nrf2+/+ males (8.9±4.6 versus 1.3±0.5), but the effect of genotype was not statistically significant (P=0.15 by t-test).
Concomitant with the testicular abnormalities, decreased epididymal sperm numbers and degenerating rounded cells were observed (Figure 2G,H). The epididymal epithelia appeared histologically normal in both wild type and Nrf2−/− males at 35 days and 7 months. Epididymal weights tended to be lower in Nrf2−/− males than in wild type littermates at 4 and 6 months of age (Table 3). The effect of genotype was statistically significant at 4 months of age.
Table 3.
Epididymal weights (mg)
| Genotype | Age (mos) | N | Paired epididymis weight (mean±SEM) | Paired epididymis weight (% body weight) |
|---|---|---|---|---|
| Nrf2−/− | 2 | 17 | 60.8±1.5 | 0.258±0.007 |
| Nrf2+/+ | 2 | 12 | 63.7±1.7 | 0.270±0.011 |
| Nrf2−/− | 3 | 6 | 67.1±2.9 | 0.236±0.010 |
| Nrf2+/+ | 3 | 6 | 68.3±3.1 | 0.245±0.010 |
| Nrf2−/− | 4 | 14 | 58.5±1.6 | 0.196±0.005 |
| Nrf2+/+ | 4 | 14 | a72.4±2.2 | a0.232±0.007 |
| Nrf2−/− | 6 | 11 | 62.4±3.1 | 0.183±0.011 |
| Nrf2+/+ | 6 | 5 | 70.2±2.6 | 0.191±0.011 |
P<0.001, Nrf2+/+ versus Nrf2−/− of same age by t-test
Testicular and epididymal sperm counts decline with age in Nrf2−/− mice
Testicular and cauda epididymal sperm counts, and cauda epididymal sperm motility in two month old Nrf2−/− mice did not differ significantly from those of Nrf2+/+ males (Figure 3A,B). Consistent with the decreased testicular weights and testicular histology findings in 6 and 7 month old Nrf2−/− male mice, 6 month old Nrf2−/− males had 44% fewer sperm heads per testis on average than wild type mice (Figure 3A; P=0.03, t-test). Sperm heads per milligram testis were also slightly lower in Nrf2−/− than wild type males, but the difference was not statistically significant (P=0.39; Figure 3B). As was noted for the testicular weights and histology, the effect of lack of Nrf2 at 6 months was variable, with some Nrf2−/− males having very low sperm counts and some having counts similar to wild type males. Cauda epididymal sperm numbers were also decreased in those males that had decreased testicular sperm head numbers. There were 65% fewer sperm per cauda and 63% fewer sperm per milligram cauda in Nrf2−/− males compared to wild type males (P=0.006 and P=0.008, respectively; Figure 3C,D). Cauda epididymal sperm motility was 66% lower in the 6 month old Nrf2−/− males than in wild type males, suggesting that lack of Nrf2 may have direct adverse effects on the epididymis in addition to affecting the testis (P=0.006; Figure 3E).
Figure 3.
Nrf2−/− males have lower testicular sperm head counts and cauda epididymal sperm counts and sperm motility than wild type males at 6 months, but not at 2 months of age. A) Mean + SEM testicular sperm heads × 106 per testis. *significantly different from wild type by t-test, P=0.03. B) Mean + SEM testicular sperm heads × 105 per mg testis. N = 5 to 11 per genotype at each age. C) Mean + SEM epididymal sperm × 106 per cauda epididymis. D) Mean + SEM epididymal sperm × 105 per mg cauda epididymis. E) Mean + SEM percent motile cauda epididymal sperm. For C–E, * significantly different from wild type by t-test, P<0.009. N = 5 to 11 per genotype at each age.
Serum testosterone levels do not differ between Nrf2−/− and Nrf2+/+ males
Serum testosterone concentrations were 4.95±2.28 ng/ml at 2 months of age and 1.49±0.75 ng/ml at 6 months in Nrf2+/+ males and 3.84±2.50 ng/ml at 2 months and 1.77±1.03 ng/ml at 6 months in Nrf2−/− males (P>0.05 by t-test at both ages; N=5–8/group). Although testosterone concentrations were lower in the 6 month old males, the effect of age was not statistically significant (P=0.18, effect of age by ANCOVA).
Decreased testicular and epididymal activities and mRNA levels of antioxidant enzymes and GSH concentrations in Nrf2−/− mice
Testicular and epididymal mRNA levels of antioxidant genes were decreased in 2 month old Nrf2−/− males relative to wild type males. Gclc, glutathione transferase a3 (Gsta3), and Gstm1 mRNA levels were significantly lower in Nrf2−/− testes compared to wild type testes (Table 4). Gclc, Gclm, Gstm1, Gstm2, and Sod2 mRNA levels were significantly lower in Nrf2−/− epididymides compared to wild type epididymides (Table 4). Hemeoxygenase 1 (Hmox1) mRNA levels were 36% lower in Nrf2−/− than in wild type testes and 33% lower in Nrf2−/− than in wild type epididymides, but the effects of genotype were not statistically significant (P=0.085 and P=0.183, respectively, by t-test). Gstp, Gstm5, Gpx1, Gpx2, Gpx3, Gpx4 and Gpx5 mRNA levels were unaffected by Nrf2 genotype.
Table 4.
Testicular and epididymal antioxidant gene expression by Nrf2 genotype (fold mean Nrf2+/+ level ± SEM)
| Testis | Epididymis | |||
|---|---|---|---|---|
| Nrf2+/+ | Nrf2−/− | Nrf2+/+ | Nrf2−/− | |
| Gclc | 1.00±0.08 | a0.77±0.02 | 1.00±0.10 | a0.63±0.07 |
| Gclm | 1.00±0.04 | 0.98±0.06 | 1.00±0.08 | a0.71±0.04 |
| Gstm1 | 1.00±0.17 | a0.40±0.08 | 1.00±0.04 | b0.53±0.05 |
| Gstm2 | 1.00±0.14 | 0.79±0.15 | 1.00±0.08 | a0.57±0.12 |
| Gstm5 | 1.00±0.09 | 0.96±0.03 | 1.00±0.09 | 1.00±0.12 |
| Gsta3 | 1.00±0.18 | b0.13±0.02 | 1.00±0.20 | 0.60±0.08 |
| Gstp | 1.00±0.05 | 1.01±0.05 | 1.00±0.11 | 0.73±0.08 |
| Gpx1 | 1.00±0.10 | 1.17±0.18 | 1.00±0.05 | 0.98±0.10 |
| Gpx2 | 1.00±0.41 | 1.08±0.16 | ND | ND |
| Gpx3 | 1.00±0.24 | 1.42±0.22 | 1.00±0.15 | 0.98±0.13 |
| Gpx4 | 1.00±0.26 | 1.19±0.18 | 1.00±0.16 | 1.06±0.20 |
| Gpx5 | ND | ND | 1.00±0.08 | 0.96±0.06 |
| Hmox1 | 1.00±0.08 | c0.64±0.15 | 1.00±0.07 | 0.67±0.21 |
| Sod2 | 1.00±0.14 | 1.00±0.08 | 1.00±0.03 | b0.72±0.06 |
N= 4 per genotype.
P<0.05 versus Nrf2+/+
P<0.01 versus Nrf2+/+
P=0.085 versus Nrf2+/+
ND: Not detected at sufficient levels to permit accurate quantification.
Total GSH and GSSG concentrations did not differ significantly between testes of Nrf2−/− mice and those of their wild type littermates (Table 5). Total GSH concentrations in epididymides of Nrf2−/− males were 34% lower when expressed per mg protein and 35% lower when expressed per mg tissue than in wild type males (P=0.005 and P=0.02, respectively, Table 5). Epididymal GSSG levels did not differ significantly by genotype.
Table 5.
Testicular and epididymal glutathione and basal antioxidant enzyme activities by genotype
| Testis | Epididymis | |||
|---|---|---|---|---|
| Nrf2+/+ | Nrf2−/− | Nrf2+/+ | Nrf2−/− | |
| GSH (nmol/mg protein) | 73.5±3.2 | 78.2±4.2 | 22.2±2.3 | a14.7±1.1 |
| GSH (nmol/mg tissue) | 4.2±0.2 | 3.9±0.1 | 0.99±0.14 | b0.64±0.06 |
| GSSG (nmol/mg protein) | 0.84±0.20 | 0.94±0.10 | 0.76±0.21 | 0.68±0.24 |
| GSSG (nmol/mg tissue) | 0.049±0.013 | 0.045±0.006 | 0.031±0.006 | 0.025±0.006 |
| GR (mU/mg protein) | 61.1±5.8 | 70.8±6.9 | 61.7±4.5 | 55.1±1.8 |
| GR (mU/mg tissue) | 3.2±0.2 | 3.1±0.1 | 3.5±0.1 | b2.4±0.2 |
| GPX (mU/mg protein) | 21.9±1.1 | 22.9±1.0 | 56.5±3.6 | 60.6±2.9 |
| GPX (mU/mg tissue) | 1.4±0.1 | c1.2±0.02 | 3.1±0.1 | a2.6±0.2 |
| GST (mU/mg protein) | 448±31 | 384±25 | 370±36 | 428±21 |
| GST (mU/mg tissue) | 22.2±1.2 | b16.8±0.2 | 20.7±1.1 | d19.0±0.4 |
N=5 to 10 two month old mice/group, except Nrf2−/− epididymal GST, N=17
P<0.05, Nrf2−/− versus Nrf2+/+ by t-test
P<0.01, Nrf2−/− versus Nrf2+/+ by t-test
P=0.06, Nrf2−/− versus Nrf2+/+ by t-test
P=0.04, Nrf2−/− versus Nrf2+/+ ANCOVA with age as covariate because measured epididymal GST in mix of 2 and 6 month old mice
The enzymatic activity of glutathione reductase was 31% lower in epididymides of Nrf2−/− males than in Nrf2+/+ males when expressed in terms of mU per milligram of tissue (P<0.01; Table 5). Similarly, the enzymatic activity of glutathione peroxidase was 16% lower in epididymides of Nrf2−/− males than in Nrf2+/+ males when expressed in terms of mU per milligram of tissue (P<0.05; Table 5). The activity of glutathione peroxidase also tended to be lower in testes of Nrf2−/− males, but the effect of genotype did not reach statistical significance (P=0.06). The GST enzymatic activity, in contrast, was 24% lower when expressed as mU per milligram tissue and 14% lower when expressed as mU per milligram protein in Nrf2−/− testes than in Nrf2+/+ testes (P=0.001 when expressed per mg tissue, Table 5). The epididymal GST activity was 8% lower when expressed as mU per milligram tissue in Nrf2−/− males than in Nrf2+/+ males (P=0.04, Table 5).
Testicular and epididymal oxidative stress in Nrf2−/− males
To test whether the spermatogenic defects observed in Nrf2−/− males are associated with oxidative stress, we measured levels of lipid hydroperoxides (malondialdehyde, MDA, and 4-hydroyalkenals, HAE) in testicular and epididymal homogenates collected from 3 month old Nrf2−/− male mice and wild type littermates. Three months corresponded to the youngest post-pubertal age at which significant differences in testes weights were observed (Table 1). MDA and HAE levels were about 23% higher in Nrf2−/− testes than in wild type testes when expressed per mg protein (P=0.006 by t-test; Table 6). Levels of MDA and HAE were more than 2-fold higher in epididymides than in testes in both Nrf2−/− and Nrf2+/+ males. More importantly, Nrf2−/− males had statistically significantly higher epididymal MDA and HAE levels than their wild type littermates (59% higher in Nrf2−/− when expressed per mg protein, P=0.001; Table 6). Immunostaining using an antibody directed against 4-hydroxynonenal revealed a trend towards increased germ cell staining in the seminiferous tubules of Nrf2−/− mice compared to Nrf2+/+ mice at 4 months of age (P=0.076), but not at 2 months of age (Supplemental Figure S2). 4-HNE positive interstitial cells were frequently observed in testes from mice of both genotypes (Supplemental Figure S2).
Table 6.
Lipid peroxidation products (MDA plus HAE) in testes and epididymes by genotype
| Testis | Epididymis | |||
|---|---|---|---|---|
| Nrf2+/+ | Nrf2−/− | Nrf2+/+ | Nrf2−/− | |
| N per group | 6 | 6 | 6 | 6 |
| pmol/mg tissue | 9.6±0.4 | a10.7±0.4 | 24.5±2.5 | b31.9±2.0 |
| pmol/mg protein | 173±9 | c212±7 | 401±36 | c637±40 |
P=0.09, Nrf2−/− versus Nrf2+/+ by t-test
P<0.05, Nrf2−/− versus Nrf2+/+ by t-test
P<0.01, Nrf2−/− versus Nrf2+/+ by t-test
We used 8-OHdG immunostaining to examine testicular DNA oxidation in 2 and 4 month old males. At both ages, testicular 8-OHdG immunostaining did not differ between wild type and Nrf2−/− males. An average of 8.5±0.5% and 6.0±1.0% of the seminiferous tubules had any 8-OHdG positive cells in wild type testes at 2 and 4 months of age, respectively, and 7.0±1.1% and 7.0±1.3% of the tubules had 8-OHdG positive cells in 2 and 4 month old Nrf2−/− testes. The average number of 8-OHdG positive cells per seminiferous tubule cross-section also did not differ by genotype (0.21±0.02 and 0.13±0.03 in wild type versus 0.32±0.14 and 0.18±0.05 in Nrf2−/− at 2 and 4 months of age, respectively).
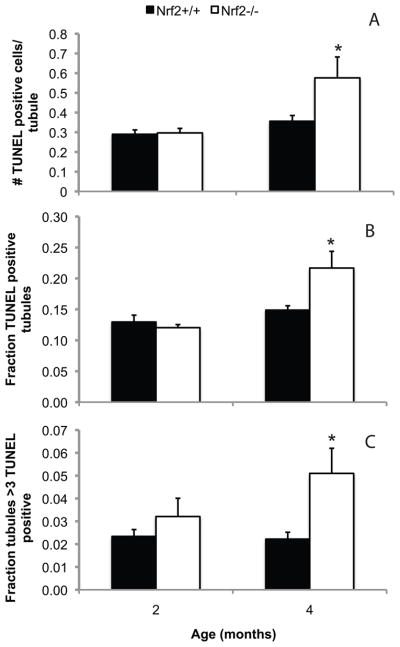
Knockout of Nrf2 increases testicular apoptosis
Apoptotic cells were rarely observed in the testes of 2 or 4 month old Nrf2+/+ males or in testes of 2 month old Nrf2−/− males (Figure 4). However, by 4 months of age, the number of apoptotic germ cells per seminiferous tubule cross-section was 1.7-fold greater, the fraction of tubule cross-sections with any apoptotic germ cells was 1.4-fold greater, and the fraction of tubule cross-sections with greater than 3 apoptotic germ cells was 2.3-fold greater in Nrf2−/− males than in their wild type littermates (P=0.027, P=0.044, P=0.018 by Mann Whitney test, respectively). In tubules that appeared to be in the early stages of degeneration, germ cells near the basement membrane were most often TUNEL positive in testes of both genotypes (Supplemental Figure S3). In tubules that appeared to be in the later stages of degeneration, in which there were few remaining germ cells, TUNEL positive spermatids were also noted.
Figure 4.
Nrf2−/− males have more germ cell apoptosis than wild type males at 4 months, but not at 2 months of age. Testes were processed for detection of apoptotic cells by TUNEL as described in Methods. Sections were counterstained with hematoxylin. A) Mean + SEM average number of TUNEL positive, apoptotic germ cells per seminiferous tubule cross-section. B) Mean + SEM fraction of seminiferous tubule cross-sections with any TUNEL positive germ cells. C) Mean + SEM fraction of seminiferous tubule cross-sections with greater than 3 TUNEL positive germ cells. *P<0.05 versus wild type of same age by Mann Whitney test. N = 4 to 6 per genotype and age.
Effects of vitamin E supplementation on spermatogenesis in Nrf2 knockout males
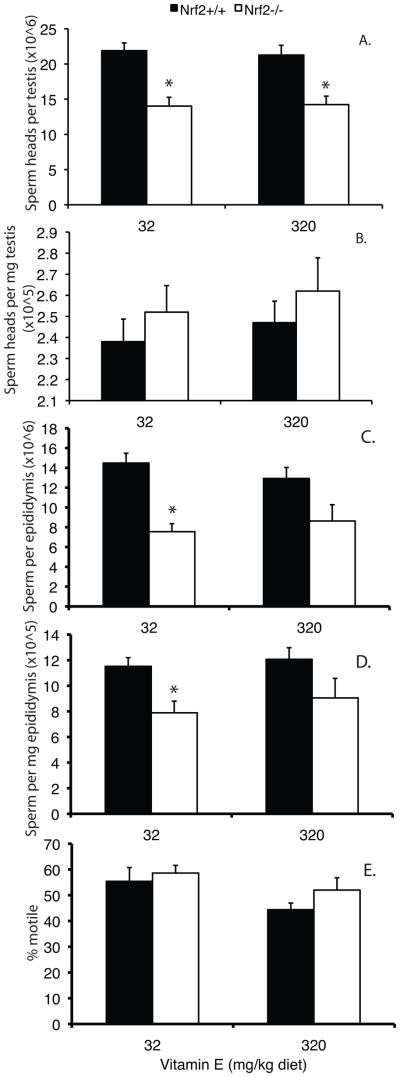
Nrf2−/− and Nrf2+/+ male mice were fed diets containing either the minimum daily requirement for vitamin E (as DL-alpha tocopherol acetate) of 32 mg/kg diet (low) or 320 mg/kg diet (high) from the age of 21 days until 4 months. Weight gain and amount of feed consumed did not differ by genotype or diet (data not shown). Total testicular sperm head counts were 51% lower in Nrf2−/− males on the low vitamin E diet than in wild type controls (Figure 5A). The high vitamin E diet did not increase testicular sperm head numbers in Nrf2−/− males. There were no significant effects of diet or genotype on sperm heads per mg testis (Figure 5B). Both sperm per epididymis and sperm per mg epididymis increased slightly in Nrf2−/− males on the high vitamin E diet compared to Nrf2−/− males on the low vitamin E diet; however, the effects of diet were not statistically significant (P=0.566 and P=0.526, respectively) (Figure 5C,D). For mice fed the low vitamin E diet, the effects of genotype on sperm per epididymis and sperm per mg epididymis were statistically significant (P<0.001 and P=0.008 by t-test); however, the effects of genotype were not statistically significant for mice fed the high vitamin E diet (P=0.051 and P=0.114).
Figure 5.
Effects of low and high vitamin E supplemented diets on testicular and epididymal sperm parameters in Nrf2−/− mice. Nrf2−/− and Nrf2+/+ male mice were fed diets supplemented with the minimum daily requirement of vitamin E (32 mg/kg diet) or 320 mg/kg diet from the age of 21 days to 4 months, when sperm parameters were measured. A) Mean + SEM sperm heads × 106 per testis. The effect of genotype was statistically significant by 2-way ANOVA (P<0.001). *significantly different from wild type on same diet by t-test, P<0.003). B) Mean + SEM sperm heads × 105 per mg testis. No statistically significant differences by 2-way ANOVA. C) Mean + SEM sperm × 106 per cauda epididymis. The effect of genotype was statistically significant by 2-way ANOVA (P<0.001). *significantly different from wild type on same diet by t-test, P<0.001. D) Mean + SEM sperm × 105 per mg cauda epididymis. The effect of genotype was statistically significant by 2-way ANOVA (P=0.004). *significantly different from wild type on same diet by t-test, P=0.008. E) Mean + SEM percent motile cauda epididymal sperm. The effect of diet was marginally statistically significant by 2-way ANOVA (P=0.044). N = 7/group.
This experiment provided additional data on the timing of the onset of the declines in sperm counts in the Nrf2−/− males. The 4 month old Nrf2−/− males had significantly fewer sperm heads per testis, sperm per epididymis, and sperm per milligram epididymis than Nrf2+/+ males (P<0.001, P<0.001, P=0.004, respectively, effects of genotype by 2-way ANOVA; Figure 5A,C,D). However, the effects on sperm counts were not as pronounced as at 6 months of age (compare with Figure 3). Interestingly, the effect of lack of Nrf2 on cauda epididymal sperm motility was not yet apparent at 4 months of age (Figure 5E).
There was no effect of either Nrf2 genotype or vitamin E supplementation on epididymal sperm morphology (Supplemental Table S1).
Effects of lack of Nrf2 on autoimmunity
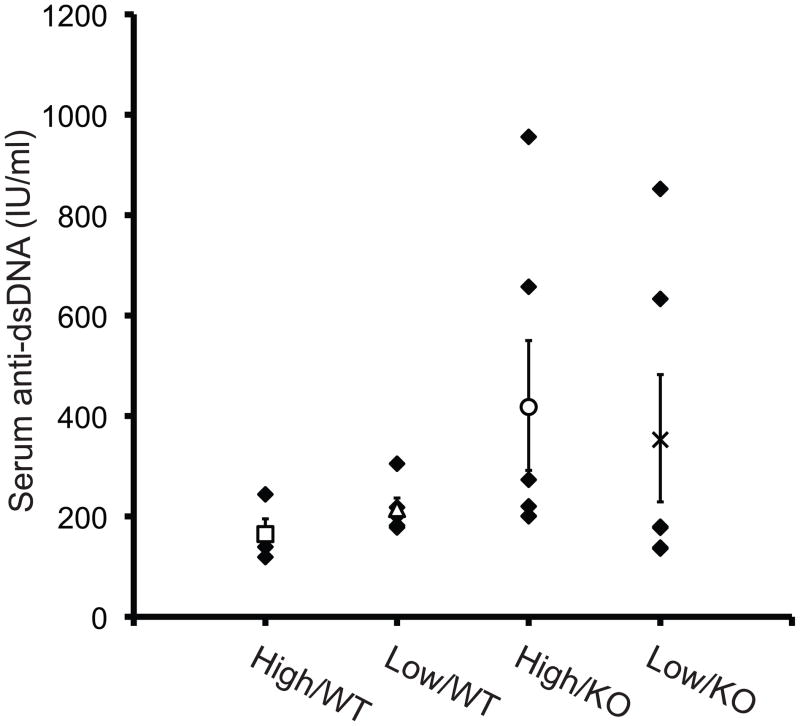
Nrf2−/− mice on a 129SvJ genetic background were previously reported to develop autoimmunity, as indicated by increased titers of antibodies against dsDNA and glomerulonephritis [23]. To investigate whether our mice on a C57BL/6NCrl genetic background had evidence of autoimmunity, we measured dsDNA antibodies and evaluated renal histology in males from the vitamin E supplementation study. Four of 12 Nrf2−/− males (two each in the high and low vitamin E diet groups) evaluated had elevated dsDNA antibodies compared to 0 of 10 wild type controls (Figure 6). However, there was no significant correlation between dsDNA antibody level and testicular weight (r=−0.040, P=0.903), testicular sperm count (r=0.091, P=0.778), or epididymal sperm count (r=−0.134, P=0.677) in the knockout males. Kidney weights were not affected by Nrf2 genotype or diet (data not shown). Glomerulonephritis was not detected in Nrf2−/− males (N=13, including the 4 males with elevated dsDNA levels) or wild type controls (N=8). The only abnormality noted was focal, minimal tubular mineralization in one of the knockout males.
Figure 6.
Serum anti-dsDNA titers. Blood was collected by cardiac puncture from mice in the vitamin E supplementation study described in Figure 5, and serum dsDNA antibodies were measured using a mouse ELISA Kit. The x-axis labels indicate high or low vitamin E diet and Nrf2+/+ (WT) or Nrf2−/− (KO) genotype. Dark symbols indicate the values for individual mice in each group. Open symbols and error bars indicate the mean and standard error of the values in that group. The effects of genotype and diet were not statistically significant by Mann Whitney test. N=4–6/group.
Discussion
Increased levels of reactive oxygen species and oxidative stress in sperm are thought to play a causal role in male infertility [5, 6]. The transcription factor NRF2 regulates the expression of genes encoding enzymes that are important for detoxification of ROS [15–19]. Polymorphisms in the promoter of the Nrf2 gene exist in humans, but their effects on male reproductive function have not been investigated [43]. This study is the first to show a role for NRF2 in male fertility. Male Nrf2−/− mice had age-related declines in testicular weights and testicular and epididymal sperm counts and increased seminiferous tubule vacuolization compared to wild type littermates between two and seven months of age. Prior to observable differences in sperm counts or testis weights, Nrf2−/− males had lower testicular and/or epididymal levels of GSH and of antioxidant gene expression and enzymatic activities than Nrf2+/+ males. Elevated levels of testicular lipid hydroperoxides and increased numbers of apoptotic germ cells were observed concomitant with the earliest significant decreases in testicular weights, at 3 to 4 months of age. These findings provide evidence that decreased testicular antioxidant capacity led to increased testicular oxidative stress, which caused germ cell apoptosis, decreased sperm production, and ultimately decreased fertility in Nrf2−/− males.
There were minimal histological abnormalities noted in Nrf2−/− testes at 35 days of age, and there were no genotype-related differences in testicular or epididymal sperm counts or testis weights at 2 months of age. However, the testes of Nrf2−/− males underwent a process of degeneration that worsened with age, as demonstrated by the declining testicular weights with increasing age from 2 to 7 months and the statistically significantly decreased sperm counts at 4 and 6 months of age. The worsening with age of the testicular defects observed in Nrf2−/− mice are reminiscent of the worsening of hemolytic anemia between 6 and 14 months of age reported by Lee and coworkers [22]. This group reported that Nrf2−/− mice have a regenerative, immune-mediated hemolytic anemia that is mild at 6 months of age and more severe at 14 months of age [22].
Our data provide evidence that the age-related decline in testicular spermatogenesis in Nrf2−/− male mice is caused by chronic oxidative stress and the accumulation of oxidative damage to the seminiferous tubules over time due to inadequate testicular antioxidant activities. Testes of young Nrf2−/− males had significantly increased levels of testicular lipid peroxidation compared to wild type males, consistent with oxidative stress. Testicular expression of Gstm1 and Gsta3 was significantly decreased in Nrf2−/− males, as was testicular GST enzymatic activity. Although the role of GSTs in Phase 2 detoxification of xenobiotics is most well-known, many GSTs also exhibit peroxidase activity and this peroxidase activity appears to be particularly critical in the testis [44]. Inhibition of GST activity in cultured rat germ cells increased their sensitivity to hydrogen peroxide-induced apoptosis and lipid peroxidation [44]. Oxidative stress has also been implicated in the pathophysiology of hemolytic anemia [22] and of autoimmune glomerulonephritis [23] in Nrf2−/− mice. A recent study by Lu and coworkers revealed a similar testicular phenotype in mice with a mutation in the inner mitochondrial membrane peptidase 2-like gene (Immp2l) [45]. The Immp2l mutant mice had increased testicular and spermatozoal superoxide generation. Although they had normal testicular histology and sperm production at 2 to 3 months of age, by 7 months they had vacuolated seminiferous tubules and reduced germ cell numbers [45]. These findings are very similar to our findings in the Nrf2−/− males. Peroxiredoxin 4 knockout mice also displayed increased testicular oxidative damage and concurrent increase in TUNEL positive cells in seminiferous tubules compared to wild type mice at 8 weeks of age [46]. Together our findings in the present study and these other studies suggest that testicular spermatogenesis is highly susceptible to disruption by chronic oxidative stress, whether it is due to decreased antioxidant capacity in the case of Nrf2 or Peroxiredoxin 4 knockouts or increased generation of ROS in the case of Immp2l mutants. Others have reported similar histological changes, including seminiferous tubule vacuolization and Sertoli cell only tubules, in the testes of very aged males [47, 48]. Oxidative stress is thought to play a role in the age-related decline in function of many organs, including the testis [7, 49]. We speculate that the testicular phenotype of the Nrf2 knockout males may represent an acceleration, due to chronically increased oxidative stress, of processes that occur during normal testicular aging.
The inability of a moderately high vitamin E-supplemented diet to prevent the age-related spermatogenic deficits in the present study does not rule out oxidative stress as the cause of the deficits for several reasons. First, it is possible that the dose of vitamin E may not have been sufficiently high. Second, multiple antioxidant supplements may be required since lack of Nrf2 affects expression of multiple antioxidant enzymes, which act by different mechanisms. It has been suggested that supplementation with antioxidant mixtures, containing compounds with different mechanisms of action and different cellular targets, may be more efficacious than supplementation with single antioxidants at preventing age-related changes. Lemon and coworkers showed that a complex anti-aging supplement containing 31 components, including many antioxidants, prevented age-related cognitive decline and extended longevity in a transgenic mouse model of accelerated aging [50, 51]. Finally, it is possible that supplementation with antioxidants must begin earlier in life than 21 days to prevent the age-related decline in spermatogenesis in Nrf2−/− males.
The lack of an effect of Nrf2 genotype on serum testosterone concentrations combined with severe histological abnormalities of seminiferous tubules suggests that Sertoli cells or germ cells and not Leydig cells are the targets of oxidative damage in the testes of Nrf2−/− males. There have been a few previous studies that showed that treatment in vivo with pharmacological agents that induce oxidative stress in the testis disrupt testicular spermatogenesis. Kumar and Muralidhara reported that male rats treated chronically with organic hydroperoxides had increased testicular ROS, lipid peroxidation, and DNA damage and decreased epididymal sperm counts compared to controls [52]. Similar effects were observed in male mice treated with tert-butyl hydroperoxide [53]. Cattan et al showed that depletion of the antioxidant GSH in male mice by treatment with buthionine sulfoximine resulted in increased testicular oxidative stress, as indicated by increased protein carbonyls, and shortened testicular telomere length [54].
In addition to the clearly adverse effect of lack of NRF2 on the seminiferous tubules, our studies also provide evidence that lack of NRF2 has detrimental effects during the epididymal phase of spermatogenesis. During the epididymal phase, sperm become capable of motility on release into physiological media. We found an age-related decline in epididymal sperm motility in Nrf2−/− males, but not in wild type males, consistent with an adverse effect in the epididymis. The observation that the rate of the age-related decline in sperm motility was slower than the rates of decline in sperm counts (sperm counts were significantly decreased by 4 months of age, but motility was not significantly decreased until 6 months) also argues for an independent effect of Nrf2 knockout on the epididymis. Epididymides of Nrf2−/− males had increased lipid peroxidation and decreased expression of several GSTs, Sod2, and the subunits of the rate-limiting enzyme in GSH synthesis (Gclc and Gclm), decreased enzymatic activities of GR, GPX, and GST, and decreased concentrations of GSH compared to Nrf2+/+ males. Previous studies have shown that spermatozoa are susceptible to oxidative damage due to their high polyunsaturated fatty acid content [55]. Consistent with our finding of decreased sperm motility in Nrf2−/− male mice, treatment of male mice with tert-butyl hydroperoxide decreased epididymal sperm motility [53]. Oxidative stress has also been implicated in decreased sperm motility in men. About 10% of infertile men had greatly decreased sperm Gpx4 expression compared to normal men, and these men also had decreased sperm motility that declined more quickly over time after ejaculation [56]. Recent work by Eddy and coworkers has shown that activation of spermatogenic cell-specific Type 1 hexokinase (HK1S) is necessary for the acquisition of motility and that HK1S activation requires cleavage of HK1S dimers to active monomers by cleavage of disulfide bonds [57]. In their study thioredoxin-1 was able to reduce HK1S to the monomeric form in vitro.
The effect of lack of Nrf2 on the expression of antioxidant genes is tissue- and gene-dependent. Decreased or no differences in basal expression of Gsta3, Gstm1, Gstm5, Gstp, Gclc, and Gclm have been reported in Nrf2−/− mice compared to wild type mice, depending on the tissue examined [15, 18, 19]. The present results add to this body of knowledge by reporting on the effects of lack of Nrf2 on the basal expression of these genes in testis and epididymis for the first time. The effects of Nrf2 knockout on enzymatic activities have been less frequently reported than effects on transcription. Basal GR and GST activities were significantly lower and basal GPX activity was non-significantly lower in Nrf2−/− compared to wild type cardiac fibroblasts [58]. Basal GST activity was also significantly lower in Nrf2−/− than wild type forestomach and liver [19]. In contrast, basal GST activity did not differ by Nrf2 genotype in small intestine [18]. The modest genotype-related differences in GPX enzymatic activity in the absence of differences in gene expression in the present study could be due to the effects of increased ROS caused by lack of NRF2 on GPX enzymatic activity. For example, hydrogen peroxide was reported to directly inhibit GPX-1 enzymatic activity in red blood cells [59].
We examined various endpoints that were previously reported to be adversely affected by lack of Nrf2−/− on a different genetic background (129SvJ versus C57BL/6NCrl in our study) [23]. We never observed any clinical signs of illness or mortality in our male Nrf2−/− mice up to 7 months of age, and there was no histological evidence of glomerulonephritis in Nrf2−/− males at 4 months of age. There are likely two reasons for these differences. First, Ma et al reported that clinical signs and mortality occurred to a much greater extent in Nrf2−/− females than in males [23]. For example, at 40 weeks only about 45% of the Nrf2−/− females were surviving, compared to about 75% of the Nrf2−/− males and 100% of wild type mice of both sexes [23]. Evidence of immunological disease, such as glomerulonephritis was also much more prevalent in females and occurred in females at younger ages than in males [23]. The second likely reason for the differences between our study and that of Ma and coworkers is the different genetic backgrounds. Genetic background has been shown to dramatically modulate the effects of deletion of other genes. For example, the effects of Dax knockout on gonadal differentiation are much more pronounced on a C57BL/6J background than on a 129/SvlmJ background [60]. The effects of lack of Sod2 also vary with background strain, with embryonic lethality occurring on a C57BL/6J background compared to survival to postnatal day 8 on a DBA/2J background [61]. Although 4 of 12 Nrf2−/− males evaluated had elevated ds-DNA antibodies compared to 0 of 10 wild type controls, there was no correlation between dsDNA antibody level and testicular weight, testicular sperm count, or epididymal sperm count in the knockout males, and there was no histological evidence of testicular inflammation in knockout males of various ages. Based on all of the above observations, we conclude that it is unlikely that autoimmune disease was the cause of the decline in spermatogenesis observed in our study.
In conclusion, our results show that lack of the transcription factor NRF2 results in profound age-related disruption of spermatogenesis. Vacuolization of seminiferous tubules, decreased testicular weights, decreased testicular and epididymal sperm counts, and decreased sperm motility were not observed in young adult Nrf2−/− males, but became pronounced with increasing age. Our results provide evidence that decreased testicular and epididymal antioxidant capacity in Nrf2−/− males result in oxidative damage to seminiferous tubules and epididymal sperm. Based on these results, the association of polymorphisms in Nrf2 with poor sperm quality and infertility in men should be investigated.
Supplementary Material
Acknowledgments
This work was supported by the University of California Irvine (UCI) Academic Senate Council on Research, Computing, and Library Resources (SIIG-2006-2007-16 to U.L.), by NIH AG032087 (to U.L.), by the UCI Chao Family Comprehensive Cancer Center Experimental Tissue Resource (NIH CA62203), by UCI Undergraduate Research Opportunities Program Fellowships (to L.O. and B.A.R.), by the UCI Office of Research, and by the UCI Center for Occupational and Environmental Health.
The authors thank Dr. Grant MacGregor for helpful discussions on the interpretation of the testicular and epididymal histology. The authors thank Lee Sutherland of the UCI Chao Family Comprehensive Cancer Center Experimental Tissue Resource for preparation of histological specimens.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanukoglu I. Antioxidant Protective Mechanisms against Reactive Oxygen Species (ROS) Generated by Mitochondrial P450 Systemes in Steroidogenic Cells. Drug Metab Rev. 2006;38:171–196. doi: 10.1080/03602530600570040. [DOI] [PubMed] [Google Scholar]
- 2.de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C. Reactive Oxygen Species and Sperm Physiology. Rev Reprod. 1997;2:48–54. doi: 10.1530/ror.0.0020048. [DOI] [PubMed] [Google Scholar]
- 3.Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, Krause K-H. A Ca2+-activated NADPH Oxidase in Testis, Spleen, and Lymph Nodes. J Biol Chem. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 4.Drevet JR. The Antioxidant Glutathione Peroxidase Family and Spermatozoa: A Complex Story. Mol Cell Endocrinol. 2006;250:70–79. doi: 10.1016/j.mce.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Baker MA, Aitken RJ. Reactive Oxygen Species in Spermatozoa: Methods for Monitoring and Significance for the Origins of Genetic Disease and Infertility. Repro Biol Endocrinol. 2005;3:67. doi: 10.1186/1477-7827-3-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griveau JF, Le Lannou D. Reactive Oxygen Species and Human Spermatozoa: Physiology and Pathology. Int J Androl. 1997;20:61–69. doi: 10.1046/j.1365-2605.1997.00044.x. [DOI] [PubMed] [Google Scholar]
- 7.Luo L, Chen H, Trush MA, Show MD, Anway MD, Zirkin BR. Aging and the Brown Norway Rat Leydig Cell Antioxidant Defense System. J Androl. 2006;27:240–247. doi: 10.2164/jandrol.05075. [DOI] [PubMed] [Google Scholar]
- 8.Weir CP, Robaire B. Spermatozoa Have Decreased Antioxidant Enzymatic Capacity and Increased Reactive Oxygen Species Production during Aging in the Brown Norway Rat. J Androl. 2007;28:229–240. doi: 10.2164/jandrol.106.001362. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Liu J, Luo L, Baig MU, Kim J-M, Zirkin BR. Vitamin E, Aging, and Leydig Cell Steroidogenesis. Exp Gerontol. 2005;40:728–736. doi: 10.1016/j.exger.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Cangello D, Benson S, Folmer J, Zhu H, Trush MA, Zirkin BR. Age-Related Increase in Mitochondrial Superoxide Generation in the Testosterone-Producing Cells of the Brown Norway Rat Testes: Relationship to Reduced Steroidogenic Function? Exp Gerontol. 2001;36:1361–1373. doi: 10.1016/s0531-5565(01)00118-8. [DOI] [PubMed] [Google Scholar]
- 11.Wild AC, Moinova HR, Mulcahy RT. Regulation of gamma-Glutamylcysteine Synthetase Subunit Gene Expression by the Transcription Factor Nrf2. J Biol Chem. 1999;274:33627–33636. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen T, Sherratt PJ, Pickett CB. Regulatory Mechanisms Controlling Gene Expression Mediated by the Antioxidant Response Element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 13.Hayes JD, McMahon M. Molecular Basis for the Contribution of the Antioxidant Responsive Element to Cancer Chemoprevention. Cancer Lett. 2001;174:103–113. doi: 10.1016/s0304-3835(01)00695-4. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi M, Yamamoto M. Molecular Mechanisms Activating the Nrf2-Keap1 Pathway of Antioxidant Gene Regulation. Antioxid Redox Signal. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 15.Chan JY, Kwong M. Impaired Expression of Glutathione Synthetic Enzyme Genes in Mice with Targeted Deletion of the Nrf2 Basic-Leucine Zipper Protein. Biochim Biophys Acta. 2000;1517:19–26. doi: 10.1016/s0167-4781(00)00238-4. [DOI] [PubMed] [Google Scholar]
- 16.Chan K, Kan YW. Nrf2 Is Essential for Protection against Acute Pulmonary Injury in Mice. Proc Natl Acad Sci U S A. 1999;96:12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwak M-K, Itoh K, Yamamoto M, Sutter TR, Kensler TW. Role of Transcription Factor Nrf2 in the Induction of Hepatic Phase 2 and Antioxidant Enzymes in vivo by the Cancer Chemoprotective Agent, 3H-1,2-Dithiole-3-thione. Mol Med. 2001;7:135–145. [PMC free article] [PubMed] [Google Scholar]
- 18.McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, Wolf CR, Cavin C, Hayes JD. The Cap ‘n’ Collar Basic Leucine Zipper Transcription Factor Nrf2 (NF-E2 p45-related factor 2) Controls both Constitutive and Inducible Expression of Intestinal Detoxification and Glutathione Biosynthesis Enzymes. Cancer Res. 2001;61:3299–3307. [PubMed] [Google Scholar]
- 19.Ramos-Gomez M, Kwak M-K, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to Carcinogenesis Is Increased and Chemoprotective Efficacy of Enzyme Inducers Is Lost in nrf2 Transcription Factor-Deficient Mice. Proc Natl Acad Sci U S A. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu X, Roberts JR, Apopa PL, Kan YW, Ma Q. Accelerated Ovarian Failure Induced by 4-Vinyl Cyclohexene Diepoxide in Nrf2 Null Mice. Mol Cell Biol. 2006;26:940–954. doi: 10.1128/MCB.26.3.940-954.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan K, Han X-D, Kan YW. An Important Function of Nrf2 in Combating Oxidative Stress: Detoxification of Acetaminophen. Proc Natl Acad Sci. 2001;98:4611–4616. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J-M, Chan K, Kan YW, Johnson JA. Targeted Disruption of Nrf2 Causes Regenerative Immune-Mediated Hemolytic Anemia. Proc Natl Acad Sci U S A. 2004;101:9751–9756. doi: 10.1073/pnas.0403620101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Q, Battelli L, Hubbs AF. Multiorgan Autoimmune Inflammation, Enhanced Lymphoproliferation, and Impaired Homeostasis of Reactive Oxygen Species in Mice Lacking the Antioxidant-Activated Transcription Factor Nrf2. Am J Pathol. 2006;168:1960–1974. doi: 10.2353/ajpath.2006.051113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan K, Lu R, Chang JC, Kan YW. NRF2, a Member of the NFE2 Family of Transcription Factors, Is not Essential for Murine Erythropoiesis, Growth, and Development. Proc Natl Acad Sci U S A. 1996;93:13493–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NRC . Guide for the Care and Use of Laboratory Animals. Washington, DC: National Research Council, National Academy of Sciences; 1996. [Google Scholar]
- 26.Chapin RE, Sloane RA. Reproductive Assessment by Continuous Breeding: Evolving Study Design and Summaries of Ninety Studies. Environ Health Perspect. 1997;105(Suppl 1):199–205. doi: 10.1289/ehp.97105s1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapin RE, Dutton SL, Ross MD, Lamb JC., IV Effects of Ethylene Glycol Monomethyl Ether (EGME) on Mating Performance and Epididymal Sperm Parameters in F344 Rats. Fundam Appl Toxicol. 1985;5:182–189. doi: 10.1016/0272-0590(85)90063-6. [DOI] [PubMed] [Google Scholar]
- 28.Filler R. Methods for Evaluation of Rat Epididymal Sperm Morphology. In: Chapin RE, Heindel JJ, editors. Male Reproductive Toxicology. San Diego, CA: Academic Press, Inc; 1993. pp. 334–343. [Google Scholar]
- 29.Revel A, Raanani H, Younglai E, Xu J, Han R, Savouret J-F, Casper RF. Resveratrol, a Natural Aryl Hydrocarbon Receptor Antagonist, Protects Sperm from DNA Damage and Apoptosis by Benzo[a]pyrene. Reprod Toxicol. 2001;15:479–486. doi: 10.1016/s0890-6238(01)00149-6. [DOI] [PubMed] [Google Scholar]
- 30.Blazak WF, Treinen KA, Juniewicz PE. Applications of Testicular Sperm Head Counts in the Assessment of Male Reproductive Toxicity. In: Chapin RE, Heindel JJ, editors. Male Reproductive Toxicology. San Diego: Academic Press; 1993. pp. 86–94. [Google Scholar]
- 31.Dunnick JK, Harris MW, Chapin RE, Hall LB, Lamb JC., IV Reproductive Toxicology of Methyldopa in Male F344/N Rats. Toxicology. 1986;41:305–318. doi: 10.1016/0300-483x(86)90184-8. [DOI] [PubMed] [Google Scholar]
- 32.Lopez SG, Luderer U. Effects of Cyclophosphamide and Buthionine Sulfoximine on Ovarian Glutathione and Apoptosis. Free Radic Biol Med. 2004;36:1366–1377. doi: 10.1016/j.freeradbiomed.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 33.Luderer U, Diaz D, Faustman EM, Kavanagh TJ. Localization of Glutamate Cysteine Ligase Subunit mRNA within the Rat Ovary and Relationship to Follicular Atresia. Mol Reprod Dev. 2003;65:254–261. doi: 10.1002/mrd.10298. [DOI] [PubMed] [Google Scholar]
- 34.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, Fitzgerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJn, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC. Biomarkers of Oxidative Stress Study II: Are Oxidation Products of Lipids, Proteins, and DNA Markers of CCl4 Poisoning? Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 35.Tsai-Turton M, Luderer U. Gonadotropin Regulation of Glutamate Cysteine Ligase Catalytic and Modifier Subunit Expression in the Rat Ovary is Subunit and Follicle Stage-Specific. Am J Physiol. 2005;289:E391–E402. doi: 10.1152/ajpendo.00531.2004. [DOI] [PubMed] [Google Scholar]
- 36.Griffith OW. Determination of Glutathione and Glutathione Disulfide using Glutathione Reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 37.Luderer U, Kavanagh TJ, White CC, Faustman EM. Gonadotropin Regulation of Glutathione Synthesis in the Rat Ovary. Reprod Toxicol. 2001;15:495–504. doi: 10.1016/s0890-6238(01)00157-5. [DOI] [PubMed] [Google Scholar]
- 38.Flohé L, Günzler WA. Assays of Glutathione Peroxidase. Methods Enzymol. 1984;105:114–120. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 39.Delides A, Spooner RJ, Goldberg DM, Neal FE. An Optimized Semi-Automatic Rate Method for Serum Glutathione Reductase Activity and Its Application to Patients with Malignant Disease. J Clin Pathol. 1976;29 doi: 10.1136/jcp.29.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaneko T, Iuchi Y, Kawachiya S, Fujii T, Saito H, Kurachi H, Fujii J. Alteration of Glutathione Reductase Expression in the Female Reproductive Organs during the Estrous Cycle. Biol Reprod. 2001;65:1410–1416. doi: 10.1095/biolreprod65.5.1410. [DOI] [PubMed] [Google Scholar]
- 41.Mannervik B, Jemth P. Measurement of Glutathione Transferases. In: Bus JS, Costa LG, Hodgson E, Lawrence DA, Reed DJ, editors. Current Protocols in Toxicology. John Wiley and Sons, Inc; 2008. pp. 6.4.1–6.4.10. [Google Scholar]
- 42.Xu Z, Chen LC, Leung L, Yen TSB, Lee C, Chan JY. Liver-Specific Inactivtion of the Nrf1 Gene in Adult Mouse Leads to Nonalcoholic Steatohepatisis and Hepatic Neoplasia. Proc Natl Acad Sci U S A. 2005;102:4120–4125. doi: 10.1073/pnas.0500660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto T, Yoh K, Kobayashi A, Ishii Y, Kure S, Koyama A, Sakamoto T, Sekizawa K, Motohashi H, Yamamoto M. Identification of Polymorphisms in the Promoter Region of the Human NRF2 Gene. Biochem Biophys Res Commun. 2004;321:72–79. doi: 10.1016/j.bbrc.2004.06.112. [DOI] [PubMed] [Google Scholar]
- 44.Rao AVS, Shaha C. Role of Glutathione S-Transferases in Oxidative Stress-Induced Male Germ Cell Apoptosis. Free Radic Biol Med. 2000;29:1015–1027. doi: 10.1016/s0891-5849(00)00408-1. [DOI] [PubMed] [Google Scholar]
- 45.Lu B, Poirier C, Gaspar T, Gratzke C, Harrison W, Busija D, Matzuk MM, Andersson K-E, Overbeek PA, Bishop CE. A Mutation in the Inner Mitochondrial Membrane Peptidase 2-Like Gene (Immp2l) Affects Mitochondrial Function and Impairs Fertility in Mice. Biol Reprod. 2008;78:601–610. doi: 10.1095/biolreprod.107.065987. [DOI] [PubMed] [Google Scholar]
- 46.Iuchi Y, Okada F, Tsunoda S, Kibe N, Shirasawa N, Ikawa M, Okabe M, Ikeda Y, Fujii J. Peroxiredoxin 4 Knockout Results in Elevated Spermatogenic Cell Death via Oxidative Stress. Biochem J. 2009;419:149–158. doi: 10.1042/BJ20081526. [DOI] [PubMed] [Google Scholar]
- 47.Levy S, Serre V, Hermo L, Robaire B. The Effects of Aging on the Seminiferous Epithelium and the Blood-Testis Barrier of the Brown Norway Rat. J Androl. 1999;20:356–365. [PubMed] [Google Scholar]
- 48.Morales E, Horn R, Pastor LM, Santamaria L, Pallarés J, Zuasti A, Ferrer CMC. Involution of Seminiferous Tubules in Aged Hamsters: An Ultrastructural, Immunohistochemical and Quantitative Morphological Study. Histol Histopathol. 2004;19:445–455. doi: 10.14670/HH-19.445. [DOI] [PubMed] [Google Scholar]
- 49.Harper M-E, Bevilacqua L, Hagopian K, Weindruch R, Ramsey JJ. Ageing, Oxidative Stress, and Mitochondrial Uncoupling. Acta Physiol Scand. 2004;182:321–331. doi: 10.1111/j.1365-201X.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- 50.Lemon JA, Boreham DR, Rollo CD. A Dietary Supplement Abolishes Age-Related Cognitive Decline in Transgenic Mice Expressing Elevated Free Radical Processes. Exp Biol Med. 2003;228:800–810. doi: 10.1177/15353702-0322807-05. [DOI] [PubMed] [Google Scholar]
- 51.Lemon JA, Boreham DR, Rollo CD. A Complex Dietary Supplement Extends Longevity of Mice. J Gerontol A Biol Sci Med Sci. 2005;60A:275–279. doi: 10.1093/gerona/60.3.275. [DOI] [PubMed] [Google Scholar]
- 52.Kumar TR, Muralidhara Induction of Oxidative Stress by Organic Hydroperoxides in Testis and Epididymal Sperm of Rats in Vivo. J Androl. 2007;28:77–85. doi: 10.2164/jandrol.106.000265. [DOI] [PubMed] [Google Scholar]
- 53.Kaur P, Kaur G, Bansal MP. Tertiary-butyl Hydroperoxide Induced Oxidative Stress and Male Reproductive Activity in Mice: Role of Transcription Factor NF-kB and Testicular Antioxidant Enzymes. Reprod Toxicol. 2006;22:479–484. doi: 10.1016/j.reprotox.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 54.Cattan V, Mercier N, Gardner JP, Regnault V, Labat C, Mäki-Jouppila J, Nzietchueng R, Benetos A, Kimura M, Aviv A, Lacolley P. Chronic Oxidative Stress Induces a Tissue-Specific Reduction in Telomere Length in CAST/Ei MIce. Free Radic Biol Med. 2008;44:1592–1598. doi: 10.1016/j.freeradbiomed.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 55.Vernet P, Aitken RJ, Drevet JR. Antioxidant Strategies in the Epididymis. Mol Cell Endocrinol. 2004;216:31–39. doi: 10.1016/j.mce.2003.10.069. [DOI] [PubMed] [Google Scholar]
- 56.Imai H, Suzuki K, Ishizaka K, Ichinose S, Oshima H, Okayasu I, Emoto K, Umeda K, Nakagawa Y. Failure of Expression of Phospholipid Hydroperoxide Glutathione Peroxidase in the Spermatozoa of Human Infertile Males. Biol Reprod. 2001;64:674–683. doi: 10.1095/biolreprod64.2.674. [DOI] [PubMed] [Google Scholar]
- 57.Nakamura N, Miranda-Vizuete A, Miki K, Mori C, Eddy EM. Cleavage of Disulfide Bonds in Mouse Spermatogenic Cell-Specific Type 1 Hexokinase Isozyme Is Associated with Increased Hexokinase Activity and Initiation of Sperm Motility. Biol Reprod. 2008;79:537–545. doi: 10.1095/biolreprod.108.067561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y. Role of Nrf2 Signaling in Regulation of Antioxidants and Phase 2 Enzymes in Cardiac Fibroblasts: Protection against Reactive Oxygen and Nitrogen Species-Induced Cell Injury. FEBS Lett. 2005;579:3029–3036. doi: 10.1016/j.febslet.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 59.Cho C-S, Lee S, Lee GT, Woo HA, Choi E-J, Rhee SG. Irreversible Inactivation of Glutathione Peroxidase 1 and Reversible Inactivation of Peroxiredoxin II by H2O2 in Red Blood Cells. Antioxid Redox Signal. 2010;12:1235–1246. doi: 10.1089/ars.2009.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park SY, Lee E-J, Emge D, Jahn CL, Jameson JL. A Phenotypic Spectrum of Sexual Development in Dax1 (Nr0b1)-Deficient Mice: Consequence of the C57BL/6J Strain on Sex Determination. Biol Reprod. 2008;79:1038–1045. doi: 10.1095/biolreprod.108.069492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang T-T, Naeemuddin M, Elchuri S, Yamaguchi M, Kozy HM, Carlson EJ, Epstein CJ. Genetic Modifiers of the Phenotype of Mice Deficient in Mitochondrial Superoxide Dismutase. Hum Mol Genet. 2006;15:1187–1194. doi: 10.1093/hmg/ddl034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.