Abstract
In a retrospective analysis of 205 patients (median age 62 years) with primary myelofibrosis and known JAK2V617F mutational status, 13.2% experienced a vaso-occlusive event at or prior to their diagnosis. After a median follow up of 31 months, post-diagnosis thrombosis occurred in 22 patients (10.7%), including 9 (4.4%) and 16 (7.8%) patients with a total of 9 arterial and 24 venous events, respectively. The majority (71%) of the venous events were temporally associated with other exogenous risk factors for thrombosis such as surgery, line placement or hormonal therapy. On multivariable analysis that included age, JAK2V617F mutation status and leukocyte count as covariates, history of thrombosis was the only predictive variable in general (P=0.04) or when arterial (P=0.007) and venous (P=0.02) thromboses were analyzed separately. The current study demonstrates a higher prevalence of venous, as opposed to arterial, events in PMF, post-diagnosis, and clarifies their nature as being mostly provoked.
Keywords: primary myelofibrosis, thrombosis, JAKV617F
Introduction
Thromboembolic events (TE) in myeloproliferative neoplasms (MPN) are well-described but incompletely understood.1,2 The manifestation of TE in MPN is characterized by involvement of large and small arterial or venous conduits. Large abdominal veins are particularly targeted by MPN-associated thrombosis.3 Although advanced age and history of thrombosis are well-established risk factors for thrombosis in essential thrombocythemia (ET) and polycythemia vera (PV), the origin of their hypercoagulable state in general remains an enigma.4,5 Putative contributors include blood hyperviscosity due to erythrocytosis, abnormal activation of platelets and leukocytes and acquired resistance to activated protein C.2,6,7 Recently, both leukocytosis and the JAK2V617F mutation have been identified as potential risk factors.8–12 There is limited information on the prevalence and distribution of TE in primary myelofibrosis (PMF).
Design and Methods
After institutional review board approval, patients (≥ 18 years) were recruited form the Mayo Clinic PMF database. Diagnosis was according to the 2001 WHO criteria.13 The “pre-fibrotic” form of PMF was excluded. Clinical and laboratory information from diagnosis was correlated with the occurrence of TE in general and arterial (AT) or venous (VT) thrombosis in particular. Qualitative JAK2V617F analysis was made according to previously published PCR-based methods on stored bone marrow.14
Only thromboses registered during follow up were considered in this analysis. Arterial events considered included acute coronary syndromes (ACS) that encompassed myocardial infarction (MI), unstable angina and objectively confirmed coronary ischemia requiring revascularization procedures, stroke, transient ischemic attack (TIA) and peripheral arterial thrombosis (PAT). Venous events included extremity deep venous thrombosis (DVT), pulmonary embolism (PE), abdominal and cerebral vein thrombosis (CVT). Abdominal vein thrombosis (AVT) included portal, splenic, hepatic and or mesenteric vein thrombosis. The diagnosis of TE was accepted only if objectively proven according to published criteria.15,16 Minor vascular occlusive events such as erythromelalgia and superficial thrombophlebitis were not included.
Cardiovascular risk factors included hypertension, hypercholesterolemia, diabetes mellitus, obesity (BMI > 30) and tobacco use. Provoked VT was defined as an objectively confirmed episode associated with reversible risk factors such as a recent (i.e. within two months) surgery with general anesthesia lasting at least 30 minutes, fracture, trauma or plaster casting, confinement to bed for more than five consecutive days, pregnancy or the puerperium, or exogenous estrogenic/progestogenic therapy. Standard statistical methods were used to test significance of associations between TE and variables previously associated with TE risk in MPN. Cox’s regression model was used for multivariable analysis of thrombosis-free survival.
Results and Discussion
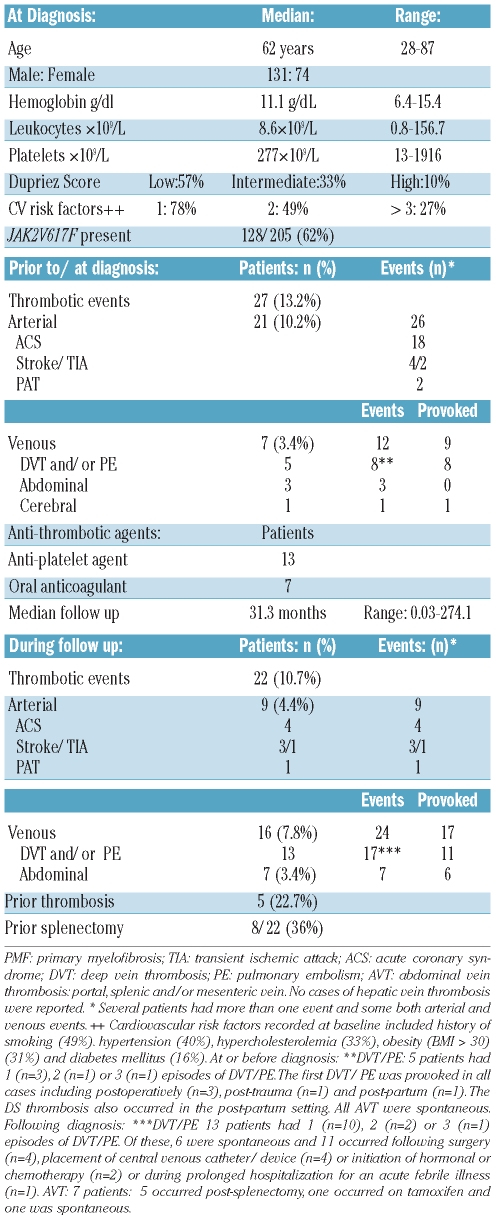
The characteristics of 205 consecutive patients with PMF meeting inclusion criteria (known JAK2V617F mutational status) diagnosed between 1982 and 2008 are outlined in Table 1. Prior to, or at diagnosis, a total of 27 subjects (13.2%) had experienced a vascular event. Of these, 21 (10.2%) and 7 (3.4%) patients had a total of 26 arterial and 12 venous events, respectively. Several patients had more than one event and some both arterial and venous events. Seven patients (3.4%) had experienced VTE. Of these, 5 patients had one (n=3), 2 (n=1) or 3 (n=1) episodes of DVT/PE, respectively. The first DVT/PE was provoked in all cases, arising post-operatively (n=3), post-trauma (n=1) and post-partum (n=1) as defined. The single case of CVT thrombosis also occurred in the post-partum setting. Three patients had a history of AVT, all of which were spontaneous.
Table 1.
Characteristics of study cohort of 205 primary myelofibrosis.
Thrombotic events post-diagnosis
After a median follow up of 31.3 months (range: 0.03–274.1), a total of 22 subjects (10.7%) had experienced a post-diagnosis vascular event. Of these, 9 (4.4%) and 16 (7.8%) patients had a total of 9 arterial and 24 venous events, respectively (Table 1). Several patients had more than one event and some both arterial and venous events. Thirteen patients (6.3%) had one (n=10), 2 (n=2) or 3 (n=1) episodes of DVT/PE. Of these, 6 were spontaneous and 11 occurred following surgery (n=4), central venous catheter/device placement (n=4), initiation of hormonal or chemotherapy (n=2) or during prolonged hospitalization for febrile illness (n=1). Seven patients (3.4%) experienced AVT, of which 5 occurred post-splenectomy, one occurred on tamoxifen for ductal carcinoma in situ of the breast (DCIS) and one occurred spontaneously.
Analysis of risk factors predicting thrombotic events in follow up
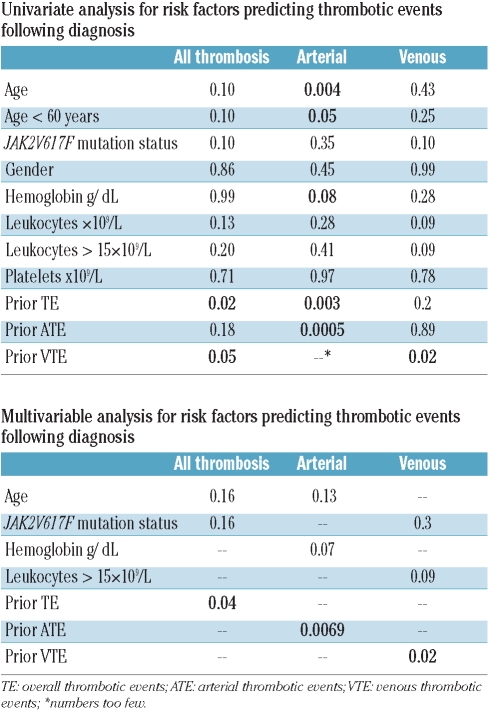
Results of the univariate analysis of risk factors for post-diagnosis TE are outlined in Table 2. Only those variables of significance, or trend thereof (<0.1) were included for the multivariable analysis. On multivariable analysis, only prior TE, ATE or VTE sustained significance for post-diagnosis TE (P=0.04), ATE (P=0.0069), or VTE (P=0.02), respectively.
Table 2.
Univariate and multivariable analysis for risk factors predicting thrombotic events following diagnosis.
The current study provides an accurate description of both arterial and venous thrombotic events in PMF. At 13.2%, the overall prevalence of TE is lower than that reported for PV (20–40%) but similar to that of ET (~13%).5,16 The characteristic propensity for AVT, as seen in PV and ET, is notable, occurring spontaneously in and accounting for 11% of prior TE.16 However, during follow up, unlike the case with PV or ET, we observed a greater frequency of VTE compared with ATE. The only baseline factor that was independently predictive of future TE was that of prior TE. As reported for ET and PV, prior vascular events in the arterial and venous distribution were predictive of subsequent ATE and VTE, respectively.8,16 The remaining factors that had shown significance or trend thereof on univariate analysis (i.e. age, leukocytes >15×109/L and JAK2V617F mutational status) lost this effect on multivariable analysis.
Recently Barbui et al. reported the frequency and predictive factors for major vascular events in 707 patients with PMF.17 They reported similar or slightly lower prevalence of prior TE (9.5%) and during follow up (7.2%) and the distribution of arterial to venous events were approximately equal during both time periods. In view of the larger patient cohort, these data are likely statistically more robust than this analysis. However, they too noted a “remarkably high rate of fatal and non-fatal venous thrombosis” and registered a high rate of DVT affecting legs, brain and splanchnic area but specific details for the non-fatal events were not reported.17 In a multivariable model, they found age over 60 years and JAK2V617F mutational status were significantly associated with thrombosis, while the strength of the association between leukocytes greater than 15×109/L became borderline. No statistically significant difference was found by separated analysis of venous or arterial events.
Prior thrombosis, advanced age and leukocytosis have been reported as risk factors for TE in PV and ET whereas the contribution of cardiovascular risk factors has not been firmly established and the role of acquired thrombophilic states has not been systematically assessed.4,5,8,9,18 With regards to the latter, only one retrospective study assessed vascular complications of surgical procedures in PV and ET, reporting that despite good control of the myeloproliferative process and antithrombotic prophylaxis, a high proportion of surgeries were complicated by vascular occlusion (7.7%) or by a major hemorrhage (7.3%). The authors estimated the risk of DVT after major surgery to be increased at least 5-fold compared to that expected.19 The observed unpredictable rate of hemorrhage highlights the challenges in the management of MPN. In the current series, we noted that the majority of VTE occurred in association with circumstantial risk factors such as surgery, line placement or hormonal therapy. As is well established in the oncology literature, VTE can be the first manifestation of occult neoplasia, represent a direct complication of hypercoagulability related to a known malignancy, or complicate hospitalization, surgery, line placement or systemic therapy.20 Patients with PMF, compared with ET and PV, a more advanced MPN, are likely exposed to more thrombotic challenges as their disease progresses. The greater frequency of VTE we observed following diagnosis would also be concordant with this well described analogy among cancer patients, although the oncology literature has largely excluded MPN.21,22
In conclusion, we confirm the hypercoagulable state, well characterized for ET and PV, also to afflict patients with PMF. Prior TE are predictive of future TE in the same vascular distribution. The observation that many VTE occur in association with circumstantial thrombotic challenges emphasizes the need for caution in the selection of therapeutic interventions and for further study to identify safe and effective prophylactic strategies for patients with MPN.
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Elliott MA, Tefferi A. Thrombosis and haemorrhage in polycythaemia vera and essential thrombocythaemia. Br J Haematol. 2005;128(3):275–90. doi: 10.1111/j.1365-2141.2004.05277.x. [DOI] [PubMed] [Google Scholar]
- 2.Landolfi R, Di Gennaro L, Falanga A. Thrombosis in myeloproliferative disorders: pathogenetic facts and speculation. Leukemia. 2008;22(11):2020–8. doi: 10.1038/leu.2008.253. [DOI] [PubMed] [Google Scholar]
- 3.Dentali F, Squizzato A, Brivio L, Appio L, Campiotti L, Crowther M, et al. JAK2V617F mutation for the early diagnosis of Phmyeloproliferative neoplasms in patients with venous thromboembolism: a meta-analysis. Blood. 2009;113(22):5617–23. doi: 10.1182/blood-2008-12-196014. [DOI] [PubMed] [Google Scholar]
- 4.Cortelazzo S, Viero P, Finazzi G, D'Emilio A, Rodeghiero F, Barbui T. Incidence and risk factors for thrombotic complications in a historical cohort of 100 patients with essential thrombocythemia. J Clin Oncol. 1990;8(3):556–62. doi: 10.1200/JCO.1990.8.3.556. [DOI] [PubMed] [Google Scholar]
- 5.Marchioli R, Finazzi G, Landolfi R, Kutti J, Gisslinger H, Patrono C, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224–32. doi: 10.1200/JCO.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 6.Falanga A, Marchetti M, Evangelista V, Vignoli A, Licini M, Balicco M, et al. Polymorphonuclear leukocyte activation and hemostasis in patients with essential thrombocythemia and polycythemia vera. Blood. 2000;96(13):4261–6. [PubMed] [Google Scholar]
- 7.Marchetti M, Castoldi E, Spronk HM, van Oerle R, Balducci D, Barbui T, et al. Thrombin generation and activated protein C resistance in patients with essential thrombocythemia and polycythemia vera. Blood. 2008;112(10):4061–8. doi: 10.1182/blood-2008-06-164087. [DOI] [PubMed] [Google Scholar]
- 8.Landolfi R, Di Gennaro L, Barbui T, De Stefano V, Finazzi G, Marfisi R, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109(6):2446–52. doi: 10.1182/blood-2006-08-042515. [DOI] [PubMed] [Google Scholar]
- 9.Carobbio A, Finazzi G, Guerini V, Spinelli O, Delaini F, Marchioli R, et al. Leukocytosis is a risk factor for thrombosis in essential thrombocythemia: interaction with treatment, standard risk factors, and Jak2 mutation status. Blood. 2007;109(6):2310–3. doi: 10.1182/blood-2006-09-046342. [DOI] [PubMed] [Google Scholar]
- 10.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352 (17):1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 11.Cheung B, Radia D, Pantelidis P, Yadegarfar G, Harrison C. The presence of the JAK2 V617F mutation is associated with a higher haemoglobin and increased risk of thrombosis in essential thrombocythaemia. Br J Haematol. 2006;132(2):244–5. doi: 10.1111/j.1365-2141.2005.05858.x. [DOI] [PubMed] [Google Scholar]
- 12.Finazzi G, Rambaldi A, Guerini V, Carobbo A, Barbui T. Risk of thrombosis in patients with essential thrombocythemia and polycythemia vera according to JAK2 V617F mutation status. Haematologica. 2007;92(1):135–6. doi: 10.3324/haematol.10634. [DOI] [PubMed] [Google Scholar]
- 13.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100(7):2292–302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 14.Tefferi A, Lasho TL, Huang J, Finke C, Mesa RA, Li CY, et al. Low JAK2V617F allele burden in primary myelofibrosis, compared to either a higher allele burden or unmutated status, is associated with inferior overall and leukemia-free survival. Leukemia. 2008;22(4):756–61. doi: 10.1038/sj.leu.2405097. [DOI] [PubMed] [Google Scholar]
- 15.Landolfi R, Marchioli R, Kutti J, Gisslinger H, Tognoni G, Patrono C, et al. Efficacy and safety of low-dose aspirin in polycythemia vera.[see comment] N Engl J Med. 2004;350(2):114–24. doi: 10.1056/NEJMoa035572. [DOI] [PubMed] [Google Scholar]
- 16.De Stefano V, Za T, Rossi E, Vannucchi AM, Ruggeri M, Elli E, et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica. 2008;93(3):372–80. doi: 10.3324/haematol.12053. [DOI] [PubMed] [Google Scholar]
- 17.Barbui T, Carobbio A, Cervantes F, Vannucchi AM, Guglielmelli P, Antonioli E, et al. Thrombosis in primary myelofibrosis: incidence and risk factors. Blood. 115(4):778–82. doi: 10.1182/blood-2009-08-238956. [DOI] [PubMed] [Google Scholar]
- 18.Cervantes F, Alvarez-Larran A, Arellano-Rodrigo E, Granell M, Domingo A, Montserrat E. Frequency and risk factors for thrombosis in idiopathic myelofibrosis: analysis in a series of 155 patients from a single institution. Leukemia. 2006;20(1):55–60. doi: 10.1038/sj.leu.2404048. [DOI] [PubMed] [Google Scholar]
- 19.Ruggeri M, Rodeghiero F, Tosetto A, Castaman G, Scognamiglio F, Finazzi G, et al. Postsurgery outcomes in patients with polycythemia vera and essential thrombocythemia: a retrospective survey. Blood. 2008;111(2):666–71. doi: 10.1182/blood-2007-07-102665. [DOI] [PubMed] [Google Scholar]
- 20.Lyman GH, Khorana AA. Cancer, clots and consensus: new understanding of an old problem. J Clin Oncol. 2009;27(29):4821–6. doi: 10.1200/JCO.2009.22.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khorana AA, Streiff MB, Farge D, Mandala M, Debourdeau P, Cajfinger F, et al. Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. J Clin Oncol. 2009;27(29):4919–26. doi: 10.1200/JCO.2009.22.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis CW. Prevention of venous thromboembolism in hospitalized patients with cancer. J Clin Oncol. 2009;27(29):4874–80. doi: 10.1200/JCO.2009.22.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]