Abstract
Based on brain imaging findings, we present a model according to which addiction emerges as an imbalance in the information processing and integration among various brain circuits and functions. The dysfunctions reflect (a) decreased sensitivity of reward circuits, (b) enhanced sensitivity of memory circuits to conditioned expectations to drugs and drug cues, stress reactivity, and (c) negative mood, and a weakened control circuit. Although initial experimentation with a drug of abuse is largely a voluntary behavior, continued drug use can eventually impair neuronal circuits in the brain that are involved in free will, turning drug use into an automatic compulsive behavior. The ability of addictive drugs to co-opt neuro-transmitter signals between neurons (including dopamine, glutamate, and GABA) modifies the function of different neuronal circuits, which begin to falter at different stages of an addiction trajectory. Upon exposure to the drug, drug cues or stress this results in unrestrained hyperactivation of the motivation/drive circuit that results in the compulsive drug intake that characterizes addiction.
Keywords: addiction, brain disease, dopamine, reward circuit
Introduction
The last 25 years of neuroscience research have produced evidence that addiction is a disease of the brain, providing a powerful argument for upholding the same standards of medical care to the addicted individual as those that are common to other diseases with a major public impact, like diabetes. Indeed, research on addiction has started to uncover the sequence of events and long-lasting sequelae that can result from the persistent abuse of an addictive substance. These studies have shown how repeated drug use can target key molecules and brain circuits, and eventually disrupt the higher order processes that underlie emotions, cognition and behavior. We have learned that addiction is characterized by an expanding cycle of dysfunction in the brain. The impairment typically begins in the evolutionarily more primitive areas of the brain that process reward, and then moves on to other areas responsible for more complex cognitive functions. Thus, in addition to reward, addicted individuals can experience severe disruptions in learning (memory, conditioning, habituation), executive function (impulse inhibition, decision making, delayed gratification), cognitive awareness (interoception) and even emotional (mood and stress reactivity) functions.
Drawing largely from the results of brain imaging studies that used positron emission tomography (PET), we introduce the key brain circuits that are affected by the chronic abuse of drugs and then present a coherent model, according to which addiction emerges as the net result of imbalanced information processing in and among these circuits. A thorough understanding of these gradual adaptive (neuroplastic) brain processes, and of the biological and environmental vulnerability factors that influence their likelihood, is critical for the development of more effective prevention and treatment approaches to combat addiction.
High, but brief, bursts of dopamine are required for addiction
Addiction is, first and foremost, a disease of the brain's reward system. This system uses the neurotransmitter dopamine (DA) as its major currency to relay information. Brain DA plays a key role in the processing of information about saliency [1, 2], which is at the heart of its ability to regulate or influence reward [3, 4], reward expectation [5], motivation, emotions, and the feelings of pleasure. Transient release of DA in the brain's ventral striatum is a necessary, albeit not sufficient, event in the complex processes that engender the sensation of reward: the increase in DA appears to be positively related to the intensity of “high” that subjects experience. Conditioned responses are only elicited when DA is repeatedly released as these sharp, transient, surges, in response to drugs or drug-associated cues.
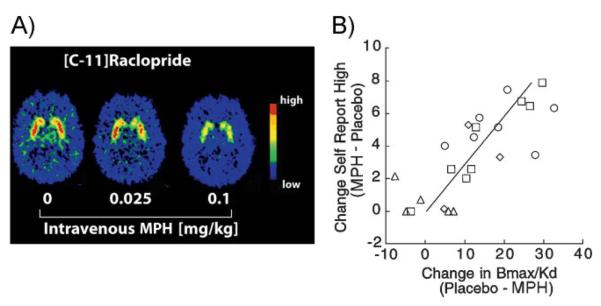
Interestingly, directly or indirectly, all addictive drugs work by triggering exaggerated but transient increases in extracellular DA in a key region of the reward (limbic) system [6, 7], specifically, in the nucleus accumbens (Nac) located in the ventral striatum. Such DA surges resemble, and in some instances greatly surpass, the physiological increases triggered by naturally pleasurable stimuli (usually referred to as natural reinforcers or rewards). As we would have expected, human brain imaging studies using positron emission tomography (PET), have clearly shown that the DA increases induced by different classes of drugs (e.g. stimulants (Fig. 1A), [8, 9], nicotine [10], and alcohol [11]) within the ventral striatum, are linked to the subjective experience of euphoria (or high) during intoxication [12, 13, 14]. Since PET studies can be done in awake human subjects it is also possible to plot the relationship between the subjective reports of drug effects and the relative changes in DA levels. Most studies have reported that those displaying the greatest DA increases following drug exposures [amphetamine, nicotine, alcohol, methylphenidate (MPH)] also report the most intense high or euphoria (Fig. 1B).
Figure 1.
Stimulant-dependent DA increases in the striatum are associated with the feeling of “high.” A: Distribution volume (DV) images of [11C]raclopride for one of the subjects at baseline and after administration of 0.025 and 0.1 mg/kg i.v. of MPH. MPH-reduced binding of [11C]raclopride dose-dependently in the striatum, where it competes with DA for binding to DA D2 receptors (D2R). B: MPH significantly increased ratings of high. Regression lines for the correlation between MPH-induced changes in D2R availability in striatum and MPH-induced changes in self-reports of high (r 0.78, df22, p < 0.0001). PET studies are carried out with a radiolabeled compound such as [11C]raclopride that binds to dopamine receptors (D2R) whenever they are not occupied by DA. Measurements are done first after injection of a placebo and then, after drug administration, such that the difference in [11C]raclopride (positron) signal between the two conditions can be used to estimate the amount of receptor occupancy and, thus, the magnitude of any drug-induced DA increase. MPH was administered IV at the following doses (in mg/kg): 0.5 (circles), 0.25 (squares), 0.1 (diamonds), and 0.025 (triangles). Modified with permission from Volkow et al. [14].
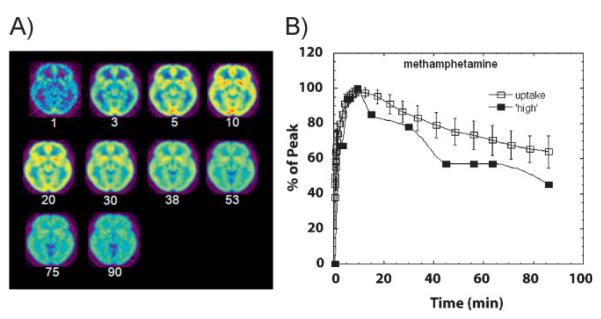
Animal and human studies have demonstrated that the speed with which a drug enters, acts upon, and leaves the brain (i.e. its pharmacokinetic profile) plays a fundamental role in determining its reinforcing effects. Indeed, every drug of abuse whose brain pharmacokinetics have been measured with PET (cocaine, MPH, methamphetamine, and nicotine) exhibits the same profile when the administration is intravenous, i.e., peak levels in the human brain are reached within 10 min (Fig. 2A)and this fast uptake is associated with the “high” (Fig. 2B). Based on this association, it follows that making sure that an addictive drug enters the brain as slowly as possible should be an effective way of minimizing its reinforcing potential, hence its abuse liability. We designed an experiment to test precisely this hypothesis with the stimulant drug MPH, which, like cocaine, increases DA by slowing down its transport back into the presynaptic neuron (i.e. by blocking DA transporters), thus magnifying the DA signal. Indeed, we found that, while intravenous administration of MPH is often euphorigenic, orally administered MPH, which also increases DA in the striatum [15], but with 6- to 12-fold slower pharmacokinetics, is not typically perceived as reinforcing [16, 17]. Thus, the failure of oral MPH – or amphetamine [18] for that matter – to induce a high is likely the reflection of their slow uptake into the brain [19]. Therefore, it is reasonable to propose the existence of a close correlation between the rate at which a drug of abuse enters the brain, which determines the speed at which DA increases in the ventral striatum, and its reinforcing effects [20, 21, 22]. In other words, for a drug to exert reinforcing effects it has to raise DA abruptly. Why should this be so?
Figure 2.
A: Axial brain images of the distribution of [11C]methamphetamine at different times (minutes) after its administration. B: Time activity curve for the concentration of [11C]methamphetamine in striatum alongside the temporal course for the “high” experienced after intravenous administration of pharmacological doses of methamphetamine. Modified with permission from Fowler et al. [55].
Based on the magnitude and duration of neuronal firing, DA signaling can take one of two basic forms: phasic or tonic. Phasic signaling is characterized by high amplitude and short burst firing, whereas tonic signaling has typically low amplitude and a more protracted or sustained time course. The distinction is important because it turns out that phasic DA signaling is necessary for drugs of abuse to induce “conditioned responses,” which is one of the initial neuroadaptations that follow exposure to reinforcing stimuli (including a drug). One of the distinguishing aspects that links phasic signaling with conditioning is the involvement of D2R and glutamate n-methyl-d-aspartic acid (NMDA) receptors [23]. On the other hand, tonic DA signaling plays a role in the modulation of working memory and other executive processes. Some of the features that distinguish this mode of signaling from the phasic type are that it operates mostly through lower affinity DA receptors (DA D1 receptors). However, and in spite of the different mechanisms involved, protracted drug exposure (and changes in tonic DA signaling through these receptors) has also been implicated in the neuroplastic changes that ultimately result in conditioning [25] through the modification of NMDA and and alpha-amino-3-hydroxyl-5-methyl-4-isoxazone-propionate (AMPA) glutamate receptors [24].
The evidence indicates that abrupt drug-induced increases in DA mimic phasic DA cell firing. This helps explain why the chronic use of an addictive substance can engender such powerful conditioned responses to the drug itself, its expectation, and myriad cues (people, things and places) associated with its use. However, while the acute reinforcing effects of drugs of abuse that depend on such fast DA increases are likely “necessary” for the development of addiction, they are clearly not “sufficient.” Repeated drug exposure causes changes in DA brain function that take time to develop because they result from secondary neuroadaptations in other neurotransmitter systems (e.g. glutamate [26] and perhaps also γ-aminobutyiric acid (GABA)) that, eventually, affect additional brain circuits modulated by DA. These circuits are the focus of the next sections.
Chronic drug abuse downregulates dopamine receptors and dopamine production: The “high” is blunted
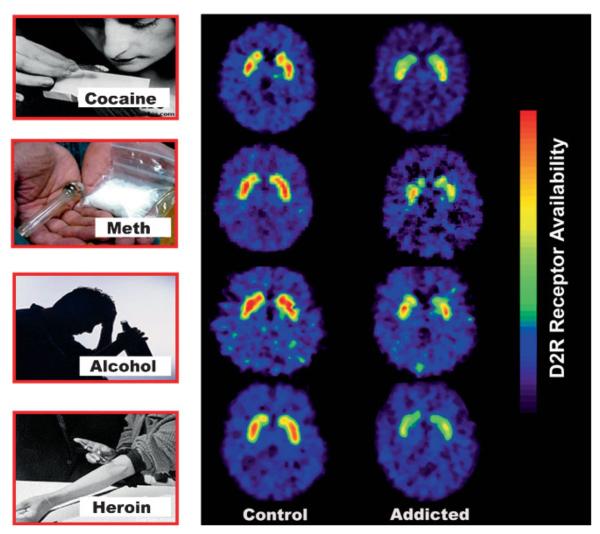
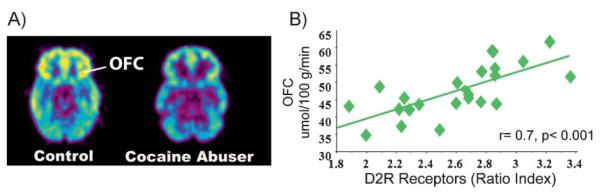
The fact that drug use must become chronic before addiction takes root is a clear indication that the disease is predicated, in vulnerable individuals, on repeated perturbations of the reward system. These perturbations can eventually lead to neuroadaptations in many other circuits (motivation/drive, inhibitory control/executive function, and memory/conditioning) that are also modulated by DA [27]. Among the neuro-adaptations that have been consistently reported in addicted subjects are the significant reductions in the levels of the D2R (high affinity) receptors and in the amount of DA released by DA cells [28] (Fig. 3). Importantly, these deficits are associated with lower regional metabolic activity in areas of the prefrontal cortex (PFC) that are critical for proper executive performance (i.e. anterior cingulate gyrus (CG) and orbitofrontal cortex (OFC)) (Fig. 4A). This observation led us to postulate that this may be one of the mechanisms that connect the drug-induced disruption in DA signaling with the compulsive drug administration and the lack of control over drug intake that characterizes addiction [29]. Also, the resulting hypodopaminergic state would explain an addicted individual's decreased sensitivity to natural rewards (e.g. food, sex, etc) and the perpetuation of drug use as a means to temporarily compensate for this deficit [30]. An important corollary of this knowledge is that addressing these deficits (by increasing striatal D2R levels and increasing DA release in striatum and prefrontal regions) could offer a clinical strategy to ameliorate the impact of addiction [31]. Is there any evidence that reversing the hypodopaminergic state can have a positive impact on substance-abuse-related behaviors? The answer is yes. Our studies show that by forcing the overproduction of D2R, deep inside the reward system of cocaine- or alcohol-experienced rats, we can significantly reduce the self-administration of cocaine [31] or alcohol [32], respectively. Moreover, in rodents, as well as in human methamphetamine abusers [33], a reduced striatal level of D2R is also associated with impulsivity, and in rodents it predicts compulsive patterns of drug self-administration (see below).
Figure 3.
Brain images of DA D2 receptors (D2R) at the level of the striatum in control subjects and substance drug abusers. Images were obtained with [11C]raclopride. Modified with permission from Volkow et al. [30].
Figure 4.
A: Images obtained with fluorodeoxyglucose (FDG) to measure brain metabolism in a control and in a cocaine abuser. Note the reduced metabolism in the orbitofrontal cortex (OFC) in the cocaine abuser when compared with the control. B: Correlations between DA D2 Receptors (D2R) in striatum and glucose metabolism in orbito-frontal cortex (OFC) in cocaine abusers. Modified with permission from Volkow et al. [29].
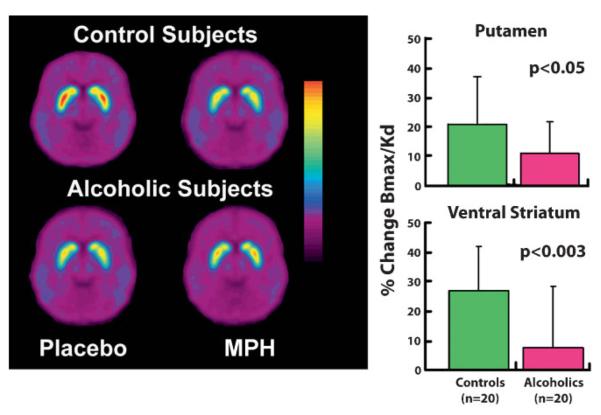
Imaging studies have also shown that, in humans, addiction is associated with a decrease in DA release in the ventral striatum and in other regions of the striatum, and in blunted pleasurable responses to the drug in active and in detoxified drug users (Fig. 5) [34]. This was an unexpected finding since it had been hypothesized that addiction reflected an enhanced sensitivity to the rewarding (and hence the dopaminergic) responses to drugs. In drug abusers, decreases in DA release could reflect either disrupted neurophysiology within the reward circuitry (i.e. in the DA neurons that release DA in the striatum) or, alternatively, a disrupted feedback regulation of the reward circuit by prefrontal (executive control) or amygdalar (emotional) pathways (prefrontal-striatal, amygdalarstriatal glutamatergic pathways). Since a pure dopaminergic dysfunction in the striatum, as seen in the chronic drug abuser, fails to account for the traits that characterize addictive behaviors, like impulsivity, cravings, and the relapse triggered by drug cues, it is very likely that prefrontal regions (as well as the amygdala) are also involved here, because their disruption would enable or at least influence these behavioral traits.
Figure 5.
MPH induced increases (assessed by its inhibition of raclopride's specific binding or Bmax/Kd) in controls and in detoxified alcoholics. Alcoholics show decreased DA release. Modified with permission from Volkow et al. [34].
Lowered dopamine receptor (DR2) levels impair the control of impulsivity by the prefrontal cortex
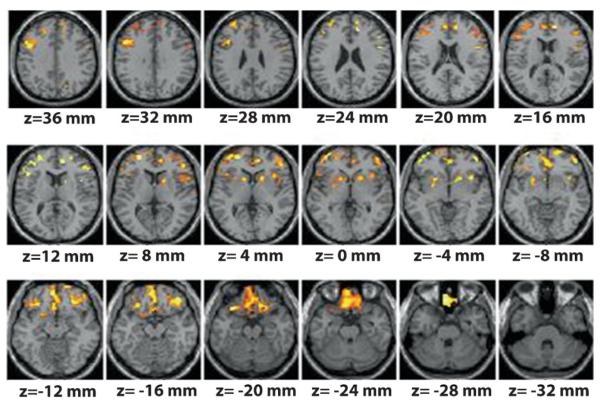
It has been hypothesized that the impaired control over compulsive drug taking behaviors that characterizes addiction may be due in part to specific dysfunctions in frontal regions of the brain [35]. There is now a significant amount of evidence that supports this notion, beginning with animal studies that explore the connection between D2R and behavioral control. Experiments with rats clearly show a correlation between low D2R and impulsivity [36], and between impulsivity and drug self administration [37]. But what is the connection? As previously mentioned, in drug abusers, lower striatal D2R significantly correlates with lower brain glucose metabolism in key regions of the PFC, such as the OFC (involved with salience attribution and whose disruption results in compulsive behaviors) and in CG (involved with inhibitory control and error monitoring and whose disruption results in impulsivity) (Fig. 4B) [38, 39]. Moreover, in a study we performed in individuals (mean SD ± age, 24 ± 3 years) a family history of alcoholism, but who were with not alcoholics themselves, we also uncovered a significant association between striatal D2R and metabolism in frontal regions (CG, OFC, and dorsolateral PFC) and also in anterior insula (involved in interoception, self-awareness, and drug craving) [40] (Fig. 6). Interestingly, these individuals had higher striatal D2R than matched controls with no family history of alcoholism, although they did not differ in frontal metabolism. Also, in the controls, striatal D2R did not correlate with frontal metabolism. This led us to speculate that the higher than normal striatal D2R in subjects with a high genetic risk for alcoholism protects them against alcoholism in part by strengthening activity in prefrontal regions. When combined, these data suggest that high levels of D2R in striatum could protect against drug abuse and addiction by keeping impulsivity traits under control, i.e., by regulating circuits involved in inhibiting behavioral responses and in controlling emotions.
Figure 6.
Areas of the brain where DA D2 receptors (D2R) were significantly correlated with brain metabolism in subjects with family history of alcoholism. Modified with permission from Volkow et al. [40].
Similarly, we hypothesized that the prefrontal regions are also involved in the reduction of striatal DA release (and reinforcement) observed in addicted subjects since they regulate DA cell firing in midbrain and DA release in striatum. To test this hypothesis we assessed the relationship between baseline metabolism in the PFC and the increases in striatal DA induced by the intravenous administration of MPH in controls and in detoxified alcoholics. Consistent with the hypothesis, in alcoholics we failed to detect the normal association between baseline prefrontal metabolism and DA release in striatum, suggesting that the marked decreases in DA release in striatum seen in alcoholics reflect in part improper regulation of brain activity by prefrontal brain regions [34].
Thus, we have found an association between reduced baseline activity in PFC and reduced striatal D2R in drug-addicted subjects, and between baseline PFC activity and DA release in controls that is not present in addicted individuals. These associations evince the strong connections between neuroadaptations in PFC pathways and downstream dysfunctions in the DA reward and motivational system, likely because of the influence of PFC on impulsivity and compulsivity. However, these fail to account for additional behavioral phenomena, such as the effects of drug-associated cues in triggering craving, which would presumably implicate memory and learning circuits.
Conditioned memories and stereotypic behaviors replace the “high” as the driver
Over-stimulation of DA cells in the ventral striatum eventually establishes new functional connections in the brain between the act of satisfying the urge, and the situational events surrounding it (e.g., environment, routine of preparing the drug, etc.), laying down new, powerful learned associations that can trigger behavior. Ultimately, the mere memory or anticipation of the drug can trigger the impulsive behaviors that characterize addicted individuals. With repeated drug use, the firing of DA cells in the striatum begins to change the neurochemistry underlying associative learning. This facilitates the consolidation of maladaptive memory traces connected to the drug, which helps explain the ability of all sorts of drug-associated stimuli (in the learned expectation of receiving the drug reward when exposed to these stimuli) [41] to readily trigger DA cells firing. And because of the role of DA in motivation, these DA increases trigger the motivation drive required to secure the reward [42]. Indeed, when rats are exposed repeatedly to a neutral stimulus that is paired with the drug (conditioned), it can elicit DA increases and reinstate drug self- administration [43]. Such conditioned responses are clinically relevant in substance-use disorders because they are responsible for the high likelihood of an addicted person to relapse even after protracted periods of detoxification. Now, brain imaging techniques allow us to test whether exposure of humans to drug-associated cues can trigger drug craving just as shown in laboratory animals.
With repeated drug use, the firing of DA cells in the striatum begins to change the neurochemistry underlying associative learning. This facilitates the consolidation of maladaptive memory traces connected to the drug, which helps explain the ability of all sorts of drug-associated stimuli (in the learned expectation of receiving the drug reward when exposed to these stimuli) [41] to readily trigger DA cells firing. And because of the role of DA in motivation, these DA increases trigger the motivation drive required to secure the reward [42]. Indeed, when rats are exposed repeatedly to a neutral stimulus that is paired with the drug (conditioned), it can elicit DA increases and reinstate drug self- administration [43]. Such conditioned responses are clinically relevant in substance-use disorders because they are responsible for the high likelihood of an addicted person to relapse even after protracted periods of detoxification. Now, brain imaging techniques allow us to test whether exposure of humans to drug-associated cues can trigger drug craving just as shown in laboratory animals.
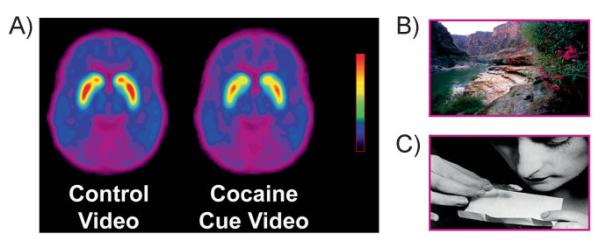
This question has been investigated in active cocaine abusers. Using PET and [11C]raclopride, two independent studies showed that exposure to a cocaine-cues video (of subjects smoking cocaine) but not to a neutral video (of nature scenes) increased striatal DA in human subjects addicted to cocaine (Fig. 7) and that the DA increases were associated with subjective reports of drug craving [44, 45]. The higher the DA increases triggered by exposure to the cocaine-cues video, the more intense the drug craving. Moreover, the magnitude of the DA increases was also correlated with addiction severity scores, highlighting the relevance of conditioned responses in the clinical syndrome of addiction.
Figure 7.
A: Average DV images of [11C]raclopride in a group of active cocaine abusers (n = 17) tested while viewing a (B) neutral video (nature scenes), and while viewing a (C) video with cocaine cues (subjects procuring and administering cocaine). Modified with permission from Volkow et al. [44].
It is important to emphasize, however, that in spite of the presumed strength of these maladaptive associations, we have recently gathered new evidence suggesting that cocaine abusers retain some ability to purposefully inhibit craving. Therefore, strategies to strengthen fronto-striatal regulation may offer potential therapeutic benefits [46].
Putting it all together
Some of the most pernicious features of drug addiction are the overwhelming craving to take drugs that can reemerge even after years of abstinence, and the severely compromised ability of addicted individuals to inhibit drug seeking once the craving erupts in spite of well-known negative consequences.
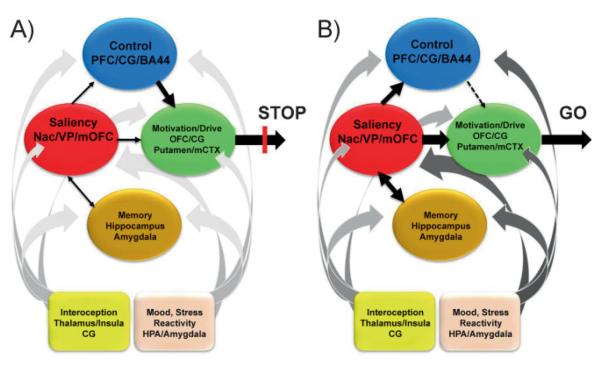
We have proposed a model of addiction [47] that explains the multidimensional nature of this disease by proposing a network of four interrelated circuits, whose combined dys-functional output can explain many of the stereotypic behavioral features of addiction: (a) reward, including several nuclei in the basal ganglia, especially the ventral striatum, whose Nac receives input from the ventral tegmental area and relays the information to the ventral pallidum (VP); (b) motivation/drive, located in the OFC, subcallosal cortex, dorsal striatum and motor cortex; (c) memory and learning, located in the amygdala and the hippocampus; and (d) planning and control, located in the dorsolateral prefrontal cortex, anterior CG and inferior frontal cortex. These four circuits receive direct innervations from DA neurons but are also connected with one another through direct or indirect projections (mostly glutamatergic).
The four circuits in this model work together and their operations change with experience. Each is linked to an important concept, respectively: saliency (reward), internal state (motivation/drive), learned associations (memory, conditioning), and conflict resolution (control). In addition, these circuits also interact with circuits involved with mood (including stress reactivity) [48] and with interoception (which result in awareness of drug craving and mood) [49]. We have proposed that the pattern of activity in the four-circuit network outlined here influences how a normal individual makes choices among competing alternatives. These choices are influenced systematically by the reward, memory/conditioning, motivation, and control circuits and these in turn are modulated by circuits that underlie mood and conscious awareness (Fig. 8A).
Figure 8.
Model proposing a network of four circuits underlying addiction: reward (red: located in the nucleus accumbens of the ventral astriatum and VP); motivation (green: located in OFC, subcallosal cortex, dorsal striatum, and motor cortex); memory (gold: located in the amygdala and hippocampus); and executive control (blue: located in dorsolateral prefrontal, anterior CG, and inferior frontal cortex). These circuits work together and change with experience. Each is linked to an important concept: saliency (reward), internal state (motivation/drive), learned associations (memory), and conflict resolution (control). A: When these circuits operate in an integrated and balanced fashion the result is manifested as the execution of appropriate behaviors (proper inhibitory control and decision making) in a broad range of circumstances. B: During addiction, the enhanced value of the drug in the reward, motivation, and memory circuits overcomes the inhibitory control exerted by the PFC, thereby favoring a positive-feedback loop initiated by the consumption of the drug and perpetuated by the enhanced activation of the motivation/drive and memory circuits [47]. In addition, these circuits also interact with circuits involved in the regulation of mood (pink: including stress reactivity) and interoception (yellow: that contributes to the awareness of drug craving and mood), which also become recalibrated during the process of addiction (represented by a darker shade of gray in the ascending arrows) further tilting the balance away from inhibitory control towards craving. Modified with permission from Volkow et al. [47].
The response to a stimulus is affected by its momentary saliency, i.e. its expected reward. In turn, reward expectation is processed in part by DA neurons projecting into the ventral striatum and influenced by glutamatergic projections from the OFC (which assigns saliency value as a function of context) and amygdala/hippocampus (which mediate conditioned responses and memory recollections). The value of the stimulus is weighted (compared) against that of other alternative stimuli, but also changes as a function of the internal needs of the individual, which are modulated by mood (including stress reactivity) and interoceptive awareness. In particular, stress exposure enhances the saliency value of drugs while at the same time it decreases prefrontal regulation of the amygdala [50]. In addition, since chronic drug exposure is linked to enhanced sensitization to stress responses this explains why stress can trigger a drug relapse so often in clinical situations. The stronger the saliency value of the stimulus, in part shaped by previously memorized experiences, the greater the activation of the motivational circuit and the stronger the drive to procure it. The cognitive decision to act (or not) to procure the stimulus is processed in part by the PFC and the CG, which weigh the balance between the immediate positive versus the delayed negative outcomes, and by the inferior frontal cortex (Broadmann Area 44), which works to inhibit the prepotent response to act [51].
According to this model, in the addicted subject (Fig. 8B), the saliency value of the drug of abuse and its associated cues is enhanced at the expense of other (natural) rewards, whose saliency is markedly reduced. This would explain the increased motivation to seek the drug. However, acute drug exposure also resets reward thresholds, resulting in decreased sensitivity of the reward circuit to reinforcers [52], which also helps explain the decreasing value of non-drug reinforcers in the addicted person. Another reason for the enhanced saliency of a drug is the lack of habituation of DA responses to drugs of abuse (tolerance) compared with the normal habituation that exists for natural rewards and that results in satiety [53].
Moreover, exposure to conditioned stimuli is sufficient to increase reward thresholds [54]; thus, we would predict that in an addicted person, exposure to an environment with conditioned cues would further exacerbate their decreased sensitivity to natural rewards. In the absence of competition by other reinforcers, conditioned learning elevates the acquiring of the drug to the level of a main motivational drive for the individual. We hypothesize that drug cues (or stress) result in fast DA increases in the Nac in the ventral striatum and in the dorsal striatum that drive the motivation to take the drug and cannot be properly opposed by a dysfunctional PFC. Thus, upon drug consumption and intoxication the enhancement of the DA signals would result in a corresponding overactivation of the motivational/drive and memory circuits, which deactivate the PFC (prefrontal inhibition occurs with intense amygdala activation) [50], blocking the power of the PFC to control the motivational/drive circuit. Without this inhibitory control, a positive-feedback loop is established, which results in compulsive drug intake. Because the interactions between the circuits are bidirectional, the activation of the network during intoxication serves to further strengthen the saliency value of the drug and the conditioning to drug cues.
Conclusions
In short, we propose a model that accounts for addiction as follows: During addiction, the enhanced value of drug cues in the memory circuit drives reward expectation and enhances the motivation to consume the drug, overcoming the inhibitory control exerted by an already dysfunctional PFC. Although the drug-induced DA increase is markedly attenuated in drug-addicted subjects, the pharmacological effects of the drug become conditioned responses in themselves, further driving the motivation to take the drug and favoring a positive-feedback loop now unopposed, because of the disconnection of the prefrontal control circuit. At the same time, addiction is likely to also recalibrate the circuits that instantiate mood and conscious awareness (represented by darker shades of gray) (Fig. 8B) in ways that, if experimentally corroborated, would further tilt the balance away from inhibitory control and towards craving and compulsive drug taking.
We readily admit that this is a simplified model: we realize that other brain regions must also be involved in these circuits, that one region may contribute to several circuits, and that other circuits are likely to also be involved in addiction. In addition, though this model focuses on DA, it is evident from preclinical studies that modifications in glutamatergic projections mediate many of the adaptations observed in addiction and that we discussed here. It is also evident from preclinical studies that other neurotransmitters are involved in the reinforcing effects of drugs including cannabinoids and opioids. Unfortunately, until recently, the limited access to radio-tracers for PET imaging has limited the capacity to investigate the involvement of other neurotransmitters in drug reward and in addiction.
Abbreviations
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- CG
cingulate gyrus
- CTX
cortex
- D2R
dopamine type 2/3 receptor
- DA
dopamine
- FDG
fluorodeoxyglucose
- GABA
γ-aminobutyiric acid
- HPA
hypothalamic pituitary axis
- MPH
methylphenidate
- Nac
nucleus accumbens
- NMDA
n-methyl-d-aspartic acid
- OFC
orbitofrontal cortex
- PET
positron emission tomography
- PFC
prefrontal cortex
- VP
ventral pallidum
References
- 1.Zink CF, Pagnoni G, Martin ME, et al. Human striatal response to salient nonrewarding stimuli. J Neurosci. 2003;23:8092–7. doi: 10.1523/JNEUROSCI.23-22-08092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–6. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- 3.Tobler PN, O'Doherty JP, Dolan RJ, et al. Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. J Neurophysiol. 2007;97:1621–32. doi: 10.1152/jn.00745.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–84. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- 5.Volkow ND, Wang GJ, Ma Y, et al. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci. 2003;23:11461–8. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–23. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 7.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villemagne VL, Wong DF, Yokoi F, et al. GBR12909 attenuates amphetamine-induced striatal dopamine release as measured by [(11)C]raclopride continuous infusion PET scans. Synapse. 1999;33:268–73. doi: 10.1002/(SICI)1098-2396(19990915)33:4<268::AID-SYN3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 9.Hemby SE. Drug addiction and its treatment: Nexus of neuro-science and behavior. In: Johnson BA, Dworkin SI, editors. Neurobiological Basis of Drug Reinforcement. Lippincott-Raven; Philadelphia: 1997. [Google Scholar]
- 10.Brody AL, Mandelkern MA, Olmstead RE, et al. Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology. 2009;34:282–9. doi: 10.1038/npp.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boileau I, Assaad JM, Pihl RO, et al. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–31. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- 12.Drevets WC, Gautier C, Price JC, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- 13.Volkow ND, Wang GJ, Fowler JS, et al. Relationship between psychostimulant-induced “high” and dopamine transporter occupancy. Proc Natl Acad Sci USA. 1996;93:10388–92. doi: 10.1073/pnas.93.19.10388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volkow ND, Wang GJ, Fowler JS, et al. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther. 1999;291:409–15. [PubMed] [Google Scholar]
- 15.Volkow ND, Wang GJ, Fowler JS, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155:1325–31. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- 16.Chait LD. Reinforcing and subjective effects of methylphenidate in humans. Behav Pharmacol. 1994;5:281–8. doi: 10.1097/00008877-199406000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoops WW, Vansickel AR, Lile JA, et al. Acute d-amphetamine pretreatment does not alter stimulant self-administration in humans. Pharmacol Biochem Behav. 2007;87:20–9. doi: 10.1016/j.pbb.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parasrampuria DA, Schoedel KA, Schuller R, et al. Assessment of pharmacokinetics and pharmacodynamic effects related to abuse potential of a unique oral osmotic-controlled extended-release methylphenidate formulation in humans. J Clin Pharmacol. 2007;47:1476–88. doi: 10.1177/0091270007308615. [DOI] [PubMed] [Google Scholar]
- 20.Balster RL, Schuster CR. Fixed-interval schedule of cocaine reinforcement: effect of dose and infusion duration. J Exp Anal Behav. 1973;20:119–29. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volkow ND, Wang GJ, Fischman MW, et al. Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci. 2000;67:1507–15. doi: 10.1016/s0024-3205(00)00731-1. [DOI] [PubMed] [Google Scholar]
- 22.Volkow ND, Ding YS, Fowler JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry. 1995;52:456–63. doi: 10.1001/archpsyc.1995.03950180042006. [DOI] [PubMed] [Google Scholar]
- 23.Zweifel LS, Parker JG, Lobb CJ, et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci USA. 2009;106:7281–8. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lane DA, Lessard AA, Chan J, et al. Region-specific changes in the subcellular distribution of AMPA receptor GluR1 subunit in the rat ventral tegmental area after acute or chronic morphine administration. J Neurosci. 2008;28:9670–81. doi: 10.1523/JNEUROSCI.2151-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong Y, Saal D, Thomas M, et al. Cocaine-induced potentiation of synaptic strength in dopamine neurons: behavioral correlates in GluRA(−/−) mice. Proc Natl Acad Sci USA. 2004;101:14282–7. doi: 10.1073/pnas.0401553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–58. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 27.Di Chiara G, Bassareo V, Fenu S, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47:227–41. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 28.Volkow ND, Wang GJ, Fowler JS, et al. Cocaine uptake is decreased in the brain of detoxified cocaine abusers. Neuropsychopharmacology. 1996;14:159–68. doi: 10.1016/0893-133X(95)00073-M. [DOI] [PubMed] [Google Scholar]
- 29.Volkow ND, Fowler JS, Wang GJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–77. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- 30.Volkow ND, Fowler JS, Wang GJ, et al. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–24. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- 31.Thanos PK, Michaelides M, Umegaki H, et al. D2R DNA transfer into the nucleus accumbens attenuates cocaine self-administration in rats. Synapse. 2008;62:481–6. doi: 10.1002/syn.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thanos PK, Taintor NB, Rivera SN, et al. DRD2 gene transfer into the nucleus accumbens core of the alcohol preferring and nonpreferring rats attenuates alcohol drinking. Alcohol Clin Exp Res. 2004;28:720–8. doi: 10.1097/01.alc.0000125270.30501.08. [DOI] [PubMed] [Google Scholar]
- 33.Lee B, London ED, Poldrack RA, et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–40. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volkow ND, Wang GJ, Telang F, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbito-frontal involvement. J Neurosci. 2007;27:12700–6. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–9. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Dalley JW, Fryer TD, Brichard L, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belin D, Mar AC, Dalley JW, et al. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–5. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volkow ND, Chang L, Wang GJ, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–82. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 39.Volkow ND, Wang GJ, Fowler JS, et al. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry. 1999;156:19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- 40.Volkow ND, Wang GJ, Begleiter H, et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- 41.Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412:43–8. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- 42.McClure SM, Daw ND, Montague PR. A computational substrate for incentive salience. Trends Neurosci. 2003;26:423–8. doi: 10.1016/s0166-2236(03)00177-2. [DOI] [PubMed] [Google Scholar]
- 43.Phillips PE, Stuber GD, Heien ML, et al. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–8. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- 44.Volkow ND, Wang GJ, Telang F, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–8. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong DF, Kuwabara H, Schretlen DJ, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–27. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- 46.Volkow ND, Fowler JS, Wang GJ, et al. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49:2536–43. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–51. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldstein RZ, Craig AD, Bechara A, et al. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–80. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grace AA. Disruption of cortical-limbic interaction as a substrate for comorbidity. Neurotox Res. 2006;10:93–101. doi: 10.1007/BF03033238. [DOI] [PubMed] [Google Scholar]
- 51.Volkow ND, Fowler JS, Wang GJ, et al. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49:2536–43. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barr AM, Markou A. Psychostimulant withdrawal as an inducing condition in animal models of depression. Neurosci Biobehav Rev. 2005;29:675–706. doi: 10.1016/j.neubiorev.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Di Chiara G. Dopamine in disturbances of food and drug motivated behavior: a case of homology? Physiol Behav. 2005;86:9–10. doi: 10.1016/j.physbeh.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 54.Kenny PJ, Markou A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. J Neurosci. 2005;25:6208–12. doi: 10.1523/JNEUROSCI.4785-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fowler JS, Volkow ND, Logan J, et al. Fast uptake and long-lasting binding of methamphetamine in the human brain: comparison with cocaine. Neuroimage. 2008;43:756–63. doi: 10.1016/j.neuroimage.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]