Abstract
Background
A major unmet challenge is to reduce the islet mass needed for insulin independence in type 1 diabetic recipients following islet transplantation. The recombinant homodimer of human annexin V, Diannexin has completed a Phase II Clinical Trial in Kidney Transplantation (NCT00615966).
Methods
We developed a marginal islet mass transplantation model (10-12 islets per gram of recipient body weight) and investigated whether Diannexin prevents beta-cell apoptosis and improves islet graft function. Diannexin was administered to islet cell donors shortly before pancreas harvest, added to isolation reagents, and infused into recipients at the time of transplantation and repeated daily until day 4.
Results
In the syngeneic marginal islet mass transplantation model, the median time needed to achieve normoglycemia was reduced from 17.0 days among untreated controls to 3.5 days among Diannexin-treated recipients (P=0.004). Histologic analysis of islet grafts harvested on day 3 post-transplantation revealed decreased macrophage (44.7 ± 9.8% vs. 19.2 ± 3.2%, P=0.007) and T-cell infiltration (25.9 ± 5.5% vs. 9.1 ± 1.1%, P=0.004), as well as a lower rate of islet cell apoptosis (20.5 ± 2.8% vs. 7.6 ± 2.3%, P=0.01) with Diannexin treatment. Expression profiling of the islet grafts showed significantly lower levels of mRNA for the proapoptotic molecule Bid, but higher levels of IL-6, IFN-γ, and of the immunosuppressive cytokine IL-10.
Conclusions
Our findings demonstrate that Diannexin improves the early function of marginal mass islet grafts, and its effects are associated with reductions in inflammatory cell infiltration as well as beta-cell death by apoptosis following islet transplantation.
Keywords: Islet transplantation; Diabetes mellitus, experimental; Diannexin/therapeutic use; Apoptosis/drug effects; Mice
INTRODUCTION
Pancreatic islet cell transplantation may improve glycemic control in patients with type 1 diabetes (1). The need for life-long immunosuppression and the gradual decline in islet function over time, however, limits the clinical applicability of islet cell transplantation (2). Furthermore, up to 60% of the transplanted islets are lost in the early post-transplant period, contributing to the need for islets from multiple pancreata to achieve insulin independence in a single recipient (3). The need for multiple pancreata exacerbates the current shortage of pancreas supply, and remains a critical barrier to the widespread availability of islet cell transplantation.
During pancreas procurement, islet isolation and subsequent transplantation, islet cells are subjected to various physiologic stressors, which ultimately lead to islet cell dysfunction and destruction. Islets normally receive 10-15% of the blood supply in the native pancreas, even though they only account for 1% of the organ’s weight (4). Collagenase digestion and mechanical separation of the pancreas causes disruption of the islet microvasculature, and places the islet under hypoxic stress. Although revascularization may begin as early as 7 days after transplantation (5), experimental evidence suggests that oxygen tension within the islet graft can remain decreased for up to 9 months post-transplantation (6).
Prolonged hypoxic conditions, in turn, trigger inflammatory responses in the microenvironment of the islet graft. Elevated levels of the proinflammatory cytokines such as IL-1β, TNF-α and IFN-γ promote insulitis and induce beta-cell damage. Furthermore, recruitment of macrophages, and production of toxic intermediates exacerbate cellular injury and DNA damage in beta-cells (7), and activate death-signaling pathways within the islet cells (8).
Islets possess lower levels of endogenous antioxidants such as superoxide dismutase and glutathione peroxidase, and their defenses become easily overwhelmed under conditions of stress (9). Since oxidative stress plays a major role in triggering islet cell death, we previously examined the effects of SS-31, a novel peptide targeting the inner mitochondrial membrane, on islet graft function (10). We found that SS-31 optimized the isolation process by increasing islet cell yield and reducing islet apoptosis. In a marginal islet cell mass transplantation model, pretreatment of pancreas donor with SS-31 resulted in prompt and sustained achievement of normoglycemia following transplantation.
Apart from the production of toxic intermediates, the upregulation of proinflammatory cytokines and the recruitment of macrophages also contribute to islet cell demise. The objective of our current study is to examine the effects of Diannexin, a recombinant homodimer of the endogenous anticoagulant molecule annexin V, on islet cell preservation during islet isolation and immediately following transplantation. Diannexin has several advantages, including an increased serum half-life (6-7 hours in rat and 2.5 hours in human circulation) and enhanced affinity compared with its endogenous monomeric counterpart (11). Diannexin binds to phosphatidylserine residues, which are everted onto the outer layer of the plasma membrane during the early stages of apoptosis, when cellular injury may be reversible (12). Importantly, Diannexin has recently completed a Phase II study in kidney transplantation (NCT00615966). The administration of Diannexin up to 400μg/kg did not increase the frequency or severity of adverse events in recipients of marginal deceased donor kidneys, while the incidence of delayed graft function and days on dialysis was significantly reduced in recipients treated with 400μg/kg Diannexin compared with control subjects treated with placebo (13). Trends for higher 24-hour urine output and estimated glomerular filtration rates at 1 month post-transplant were also observed among Diannexin-treated recipients (13).
In this study, we demonstrate that Diannexin improves early post-transplant islet graft function, decreases inflammatory responses and reduces apoptotic cell death within mouse islet isografts. Administration of Diannexin may represent a novel and immediately adaptable strategy to reduce the islet mass required for achieving insulin independence in type 1 diabetic recipients.
RESULTS
Effect of Diannexin on post-transplant islet graft function
Since Diannexin has been shown to engage phosphatidylserine residues on the plasma membrane and prevent leukocyte attachment, we hypothesized that Diannexin treatment would reduce local inflammation and enhance islet graft viability and function in-vivo. We developed a syngeneic marginal islet mass transplantation model in which a total of 10 to 12 islets per gram recipient body weight was transplanted in the renal subcapsular space of diabetic recipients and used this model to investigate the in-vivo efficacy of Diannexin.
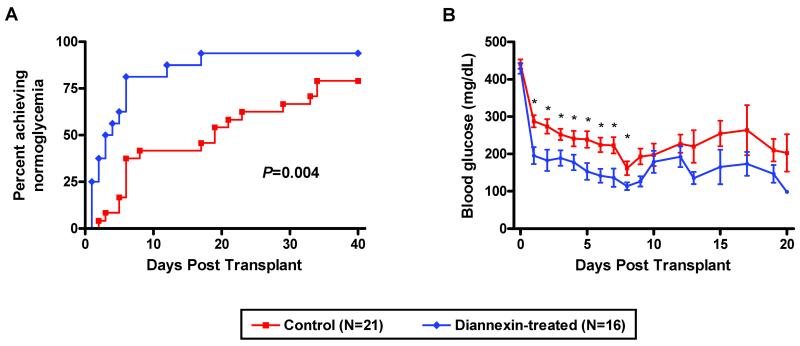
Diannexin was administered to islet donors intravenously, added to isolation reagents, and injected into the tail veins of recipient mice immediately before transplantation and on days 1 – 4 post-transplantation. Figure 1A illustrates that Diannexin reduced the median time needed to achieve normoglycemia from 17.0 days among untreated control mice (N=21) to 3.5 days among Diannexin-treated mice (N=16) (P=0.004). The mean blood glucose levels were substantially lower in the Diannexin-treated mice compared with untreated controls in the early post-transplant period (Fig. 1B).
Figure 1. Diannexin accelerates the reversal of hyperglycemia following marginal islet mass transplantation.
Streptozotocin-induced diabetic BALB/c mice received a marginal mass of syngeneic islets under the kidney capsule. Diannexin was given to donors, added to islet isolation reagents, and injected into recipients on days 0-4 post-transplantation. (A) Reversal of diabetes, as defined by blood glucose levels <200mg/dl on three consecutive measurements, after marginal islet mass transplantation in Diannexin treatment vs. control. Two-tailed P values calculated using the log rank test. (B) Mean (±SE) blood glucose levels of Diannexin-treated and control recipients following transplantation of a marginal islet cell mass. * P<0.05 by the Mann-Whitney test.
Effect of Diannexin on inflammatory cell recruitment and islet cell apoptosis
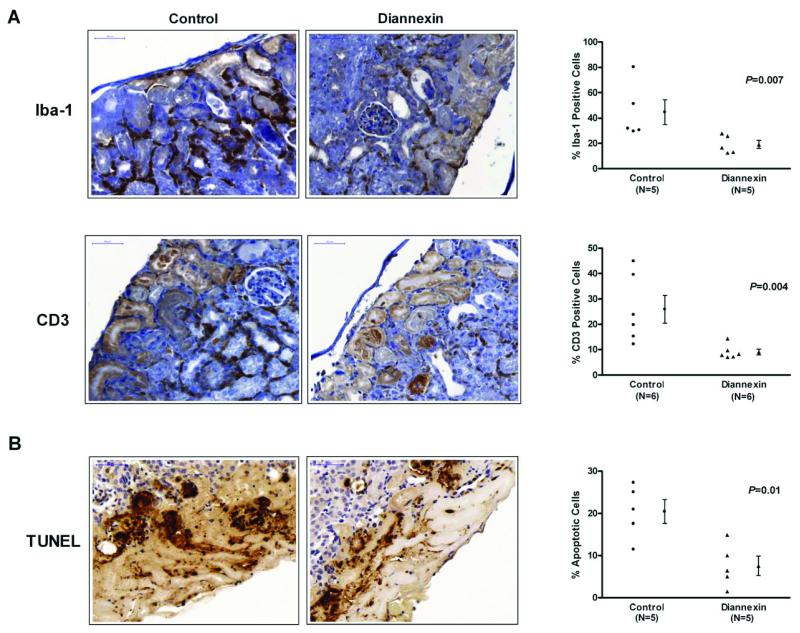
To identify the mechanisms responsible for improved islet graft function following Diannexin treatment, we examined cellular infiltration into the islet grafts. The islet grafts were harvested on day 3 after transplantation and immunohistochemical staining for Iba-1 protein, a specific marker for macrophages, and CD3 protein were performed on islet grafts from control and Diannexin-treated mice. Whereas a marked mononuclear cell infiltrate can be seen in and around the islet graft by day 3 post-transplantation in the control group, the magnitude of cellular infiltration was significantly less in the Diannexin group (Fig. 2A). The percentage of cells that stained positively for the macrophage marker Iba-1 was 44.7 ± 9.8% in the islet grafts from the control group (N=5) and 19.2 ± 3.2% in the grafts from the Diannexin-treated mice (N=5; P=0.007). T-cell infiltration in to the islet graft was also decreased with Diannexin treatment, as evidenced by 25.9 ± 5.5% and 9.1 ± 1.1% of cells staining positively for CD3 in the control (N=6) and treated (N=6) grafts, respectively (Fig. 2A, P=0.004).
Figure 2. Diannexin reduces inflammatory cell recruitment and beta-cell apoptosis within syngeneic islet grafts.
(A) Immunostaining of syngeneic islets retrieved on day 3 post-transplantation decreased macrophage (Iba-1) and T-cell (CD3) traffic into islets grafts treated with Diannexin. (B) Apoptosis detection by TUNEL demonstrates that Diannexin treatment is associated with a reduction of islet cell apoptosis in syngeneic grafts. Five or six histologic sections taken from different locations within three islet grafts were included in the analysis. Sections were counterstained with hematoxylin and shown at x400 magnifications. Two-tailed P values calculated using the Mann-Whitney test.
The TUNEL assay was used to detect apoptotic cells within the islet graft. The apoptotic rate was 20.5 ± 2.8% in the islet grafts from the control mice (N=5) vs. 7.6 ± 2.3% in the grafts from Diannexin-treated mice (N=5; Fig.2B, P=0.01). Taken together, our histological analyses demonstrate that Diannexin treatment is associated with a reduction in the recruitment of macrophages and T-cells to the islet microenvironment, and a reduction in islet cell apoptosis in-vivo.
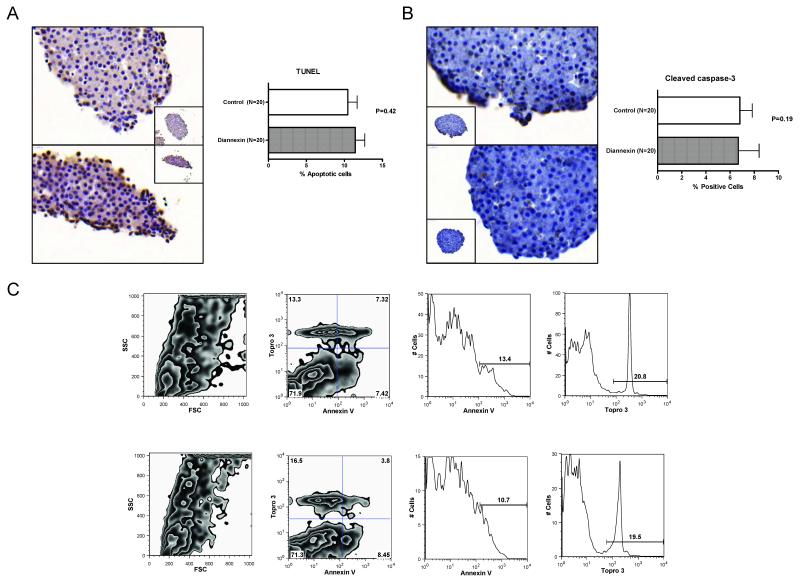
One potential mechanism for the reduction in islet cell apoptosis observed in-vivo is that donor pretreatment and the addition of Diannexin to islet isolation reagents resulted in the retrieval of healthy islets with less apoptosis. We addressed this possibility by performing the TUNEL assay on freshly isolated islets. Pretreatment of donor mice and the addition of Diannexin to the islet isolation reagents was not associated with a reduction in islet cell apoptosis, and an examination of 20 islets from three to four separate isolations with the use of TUNEL assay showed a similar rate of apoptosis between the treated group and the control group (11.4 ± 1.2% vs. 10.4 ± 1.2%, mean ± SE, N=20, P=0.42) (Fig. 3A). In accord with data illustrated in Figure 3A, immunostaining with monoclonal antibodies directed against cleaved caspase-3 showed that the percentage of cells expressing cleaved caspase-3 was similar between the two groups (6.8 ± 1.0% in the control group, mean ± SE, N=20 vs. 6.7 ± 1.7% in the Diannexin group, N=20, P=0.19) (Fig. 3B).
Figure 3. Effect of Diannexin on islet cell apoptosis.
Diannexin (400μg/kg IV) was administered to BALB/c donor mice prior to pancreas harvest and also added at a concentration of 100ng/ml to all reagents used for islet isolation. (A) TUNEL-assisted detection of apoptosis revealed a similar apoptotic rate between control (top) and Diannexin-treated (bottom) islets. Sections were counterstained with hematoxylin and shown at x500 magnifications (inset, x400). (B) Histologic analysis of mouse islet samples. Paraffin-embedded sections of freshly isolated mouse islets demonstrate comparable levels of cleaved caspase-3 expression between untreated (top) and Diannexin-treated (bottom) islets. For (A) and (B), a total of 20 islets from three or four separate isolations were included in the analysis. Sections were counterstained with hematoxylin and shown at x500 magnifications (inset, x400). (C) The islets were dissociated with trypsin/EDTA and stained with annexin V-FITC (annexin V) and TO-PRO-3 iodide (Topro 3). Representative flow cytometry analysis from two independent experiments comprised of 4 untreated (top panels) and 3 Diannexin-pretreated (bottom) islet isolates. Panels on the left show size and granularity of the dissociated islet cells, and dual-parameter analyses for annexin V and Topro 3 to categorize viable cells (annexin V/TO-PRO-3 double-negative cells), early apoptotic cells (annexin V single-positive cells), late apoptotic/necrotic cells (annexin V/TO-PRO-3 double-positive cells), and necrotic cells (TO-PRO-3 single-positive cells), as previously described (32). Two-tailed P values were calculated using the Mann-Whitney test.
We also stained dissociated islet cells with both annexin V and TO-PRO-3 iodide and performed dual-parameter flow cytometry analysis to investigate islet cell apoptosis at the single cell level. Figure 3C shows data from a representative of two independent experiments composed of 4 untreated and 3 Diannexin-pretreated islet cell samples. The mean (±SE) percentage of viable islet cells (annexin V/TO-PRO-3 double-negative cells) was 74.2 ± 3.4% with islets from the control mice and 71.3 ± 4.1% with islets from mice that were pretreated with Diannexin (P=0.85). Diannexin treatment had no effects on early apoptosis (annexin V single-positive cells; 7.7 ± 1.1% vs. 7.3 ± 1.4%; P=0.85), late apoptosis/necrosis (annexin V/TO-PRO-3 double-positive cells; 6.6 ± 0.8% vs. 5.7 ± 1.1%; P=0.62), or necrosis (TO-PRO-3 single-positive cells; 11.5 ± 2.0% vs. 15.7 ± 2.2%; P=0.22).
Our data, illustrated in Figures 2 and 3, suggest that the reduction in islet cell apoptosis observed in-vivo is not due to donor pretreatment and the addition of Diannexin to islet isolation reagents resulting in the isolation of healthier islets with less apoptosis, and support the hypothesis that the reduction in cellular traffic to the islet graft is responsible for the decrease in islet cell apoptosis in-vivo.
Effect of Diannexin on mRNA expression patterns
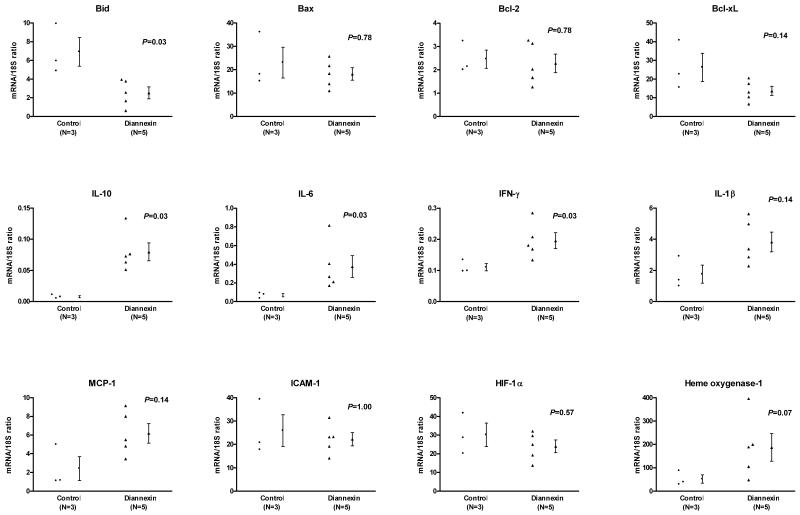
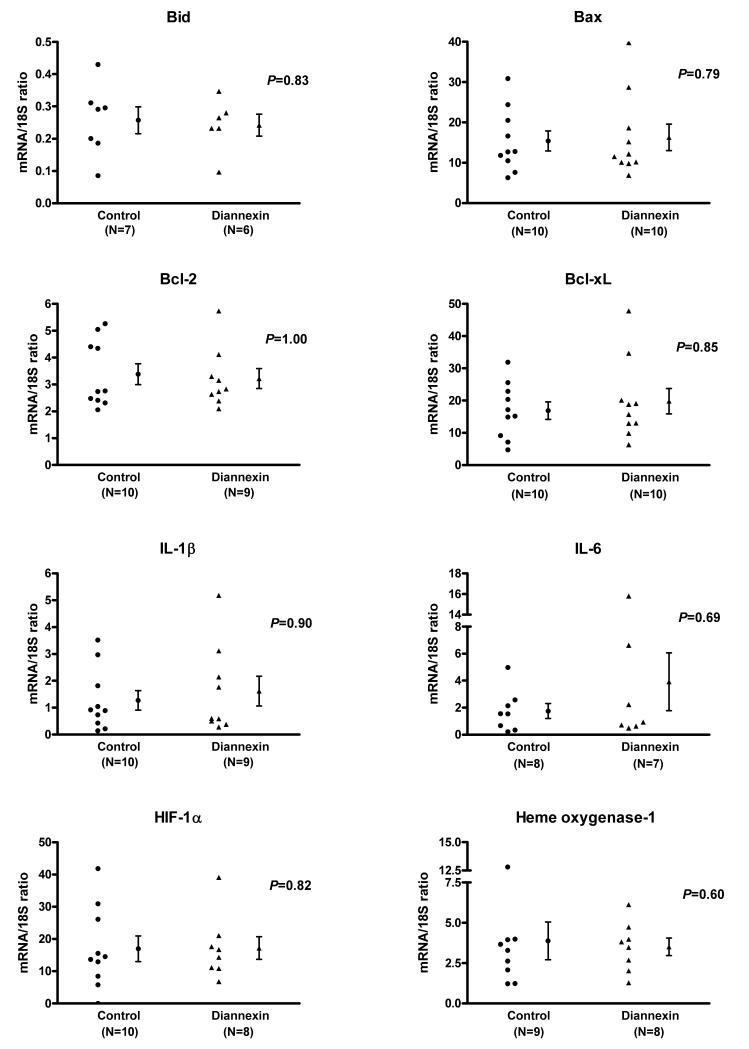
To further characterize the molecular mechanisms associated with the beneficial effects of Diannexin in-vivo, we studied mRNA expression patterns of islet grafts harvested on day 3 post-transplantation (Fig. 4). Absolute quantification of mRNAs using real-time quantitative PCR assays showed that Diannexin treatment is associated with a significantly lower level of mRNA for pro-apoptotic Bid (3-fold, P=0.03), but does not affect the mRNA levels of Bax, Bcl-2, or Bcl-xL. In addition, the levels of mRNA for IL-10 (10-fold, P=0.03) and IL-6 (5.5-fold, P=0.03) were substantially higher, and IFN-γ transcripts modestly increased (1.8-fold, P=0.03) in the islet grafts from Diannexin group compared to islet grafts from the control group. Levels of IL-1β, MCP-1, ICAM-1 and HIF-1α mRNA were similar between the two groups (P>0.05). Diannexin also appeared to upregulate the expression of heme oxygenase-1 (HO-1) (3.6-fold) in the islet grafts, although this difference did not reach statistical significance (P=0.07).
Figure 4. Intragraft mRNA expression patterns of islets from Diannexin-treated mice or control mice, as measured by real-time quantitative PCR assays.
Absolute levels of intragraft mRNA levels were measured with the use of real-time quantitative PCR assays. Individual and mean (±SE) mRNA levels for control (N=3) and Diannexin-treated (N=5) recipients are shown. mRNA expression levels are expressed as a ratio of mRNA copies in 1μg of RNA to18S ribosomal RNA copies in 1pg of RNA. Two-tailed P values calculated using the Mann-Whitney test.
We also compared mRNA expression profiles of fresh islets isolated from pancreata of mice treated with Diannexin and islets from pancreata of control mice to ascertain whether the superior islet graft mRNA expression pattern observed in-vivo is the result of donor pretreatment and the addition of Diannexin to islet isolation reagents. Our investigation of mRNA expression patterns of freshly isolated islets showed that there are no significant differences (P>0.05) between the Diannexin-treated group and control group in the number of transcripts for the pro-apoptotic Bid and Bax, the anti-apoptotic Bcl-2 and Bcl-xL, the cytokines IL-1β and IL-6, and HIF-1α, HO-1 (Fig. 5).
Figure 5. mRNA expression patterns of islet isolates from Diannexin-treated mice or untreated control mice.
Diannexin (400μg/kg IV) was administered to BALB/c donor mice prior to pancreas harvest and also added at a concentration of 100ng/ml to all reagents used for islet isolation. mRNA levels in fresh mouse islets were measured with the use of real-time quantitative PCR assays. Individual and mean (±SE) mRNA levels are expressed as a ratio of mRNA copies in 1μg of total RNA to 18S ribosomal RNA copies in 1pg of RNA. Two-tailed P values were calculated using the Mann-Whitney test.
DISCUSSION
The islet isolation and transplantation procedures induce ischemic, cytokine, and oxidative stress, resulting in pancreatic beta-cell dysfunction and destruction. Our findings demonstrate that Diannexin decreases inflammatory cell infiltration, alters cytokine expression profiles, and reduces apoptotic cell death within transplanted islet isografts and improves early post-transplant islet graft function.
Diannexin is a biosynthesized homodimer of human annexin V, an endogenous protein with anti-thrombotic properties, joined by a non-immunogenic peptide linker (11). The dimeric molecule binds externalized phosphatidylserine residues on the surface of early apoptotic cells, thereby suppressing the attachment of leukocytes and platelet aggregates (12). In preclinical models of liver and muscle ischemia-reperfusion injury (IRI), Diannexin has been found to inhibit the attachment and transmigration of leukocytes across the endothelial cell wall (12, 14-15). Through the inhibition of inflammatory responses, Diannexin is capable of limiting tissue damage following IRI (12, 14). Although islet cell transplants differs from the liver and muscle models in that no direct blood flow is established at the time of transplantation, our findings suggest that Diannexin nonetheless attenuates hypoxia-induced injury among grafted islets.
In the Phase II clinical trial in kidney transplantation, the effects of Diannexin were most prominent in the early post-transplantation period, with a reduction in the need for dialysis during the first week (13). In our murine studies, the superior islet graft function associated with Diannexin treatment was observed only in the early post-transplantation period, and the glucose levels were similar in the Diannexin-treated group and the control group at later time points. While the basis for our findings is not resolved by our study, it is possible that revascularization over time optimized islet graft function in the control group and minimized the benefit observed due to a reduction in apoptosis in the Diannexin-treated group. This hypothesis requires further investigation.
In our histologic analysis, an extensive inflammatory infiltrate was seen around the mouse islet isograft by day 3 post-transplantation, consistent with findings from previous studies of islet graft architecture following transplantation (5, 16). Macrophages within pancreatic islets produce toxic intermediates and cause beta-cell injury (17-18). In our study, macrophage and T-cell recruitment was noticeably reduced among islet grafts harvested from Diannexin-treated recipients. Importantly, Diannexin treatment increased the expression level of the anti-inflammatory cytokine IL-10 by 10-fold. IL-10 is a potent immunosuppressive cytokine and is believed to protect against beta-cell destruction and delay autoimmune diabetes in nonobese diabetic mice (19-20).
The expression of IL-6 mRNA was also increased by 5.5-fold in islet grafts receiving Diannexin treatment. IL-6 is an acute phase reactant and a pleiotropic cytokine playing a central role in hematopoiesis, host defense, and in modulating inflammation (21). The elevated expression of IL-6, along with IL-8 and MCP-1, has been found to coincide with impaired insulin secretory function in human islet isolates (9). However, other studies have implicated IL-6 in the suppression of beta-cell apoptosis by upregulating Bcl-xL and by reducing nitric oxide (NO) production (22-23). Furthermore, IL-6 has been widely reported to be protective against IRI in the liver (21, 24-26). Therefore, IL-6 may play a beneficial role in islet transplantation, but its principle effects and precise mechanisms of action await further elucidation.
In the present study, HO-1 mRNA was upregulated in the Diannexin group. Treatment with Diannexin has also been shown to increase intragraft expression of HO-1 in a syngeneic rat liver transplantation model (12). HO-1 is a ubiquitous stress protein induced in response to IL-1β, TNF-α, and NO. During islet isolation, HO-1 is upregulated and provides antioxidant activity, protecting beta-cells from cytokine-mediated apoptosis and oxidative stress. In a rodent marginal islet mass transplantation model, the administration of HO-1 alone has been shown to reduce the time needed to achieve normoglycemia (27).
While the extracellular release of proinflammatory cytokines initiates beta-cell death via the extrinsic pathway of apoptosis, intrinsic cues of cellular stress such as hypoxia, ROIs, DNA damage and nutrient deprivation activate the mitochondrial pathway regulated by BCL-2 proteins (28). Previous experiments in our laboratory have demonstrated that islet cells express pro-apoptotic Bax at a higher level compared with antiapoptotic Bcl-2 (29), a finding corroborated by the mRNA profiling results in our current study. The reduced Bcl-2 to Bax ratio, along with lower levels of endogenous antioxidants (9), renders islet cells exceptionally vulnerable to apoptotic death upon exposure to conditions of stress.
By decreasing inflammatory cell recruitment and altering the cytokine profile within the islet microenvironment, Diannexin appears to prevent beta-cell death following islet transplantation by targeting the extrinsic pathway of apoptosis. The mitochondrial pathway may also be involved based on the downregulation of Bid (30) and the upregulation of HO-1 in Diannexin-treated animals. Nevertheless, Diannexin does not completely abrogate apoptosis within islet grafts, and recent evidence suggests that agents targeting both the extrinsic and intrinsic pathways may be more efficacious in promoting beta-cell survival (28). A combination of Diannexin and SS-31 treatments, therefore, may enhance beta-cell protection and reduce islet mass requirements in islet transplantation.
We have initiated studies to investigate whether Diannexin treatment prevents allograft rejection. Islets from C57BL/6 donor mice were transplanted into diabetic BALB/c recipients, and the recipients were either treated with Diannexin (number of recipients = 6) or untreated (N=5). The blood glucose levels were lower in the Diannexin-treated mice in the initial few days after transplant compared to untreated control mice, but this difference was not observed after day 8 post-transplantation, and the median islet allograft survival was similar between the Diannexin group and the control group (14 days vs. 15 days, P>0.05). Our findings suggest that Diannexin alone is insufficient to counter the potent immune responses elicited in the presence of an allograft. It is possible, however, that the Diannexin-associated lessening of inflammation may potentiate tolerogenic interventions.
In summary, Diannexin inhibits cellular traffic to the islet graft, reduces beta cell apoptosis, and improves early islet graft function. Since Diannexin has completed a Phase II study in kidney transplantation, our research strategy may be immediately translatable to help reduce the islet mass requirements for achieving insulin independence in type 1 diabetic recipients.
METHODS
Animals
Male BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA) at 10-12 weeks of age. Mice were housed in a pathogen-free facility and used according to the guidelines set forth by the Institutional Animal Care and Use Committee at Weill Cornell Medical College.
Male BALB/c mice at 12-18 weeks of age were used as both islet donors and islet graft recipients. Recipient mice were rendered diabetic by a single intraperitoneal injection of streptozotocin (Sigma Aldrich, St. Louis, MO) at 160-210 mg/kg. Blood glucose levels were monitored by tail-vein bleed using Precision Xtra test strips (Abbott Laboratories, Abbott Park, IL). Diabetes was confirmed by two consecutive blood glucose readings >300mg/dl.
Islet isolation and transplantation
Islet isolation was performed as described (31). Briefly, the donor pancreas was distended by infusing 0.75mg/ml of collagenase solution into the common bile duct. The pancreas was then removed and incubated at 37°C for 13-15 minutes, and islets were separated by Ficoll density centrifugation.
A syngeneic marginal mass islet transplantation model was developed after extensive titration experiments in our laboratory. In this study, we transplanted 10-12 islets per gram body weight (200-300 islets per animal) under the renal capsule of diabetic BALB/c recipients. Return to normoglycemia was defined as three consecutive blood glucose measurements <200 mg/dl.
Administration of Diannexin
Diannexin was administered to islet donors, added to isolation reagents, and given to islet graft recipients assigned to the Diannexin treatment group. BALB/c donor mice received 400μg/kg Diannexin by tail-vein injection 30 minutes prior to pancreas harvest for islet isolation. All reagents used for islet isolation were supplemented with 100ng/ml of Diannexin. The recipient mice were treated with 400μg/kg Diannexin intravenously immediately before transplantation, then daily for 4 days.
Measurement of mRNA by real-time quantitative polymerase chain reaction (PCR) assay
RNA was extracted using the PureLink™ Micro-to-Midi Total RNA Purification System (Invitrogen, Carlsbad, CA). Levels of mRNA were measured by a real-time quantitative PCR assays developed and validated in our laboratory (29).
Determination of islet cell apoptosis by flow cytometry
Islet cells were dissociated into single cells by incubation with trypsin-EDTA for 5 minutes. The dissociated islet cells were incubated with annexin V-FITC (annexin V) for 15 minutes at room temperature, and TO-PRO-3 iodide was added immediately before analysis. Samples were analyzed on FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). Dual-parameter flow cytometry was used to categorize cells as described (32).
Immunohistology
Freshly isolated islets or islet graft-bearing kidney specimens were fixed in 4% paraformaldehyde, and all histologic staining was performed using protocols established at the Memorial Sloan-Kettering Cancer Center Molecular Cytology Core facility. Paraffin-embedded sections were processed for immunohistochemistry using Discovery staining module (Ventana Medical Systems, Oro Valley, AZ) after incubation with commercial rabbit anti-mouse cleaved caspase-3, Iba-1 and CD3 antibodies. Control sections stained with purified rabbit immunoglobulin (Ig) G were included in each experiment. Cells undergoing apoptosis were detected by the terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL) assay.
One or two representative histologic sections from different locations within each islet graft were included in the analysis. For the assessment of islet cell sections, a total of 20 islets from three or four separate isolations were analyzed. Using the MetaMorph 7.0 image analysis software (Molecular Devices, Downingtown, PA), the area occupied by positively stained cells in each high-powered field image was measured, and divided by the area occupied by islet cells in the same image to obtain the percentage of positive cells.
Statistical Analysis
All data are expressed as mean ± SE, and the GraphPad Prism 4.0 statistical software (La Jolla, CA) was used for data analysis. Two-tailed P values for continuous variables were calculated using the Mann-Whitney test, and the log-rank test was used to detect differences in median times to normoglycemia between the treated and untreated groups.
ACKNOWLEDGEMENTS
We thank Dr. Katia Manova and the MSKCC Molecular Cytology Core for technical assistance. This investigation was supported by grant TL1RR024997 of the Clinical and Translational Science Center at Weill Cornell Medical College. The research reported in this manuscript is in partial fulfillment of Dr. Elaine Y. Cheng’s KL2 Masters of Science in Clinical Investigation.
ABBREVIATIONS
- HIF-1α
hypoxia-inducible factor-1 alpha
- HO-1
heme oxygenase-1
- IRI
ischemia-reperfusion injury
- MCP-1
monocyte chemoattractant protein-1
- NO
nitric oxide
Footnotes
No conflicts of interest to disclose
Allison is an employee and shareholder of Alavita Inc.
Current address: 525 East 68th Street, Box 3, New York, NY 10065
Current address: 325 East Middlefield Road, Mountain View, CA 94043
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock G, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson P, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 3.Harlan DM, Kenyon NS, Korsgren O, Roep BO. Current advances and travails in islet transplantation. Diabetes. 2009;58:2175. doi: 10.2337/db09-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77:587. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 5.Morini S, Brown ML, Cicalese L, Elias G, Carotti S, Gaudio E, et al. Revascularization and remodelling of pancreatic islets grafted under the kidney capsule. J Anat. 2007;210:565. doi: 10.1111/j.1469-7580.2007.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlsson P, Palm F, Andersson A, Liss P. Chronically decreased oxygen tension in rat pancreatic islets transplanted under the kidney capsule. Transplantation. 2000;69:761. doi: 10.1097/00007890-200003150-00015. [DOI] [PubMed] [Google Scholar]
- 7.Linn T, Schmitz J, Hauck-Schmalenberger I, Lai Y, Bretzel RG, Brandhorst, et al. Ischaemia is linked to inflammation and induction of angiogenesis in pancreatic islets. Clin Exp Immunol. 2006;144:179. doi: 10.1111/j.1365-2249.2006.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdelli S, Ansite J, Roduit R, Borsello T, Matsumoto I, Sawada T, et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes. 2004;53:2815. doi: 10.2337/diabetes.53.11.2815. [DOI] [PubMed] [Google Scholar]
- 9.Bottino R, Balamurugan AN, Tse H, Thirunavukkarasu C, Ge X, Profozich J, et al. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004;53:2559. doi: 10.2337/diabetes.53.10.2559. [DOI] [PubMed] [Google Scholar]
- 10.Thomas DA, Stauffer C, Zhao K, Yang H, Sharma VK, Szeto HH, et al. Mitochondrial targeting with antioxidant peptide SS-31 prevents mitochondrial depolarization, reduces islet cell apoptosis, increases islet cell yield, and improves posttransplantation function. J Am Soc Nephrol. 2007;18:213. doi: 10.1681/ASN.2006080825. [DOI] [PubMed] [Google Scholar]
- 11.Kuypers FA, Larkin SK, Emeis JJ, Allison AC. Interaction of an annexin V homodimer (Diannexin) with phosphatidylserine on cell surfaces and consequent antithrombotic activity. Thromb Haemost. 2007;97:478. [PubMed] [Google Scholar]
- 12.Shen XD, Ke B, Zhai Y, Tsuchihashi SI, Gao F, Duarte S, et al. Diannexin, a novel annexin V homodimer, protects rat liver transplants against cold ischemia-reperfusion injury. Am J Transplant. 2007;7:2463. doi: 10.1111/j.1600-6143.2007.01967.x. [DOI] [PubMed] [Google Scholar]
- 13.Cooper M, Kapur S, Stratta R, D’Alessandro A, Malgaonkar S, Allison A, et al. Diannexin, a novel ischemia/reperfusion therapeutic agent, reduces delayed graft function (DGF) in renal transplant recipients from marginal donors. Am J Transplant. 2010;10:S83. [Google Scholar]
- 14.Teoh NC, Ito Y, Field J, Bethea NW, Amr D, McCuskey MK, et al. Diannexin, a novel annexin V homodimer, provides prolonged protection against hepatic ischemia-reperfusion injury in mice. Gastroenterology. 2007;133:632. doi: 10.1053/j.gastro.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 15.Molski M, Groth A, Allison AC, Hendrickson M, Siemionow M. Diannexin treatment decreases ischemia-reperfusion injury at the endothelial cell level of the microvascular bed in muscle flaps. Ann Plast Surg. 2009;63:564. doi: 10.1097/SAP.0b013e3181935a4e. [DOI] [PubMed] [Google Scholar]
- 16.Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Vulnerability of islets in the immediate posttransplantation period. Diabetes. 1996;45:1161. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- 17.Heitmeier MR, Scarim AL, Corbett JA. Interferon-γ increases the sensitivity of islets of Langerhans for inducible nitric-oxide synthase expression induced by interleukin-1. J Biol Chem. 1997;272:13697. doi: 10.1074/jbc.272.21.13697. [DOI] [PubMed] [Google Scholar]
- 18.Arnush M, Heitmeier MR, Scarim AL, Marino MH, Manning PT, Corbett JA. IL-1 produced and released endogenously within human islets inhibits β cell function. J Clin Invest. 1998;102:516. doi: 10.1172/JCI844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balasa B, Gunst KV, Jung N, Katz JD, Ssarvetnick N. IL-10 deficiency does not inhibit insulitis and accelerates cyclophosphamide-induced diabetes in the nonobese diabetic mouse. Cell Immunol. 2000;202:97. doi: 10.1006/cimm.2000.1658. [DOI] [PubMed] [Google Scholar]
- 20.Pennline KJ, Roque-Gaffney E, Monohan M. Recombinant human IL-10 prevents the onset of diabetes in the nonobese diabetic mouse. Clin Immunol Immunopathol. 1994;71:169. doi: 10.1006/clin.1994.1068. [DOI] [PubMed] [Google Scholar]
- 21.Camargo CA, Madden JF, Gao W, Selvan RS, Clavien P. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology. 1997;26:1513. doi: 10.1002/hep.510260619. [DOI] [PubMed] [Google Scholar]
- 22.Choi S, Choi K, Yoon I, Shin J, Kim J, Park W, et al. IL-6 protects pancreatic islet beta cells from pro-inflammatory cytokines-induced cell death and functional impairment in vitro and in vivo. Transplant Immunol. 2004;13:43. doi: 10.1016/j.trim.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Park H, Ahn Y, Park C, Chung H, Park Y. Interleukin-6 protects MIN6 β cells from cytokine-induced apoptosis. Ann N Y Acad Sci. 2003;1005:242. doi: 10.1196/annals.1288.036. [DOI] [PubMed] [Google Scholar]
- 24.Teoh N, Field J, Farrell G. Interleukin-6 is a key mediator of the hepatoprotective and pro-proliferative effects of ischaemic preconditioning in mice. J Hepatol. 2006;45:20. doi: 10.1016/j.jhep.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 25.Selzner M, Camargo CA, Clavien P. Ischemia impairs liver regeneration alter major tissue loss in rodents: protective effects of interleukin-6. Hepatology. 1999;30:469. doi: 10.1002/hep.510300215. [DOI] [PubMed] [Google Scholar]
- 26.Sun Z, Klein AS, Radaeva S, Hong F, El-Assal O, Pan H, et al. In vitro interleukin-6 treatment prevents mortality associated with fatty liver transplants in rats. Gastroenterology. 2003;125:202. doi: 10.1016/s0016-5085(03)00696-6. [DOI] [PubMed] [Google Scholar]
- 27.Pileggi A, Molano RD, Berney T, Cattan P, Vizzardelli C, Oliver R, et al. Heme oxygenase-1 induction in islet cells results in protection from apoptosis and improved in vivo function after transplantation. Diabetes. 2001;50:1983. doi: 10.2337/diabetes.50.9.1983. [DOI] [PubMed] [Google Scholar]
- 28.Emamaullee JA, Shapiro AM. Interventional strategies to prevent β-cell apoptosis in islet transplantation. Diabetes. 2006;55:1907. doi: 10.2337/db05-1254. [DOI] [PubMed] [Google Scholar]
- 29.Thomas D, Yang H, Boffa DJ, Ding R, Sharma VK, Lagman M, et al. Proapoptotic Bax is hyperexpressed in isolated human islets compared with antiapoptotic Bcl-2. Transplantation. 2002;74:1489. doi: 10.1097/00007890-200212150-00003. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Zhu H, Xu C, Yuan J. Cleavage of BID by Caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 31.Yang H, Thomas D, Boffa DJ, Ding R, Li B, Muthukumar T, et al. Enforced c-Rel deficiency prolongs survival of islet allografts. Transplantation. 2002;74:291. doi: 10.1097/00007890-200208150-00002. [DOI] [PubMed] [Google Scholar]
- 32.Vermes I, Haanen C, Reutelingsperger C. Flow cytometry of apoptotic cell death. J Immunol Methods. 2000;243:167. doi: 10.1016/s0022-1759(00)00233-7. [DOI] [PubMed] [Google Scholar]