Abstract
Evolution occurring over contemporary time scales can have important effects on populations, communities, and ecosystems. Recent studies show that the magnitude of these effects can be large and can generate feedbacks that further shape evolution.
Introduction and context
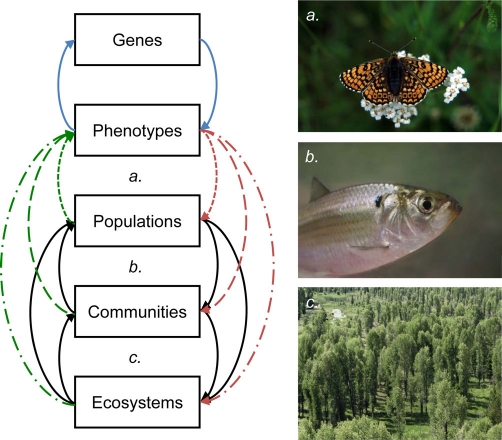
Evolutionary changes can keep pace with ecological changes [1,2]. This fundamental realization is revolutionizing our understanding of the forces governing the dynamics of natural systems. If evolution can happen at the pace of ecology, then ecological changes can directly shape evolution and vice versa [3,4]. It is this bi-directionality that intertwines ecological and evolutionary dynamics in contemporary time, leading to ‘eco-evolutionary dynamics’ (Figure 1). One direction of causality is now well established; ecological differences can cause trait evolution over years to decades (i.e., contemporary evolution) [1]. The other direction of causality - from contemporary evolution to ecological dynamics - has only recently been the focus of detailed work, although evolutionary explanations for ecological phenomena have been around for a long time [5]. The purpose of this review is to highlight some of the recent work in empirical systems that is beginning to show how contemporary evolution can influence populations, communities, and ecosystems. We also discuss empirical evidence for the dynamic feedbacks that can result from these bi-directional interactions. Finally, we discuss important areas for future work and describe some challenges facing this rapidly growing field.
Figure 1. Eco-evolutionary dynamics describe interactions between ecology and evolution occurring on contemporary time scales.
The attributes of populations, communities, and ecosystems influence phenotypes via selection and plasticity (green arrows). Selection is translated into evolutionary change via genetic inheritance (blue arrows). The resulting phenotypes can then influence the attributes of populations, communities, and ecosystems (red arrows). These effects can cascade among levels of ecological organization via ecological effects such as trophic interactions (black arrows). Note that plasticity can influence ecology by shaping phenotypes in the absence of genetic change. Eco-evolutionary feedbacks describe the effects of contemporary evolution on ecological dynamics and the reciprocal effects of ecology on the trajectory of evolution (loops represented by different dashed lines). Evidence for eco-evolutionary feedbacks comes from (a) butterflies (Melitaea cinxia) at the population level [10,11], (b) fish (Alosa pseudoharengus) at the community level [15-17], and (c) trees (Populus spp.) at the ecosystem level [13,23]. Most studies to date have relied on evolutionary inferences drawn from phenotypes. It remains a major challenge to more fully integrate molecular genetic data into the study of eco-evolutionary dynamics in the wild (but see [10,11]). Photo credits: (a) Tari Haahtela, (b) Brian Gratwicke, and (c) Joe Bailey.
Major recent advances
Recent work shows that evolutionary processes can impact ecological dynamics at multiple levels of ecological organization. Here, we describe evolutionary effects on populations, communities, and ecosystems. Some individual studies span multiple levels, and in these cases we have attempted to place studies where they are most appropriate. However, our overall goal is not to classify studies by level of organization. Rather, it is to describe the breadth of ecological processes that are influenced by contemporary evolution.
Evolutionary effects on populations
It is intuitive that natural selection on traits that influence the vital rates of populations should have ecological consequences for population dynamics. Despite this intuitive link, clear examples of contemporary evolution impacting population dynamics in the wild have come only recently. Contemporary evolution in newly founded populations can enhance survival and reproduction, as shown for introduced populations of Chinook salmon (Oncorhynchus tshawytscha) [6]. Evolution can also shape the population dynamics of multiple interacting species, as shown in simple algae-rotifer chemostats [7]. In established wild populations, natural selection has been shown to influence population growth rates. For several species of free-ranging large mammals inhabiting a wide variety of habitat types, juvenile body size contributes substantially to population growth [8]. For Soay sheep (Ovis aries) on the island of St. Kilda (Outer Hebrides, Scotland), this contribution was greatest in years when survival was lowest [9]. Ultimately, the effort to connect evolution to population dynamics should link dynamic changes in demography to changes in both phenotypes and the underlying genes. For example, population dynamics in the Glanville fritillary butterfly (Melitaea cinxia) are shaped by genetic variation at a locus that influences metabolic rate and dispersal behavior [10,11].
Evolutionary effects on communities
The traits of organisms shape the form and strength of ecological interactions. These interactions, in turn, mold the properties of communities [12]. Most empirical studies investigating the effects of evolution on communities have focused on how standing genetic and phenotypic variation in one species influences the coexisting assemblage of species. Because not all species are expected to have equal effects on communities, studies have focused primarily on strong-interacting species (e.g., foundation species, keystone species, and dominant species). For such species, a range of recent studies show that genetic and phenotypic variation can contribute substantially to community structure. For a number of plant species, the genotype of individual host plants influences the arthropod community found thereon [13]. Variation in arthropod communities can, in turn, influence interactions at higher trophic levels, including the foraging behavior of birds [14]. The majority of studies investigating the effects of evolution on communities have focused on the effects of genotype identity and genetic diversity within plant species. However, recent studies have extended these ideas to animals by comparing ecologically divergent populations that share a recent common ancestor. For example, recently diverged populations of alewives (Alosa pseudoharengus), a planktivorous fish, display phenotypic differences in trophic morphology and prey selectivity [15]. These differences drive divergence in zooplankton communities which then cascade and influence the abundance of phytoplankton [16,17].
Evolutionary effects on ecosystems
Evolution can shape the role organisms play in ecosystems by molding key processes, including consumption and nutrient cycling [4]. Effects of consumption on ecosystem processes are often indirect; they are mediated through community-level effects. For example, the evolutionary effects of feeding specialization in threespine sticklebacks (Gasterosteus aculeatus) have been shown in mesocosms to impact diverse ecosystem processes, from algal production and biomass to dissolved organic carbon and light transmission [18]. In contrast to consumption, nutrient cycling is a more direct pathway by which evolution in both plants and animals may impact ecosystem processes. In foundation plant species, such as trees of the genus Populus, heritable variation in leaf chemistry impacts soil microbial community composition, decomposition rates, and nitrogen mineralization rates [19,20]. In aquatic ecosystems, overall rates of nutrient recycling are influenced by the body-size distribution of fishes, which may be shaped by evolution. An example is provided by the guppy (Poecilia reticulata), a tropical stream fish. Populations exposed to predators mature at a smaller body size and have more numerous, smaller offspring than populations that do not face strong predation risk. These life history differences can evolve on contemporary time scales, influence rates of nutrient cycling, and may influence algal biomass [21].
Evolutionary effects versus ‘traditional’ ecological effects
Evolution shapes ecological patterns and processes over long time scales; the evolution of photosynthesis is one clear example. The above studies demonstrate that short-term evolution also influences ecological processes under at least some conditions. But are short-term evolutionary effects important and ubiquitous enough to warrant broad consideration from ecologists? One way to address this question is to examine relative effect sizes for short-term evolutionary drivers relative to well-established ‘traditional’ ecological drivers. On the basis of such comparisons, it appears that the ecological effects of short-term evolution are often on par with, and can sometimes be greater than, traditional ecological effects at the population [2,6,8], community [12,22], and ecosystem [21,22] levels. In short, contemporary evolution can be an important contributor to ecological dynamics across systems and levels of ecological organization. A major current challenge facing the field of eco-evolutionary dynamics is to broadly determine the conditions under which short-term evolutionary effects will be most important. For example, direct evolutionary effects, such as the effects of life history evolution on population dynamics, are likely to be robust and general. In contrast, indirect effects, such as the impact of consumption on ecosystem processes, may be subject to greater contingencies [22]. These contingencies include the complexity of the ecosystem, the ecological role of the evolving population, and the specific traits under selection [4].
From effects to dynamics
The ultimate goal of research on eco-evolutionary dynamics is to understand not only one-way interactions between ecology and evolution, as described above, but also the dynamic feedbacks that arise due to bi-directional interactions (Figure 1). Conclusively demonstrating these feedbacks is difficult in nature because ecological and evolutionary processes are so thoroughly intertwined. Nonetheless, several approaches have been proposed to move the field from static effects of standing genetic variation to true dynamics and feedbacks. At the population level, Zheng et al. [11] used detailed information on linkages between genes and phenotypes to construct a field-parameterized metapopulation model of the aforementioned butterfly (M. cinxia). They used this model to examine the strength of the dynamic coupling between ecology and evolution and found that, in this case, demography had a greater impact on evolution than vice versa. At the community level, Palkovacs and Post [15] proposed an approach that compares ‘coupled systems’, where bi-directional causality is present, to ‘decoupled systems’, where bi-directional causality is absent. This approach was applied to communities composed of alewives (A. pseudoharengus) and their zooplankton prey. In some habitats, zooplankton are exposed to continuous predation, whereas in other habitats they have a temporal refuge from predation. From an eco-evolutionary standpoint, habitats lacking refuges are ‘coupled’ because alewives have the opportunity to shape the zooplankton community, whereas habitats with prey refuges are ‘decoupled’. Results showed that only in the coupled systems did alewives shape the evolution of their own foraging traits via their impact on zooplankton size structure. At the ecosystem level, Fischer et al. [23] documented associations between condensed tannins in the leaves of trees (Populus angustifolia, P. fremontii, and their hybrids), nutrient release in the soil, the production of fine roots, and rates of nutrient uptake from the soil. These associations suggest the presence of an eco-evolutionary feedback driven by the effect of tree leaf chemistry on the soil microenvironment. In addition to these examples, a variety of other natural systems, including the evolution of foraging traits in seed predators and the structure of plant communities and the evolution of life history traits in fishes and the effects of nutrient cycling in aquatic ecosystems, show strong potential for eco-evolutionary feedbacks [4].
Future directions
In addition to those areas outlined above, we see several key areas where the study of eco-evolutionary dynamics could provide important new insights. Coevolution is likely a common feature of natural communities, but its effects on ecological dynamics are almost entirely unknown. The single study that has examined the ecosystem effects of coevolution found that fish species taken from the same locality (locally coevolved) reduced aquatic invertebrate biomass relative to fish species taken from different localities (non-coevolved) [21]. More work will be required to determine how often these eco-coevolutionary effects are important.
The traditional view of adaptive radiation is one of ecological opportunity, whereby lineages diversify until all available niches are filled. However, this view largely ignores the role that organisms play in shaping their environments. If this role is substantial, eco-evolutionary feedbacks may be an important driver of evolutionary diversification. Rather than lineages simply diversifying to fill available niches, ecological niches themselves may be diversifying. Evidence for this eco-evolutionary mechanism of adaptive diversification has come from laboratory experiments [24] and the fossil record [25]. However, an eco-evolutionary perspective has yet to be integrated into most working models of adaptive diversification.
Human activity causes ecological change on a global scale and also causes considerable contemporary evolution [26]. Recently, Jørgensen et al. [27] proposed ‘evolutionary impact assessments’ as tools to manage fisheries in the face of harvest-induced evolution. The ultimate goal of evolutionary impact assessments is to predict the consequences of different management options so that management decisions can provide for the greatest long-term benefit to ecosystems and society. This framework is explicitly eco-evolutionary, as it recognizes that the traits under selection by fisheries can influence ecological processes such as population dynamics, trophic interactions, and nutrient recycling [27]. The development of similar approaches for other environmental threats will be important tools for maintaining environmental health in the face of human activity. For example, eco-evolutionary strategies can be developed to prevent or slow species invasions and to stave off extinctions [6,28]. Understanding the nature and direction of selection on keystone or dominant species may enable ecological forecasting for entire communities [29], and ensuring ample scope for evolutionary responses may enhance ecosystem resilience to environmental perturbations [21,30,31]. Eco-evolutionary approaches are thus poised to contribute both to our basic understanding of natural systems and to strategies for confronting the ever-increasing threats to our global environment.
Acknowledgements
The authors thank DM Post, MT Kinnison, MTJ Johnson, F Pelletier, I Hanski, JA Schweitzer, and JK Bailey for discussions and comments that improved this manuscript.
Competing interests
The authors declare that they have no competing interests.
The electronic version of this article is the complete one and can be found at: http://f1000.com/reports/b/2/1
References
- 1.Hendry AP, Kinnison MT. Perspective: the pace of modern life: measuring rates of contemporary microevolution. Evolution. 1999;53:1637–53. doi: 10.1111/j.1558-5646.1999.tb04550.x. [DOI] [PubMed] [Google Scholar]
- 2.Hairston NGJ, Ellner SP, Geber MA, Yoshida T, Fox JA. Rapid evolution and the convergence of ecological and evolutionary time. Ecol Lett. 2005;8:1114–27. doi: 10.1111/j.1461-0248.2005.00812.x. [DOI] [Google Scholar]
- 3.Laland KN, Odling-Smee FJ, Feldman MW. Evolutionary consequences of niche construction and their implications for ecology. Proc Nat Acad Sci U S A. 1999;96:10242–7. doi: 10.1073/pnas.96.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Post DM, Palkovacs EP. Eco-evolutionary feedbacks in community and ecosystem ecology: interactions between the ecological theatre and the evolutionary play. Philos Trans R Soc Lond B Biol Sci. 2009;364:1629–40. doi: 10.1098/rstb.2009.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelletier F, Garant D, Hendry AP. Eco-evolutionary dynamics. Philos Trans R Soc Lond B Biol Sci. 2009;364:1483–9. doi: 10.1098/rstb.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinnison MT, Unwin MJ, Quinn TP. Eco-evolutionary vs. habitat contributions to invasion in salmon: experimental evaluation in the wild. Mol Ecol. 2008;17:405–14. doi: 10.1111/j.1365-294X.2007.03495.x. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida T, Jones LE, Ellner SP, Fussmann GF, Hairston NGJ. Rapid evolution drives ecological dynamics in a predator-prey system. Nature. 2003;424:303–6. doi: 10.1038/nature01767. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Richard Lenski 30 Jul 2003
- 8.Ezard THG, Cote SD, Pelletier F. Eco-evolutionary dynamics: disentangling phenotypic, environmental and population fluctuations. Philos Trans R Soc Lond B Biol Sci. 2009;364:1491–8. doi: 10.1098/rstb.2009.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelletier F, Clutton-Brock T, Pemberton J, Tuljapurkar S, Coulson T. The evolutionary demography of ecological change: linking trait variation and population growth. Science. 2007;315:1571–4. doi: 10.1126/science.1139024. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Michael Bonsall 20 Mar 2007
- 10.Hanski I, Saccheri I. Molecular-level variation affects population growth in a butterfly metapopulation. PLoS Biol. 2006;4:719–26. doi: 10.1371/journal.pbio.0040129. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 9.8 ExceptionalEvaluated by Fred Allendorf 03 May 2006, Richard Frankham 19 Jun 2006, James Cronin 26 Oct 2006
- 11.Zheng CZ, Ovaskainen O, Hanski I. Modelling single nucleotide effects in phosphoglucose isomerase on dispersal in the Glanville fritillary butterfly: coupling of ecological and evolutionary dynamics. Philos Trans R Soc Lond B Biol Sci. 2009;364:1519–32. doi: 10.1098/rstb.2009.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson MT, Stinchcombe JR. An emerging synthesis between community ecology and evolutionary biology. Trends Ecol Evol. 2007;22:250–7. doi: 10.1016/j.tree.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Whitham TG, Bailey JK, Schweitzer JA, Shuster SM, Bangert RK, Leroy CJ, Lonsdorf EV, Allan GJ, DiFazio SP, Potts BM, Fischer DG, Gehring CA, Lindroth RL, Marks JC, Hart SC, Wimp GM, Wooley SC. A framework for community and ecosystem genetics: from genes to ecosystems. Nat Rev Genet. 2006;7:510–23. doi: 10.1038/nrg1877. [DOI] [PubMed] [Google Scholar]
- 14.Bailey JK, Wooley SC, Lindroth RL, Whitham TG. Importance of species interactions to community heritability: a genetic basis to trophic-level interactions. Ecol Lett. 2006;9:78–85. doi: 10.1111/j.1461-0248.2005.00844.x. [DOI] [PubMed] [Google Scholar]
- 15.Palkovacs EP, Post DM. Eco-evolutionary interactions between predators and prey: can predator-induced changes to prey communities feed back to shape predator foraging traits? Evol Ecol Res. 2008;10:699–720. [Google Scholar]
- 16.Palkovacs EP, Post DM. Experimental evidence that phenotypic divergence in predators drives community divergence in prey. Ecology. 2009;90:300–5. doi: 10.1890/08-1673.1. [DOI] [PubMed] [Google Scholar]
- 17.Post DM, Palkovacs EP, Schielke EG, Dodson SI. Intraspecific phenotypic variation in a predator affects community structure and cascading trophic interactions. Ecology. 2008;89:2019–32. doi: 10.1890/07-1216.1. [DOI] [PubMed] [Google Scholar]
- 18.Harmon LJ, Matthews B, Des Roches S, Chase JM, Shurin JB, Schluter D. Evolutionary diversification in stickleback affects ecosystem functioning. Nature. 2009;458:1167–70. doi: 10.1038/nature07974. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.4 Must ReadEvaluated by Andrew Gonzalez 16 Apr 2009, Nelson Hairston Jr 27 Jul 2009
- 19.Schweitzer JA, Bailey JK, Fischer DG, Leroy CJ, Lonsdorf EV, Whitham TG, Hart SC. Plant-soil-microorganism interactions: heritable relationship between plant genotype and associated soil microorganisms. Ecology. 2008;89:773–81. doi: 10.1890/07-0337.1. [DOI] [PubMed] [Google Scholar]
- 20.Schweitzer JA, Madritch MD, Bailey JK, LeRoy CJ, Fischer DG, Rehill BJ, Lindroth RL, Hagerman AE, Wooley SC, Hart SC, Whitham TG. From genes to ecosystems: the genetic basis of condensed tannins and their role in nutrient regulation in a Populus model system. Ecosystems. 2008;11:1005–20. doi: 10.1007/s10021-008-9173-9. [DOI] [Google Scholar]
- 21.Palkovacs EP, Marshall MC, Lamphere BA, Lynch BR, Weese DJ, Fraser DF, Reznick DN, Pringle CM, Kinnison MT. Experimental evaluation of evolution and coevolution as agents of ecosystem change in Trinidadian streams. Philos Trans R Soc Lond B Biol Sci. 2009;364:1617–28. doi: 10.1098/rstb.2009.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey JK, Schweitzer JA, Ubeda F, Koricheva J, LeRoy CJ, Madritch MD, Rehill BJ, Bangert RK, Fischer DG, Allan GJ, Whitham TG. From genes to ecosystems: a synthesis of the effects of plant genetic factors across levels of organization. Philos Trans R Soc Lond B Biol Sci. 2009;364:1607–16. doi: 10.1098/rstb.2008.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer DG, Hart SC, Rehill BJ, Lindroth RL, Keim P, Whitham TG. Do high-tannin leaves require more roots? Oecologia. 2006;149:668–75. doi: 10.1007/s00442-006-0471-7. [DOI] [PubMed] [Google Scholar]
- 24.Habets MGJL, Rozen DE, Hoekstra RF, de Visser JAGM. The effect of population structure on the adaptive radiation of microbial populations evolving in spatially structured environments. Ecol Lett. 2006;9:1041–8. doi: 10.1111/j.1461-0248.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- 25.Erwin DH. Macroevolution of ecosystem engineering, niche construction and diversity. Trends Ecol Evol. 2008;23:304–10. doi: 10.1016/j.tree.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Darimont CT, Carlson SM, Kinnison MT, Paquet PC, Reimchen TE, Wilmers CC. Human predators outpace other agents of trait change in the wild. Proc Nat Acad Sci U S A. 2009;106:952–4. doi: 10.1073/pnas.0809235106. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by J Emmett Duffy 10 Feb 2009
- 27.Jørgensen C, Enberg K, Dunlop ES, Arlinghaus R, Boukal DS, Brander K, Ernande B, Gardmark A, Johnston F, Matsumura S, Pardoe H, Raab K, Silva A, Vainikka A, Dieckmann U, Heino M, Rijnsdorp AD. Ecology: managing evolving fish stocks. Science. 2007;318:1247–8. doi: 10.1126/science.1148089. [DOI] [PubMed] [Google Scholar]
- 28.Kinnison MT, Hairston NG. Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Funct Ecol. 2007;21:444–54. doi: 10.1111/j.1365-2435.2007.01278.x. [DOI] [Google Scholar]
- 29.Johnson MT, Vellend M, Stinchcombe JR. Evolution in plant populations as a driver of ecological changes in arthropod communities. Philos Trans R Soc Lond B Biol Sci. 2009;364:1593–605. doi: 10.1098/rstb.2008.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lennon JT, Martiny JBH. Rapid evolution buffers ecosystem impacts of viruses in a microbial food web. Ecol Lett. 2008;11:1178–88. doi: 10.1111/j.1461-0248.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- 31.Reusch TBH, Ehlers A, Hammerli A, Worm B. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proc Nat Acad Sci U S A. 2005;102:2826–31. doi: 10.1073/pnas.0500008102. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 4.8 Must ReadEvaluated by Gregor Fussmann 24 Feb 2005, Richard Frankham 29 Apr 2005