Abstract
New neurons are born and integrated into functional circuits in the brains of many adult organisms. In virtually all of these systems, serotonin is a potent regulator of neuronal proliferation. Specific neural pathways underlying these serotonergic influences have not, however, been identified and manipulated. The goal of the present study was to test whether adult neurogenesis in the crustacean brain is influenced by electrical activity in the serotonergic dorsal giant neurons (DGNs) innervating the primary olfactory processing areas, the olfactory lobes (OLs), and higher order centers, the accessory lobes (ALs). Adult-born neurons occur in two interneuronal cell clusters that are part of the olfactory pathway. The present study demonstrates that neurogenesis also continues in these areas in a dissected, perfused brain preparation, although the rate of neuronal production is lower than in brains from intact same-sized animals. Inclusion of 10−9M serotonin in the perfusate delivered to the dissected brain preparation restores the rate of neurogenesis to in vivo levels. While subthreshold stimulation of the DGN does not significantly alter the rate of neurogenesis, electrical activation of a single DGN results in significant increases in neurogenesis in Cluster 10 (CL10) on the same side of the brain, compared with levels on the contralateral, unstimulated side. Measurements of serotonin levels in the perfusate using high performance liquid chromatography established that serotonin levels are elevated about ten-fold during DGN stimulation, confirming that serotonin is released during DGN activity. This is the first identified neural pathway through which adult neurogenesis has been directly manipulated.
Keywords: Cell proliferation, serotonin, olfactory pathway, bromodeoxyuridine, neurogenic niche
Introduction
New neurons are born and incorporated into functional circuits in the brains of many adult organisms. In mammals, including humans, neurons continue to be added to the olfactory system and hippocampus (Altman and Das, 1965; Altman, 1969; Eriksson et al., 1998; Bédard and Parent, 2004). In arthropods (insects and crustaceans) the addition of new neurons is found in areas of the brain and peripheral nervous system that are, as in vertebrates, associated with olfaction (reviewed in Cayre et al., 2002; Beltz and Sandeman, 2003); in crustaceans, adult neurogenesis also is found in the optic neuropils (Sullivan and Beltz, 2005a).
Numerous environmental and endogenous factors have been shown to influence the rate at which neurogenesis in the adult brain proceeds. For example, age, diet, locomotory activity, environmental surroundings, seasonality, social interactions, the day night cycle, and levels of circulating hormones alter the rate of neurogenesis in both vertebrate and non-vertebrate systems (Beltz and Sandeman, 2003, Kempermann, 2006). Serotonin, which is a potent regulator of cell division in a variety of organisms and tissue types, also appears to play a critical role in the control of adult neural cell proliferation (Brezun and Daszuta, 2000; Benton and Beltz, 2001; Beltz et al., 2001). Dysregulation in serotonergic mechanisms in humans and reduced neurogenesis after stress may be linked to clinical depression (Jacobs et al., 2008; Paizanis et al., 2007). In no case, however, have specific neural pathways underlying the serotonergic regulation of neurogenesis been identified or manipulated. The goal of the present study was to test whether the rate of adult neurogenesis is influenced by electrical activity in identified cells, the dorsal giant neurons (DGNs), which constitute a primary serotonergic pathway in the crustacean brain.
The progenitors of the new neurons in the olfactory system of mammals are remote from the site of neuronal incorporation, and so the neuronal precursors must migrate in the rostral migratory stream over considerable distances to the olfactory bulb, where they differentiate. In contrast, neuronal precursors in the few insects in which adult neurogenesis occurs appear be asymmetrically dividing neuroblasts that persist from embryonic stages, and these are situated close to their sites of differentiation and incorporation in the brain. Asymmetrically dividing neuroblasts also form the scaffold of the developing crustacean brain, but these cells die during late embryonic and early larval life (Harzsch et al., 1998; Sandeman and Sandeman, 2003). In several decapod crustacean species (e.g., Homarus americanus, Cherax destructor, Procambarus clarkii, and the possibly related but taxonomically undefined marble crayfish), we have proposed that new neurons added to the midbrain come from precursor cells clustered in a neurogenic niche located on the ventral surface of the brain (Sullivan et al., 2007a; Zhang et al., 2009).
The influence of serotonin on neurogenesis in Crustacea has been most extensively examined in the American lobster, H. americanus. Studies have demonstrated that serotonin levels influence morphogenesis in specific brain neuropils during embryogenesis (Benton et al., 1997), and in adults alter the rate of neuronal proliferation and survival of local and projection neurons in the olfactory pathway (cell clusters 9 and 10, respectively; terminology of Sandeman et al., 1992) (Benton and Beltz, 2001; Beltz et al., 2001). Serotonin depletion results in a decrease in the rate of cell proliferation and survival, as well as abnormal differentiation in a subset of the new neurons (Sullivan et al., 2000), whereas higher levels of serotonin lead to an increase in the rate of cell proliferation (Benton et al., 2008). Possibly related to this effect of serotonin is the presence in clawed lobsters (e.g., H. americanus) and freshwater crayfish (e.g., C. destructor, P. clarkii) of a pair of large serotonergic interneurons, the dorsal giant neurons (DGNs), one on each side of the midbrain. These neurons can be considered homologs in the three species because of their many common features: the cell somata are dorsal, located in the same cluster of neurons, are the largest on the dorsal side of the brain, and project ipsilaterally to innervate the OLs and ALs. The primary neurite descends ventrally from the soma; branches from this process ramify amongst the axons of the olfactory globular tract that is composed of fibers of the CL10 projection neurons. The primary neurite then branches into the OLs and ALs, projecting to each and every glomerulus in both lobes. The projections of these neurons in all three species are strictly unilateral and label with antibodies raised against serotonin (Beltz and Sandeman 2003).
The DGNs in the crayfish C. destructor and the lobster H. americanus are ideal subjects for anatomical, ultrastructural, physiological and developmental studies because of their large size and accessibility (Sandeman and Sandeman, 1994; Sandeman DC et al., 1995; Benton and Beltz 2001). Of particular interest, however, is their proximity to the interneurons in Clusters 9 and 10 which arborize in the same neuropils as the DGNs, and which are the only two populations of interneurons in the decapod midbrain where new neurons continue to be produced throughout these animals' lives. In lobsters, fine fibers from the DGN terminate in the proliferation zone of CL10 (Beltz et al., 2001). Ultrastructural studies of the DGN have shown vesicles lined up along membranes that do not have synaptic specializations, suggesting as in other serotonergic systems that these cells may release serotonin locally into the surrounding intercellular space (Sandeman RE et al., 1995). In lobsters, the rate of neurogenesis is highly sensitive to serotonin, with the greatest increase at very low levels (10−10M) of the amine, a concentration that is consistent with circulating levels of hormones (Benton et al., 2008). Serotonin released from the DGNs could be distributed via the extensive blood capillary system in the AL that exits into the vascular sinus around the brain; serotonin circulating in the hemolymph could thereby gain access to the niche progenitor cells via the vascular cavity that connects with the blood system (Sullivan et al., 2007a, b).
The DGNs therefore have the potential to regulate neurogenesis by release of serotonin that could influence the niche progenitors as a hormone carried in the blood, or by local access to the precursors in the proliferation zones via fibers of the DGN that terminate in the proliferation zone (Beltz et al., 2001). Because the circumstantial evidence implicates the DGNs in the serotonergic regulation of neurogenesis, in the present study we tested directly whether electrical activation of the DGN alters neurogenesis in the adult crustacean brain.
Material and Methods
Animals
An Australian freshwater crayfish, C. destructor, was used for this study. The animals are reared commercially for food in ponds in New South Wales, Australia, and were shipped by air to Boston, MA. They were maintained in the animal facility at Wellesley College in circulating, filtered pond water at 20°C with a 12:12 light/dark cycle, and were fed twice each week with crayfish pellets. Experiments were restricted to sexually mature animals having a carapace length of 27mm to 29mm.
The perfused brain preparation
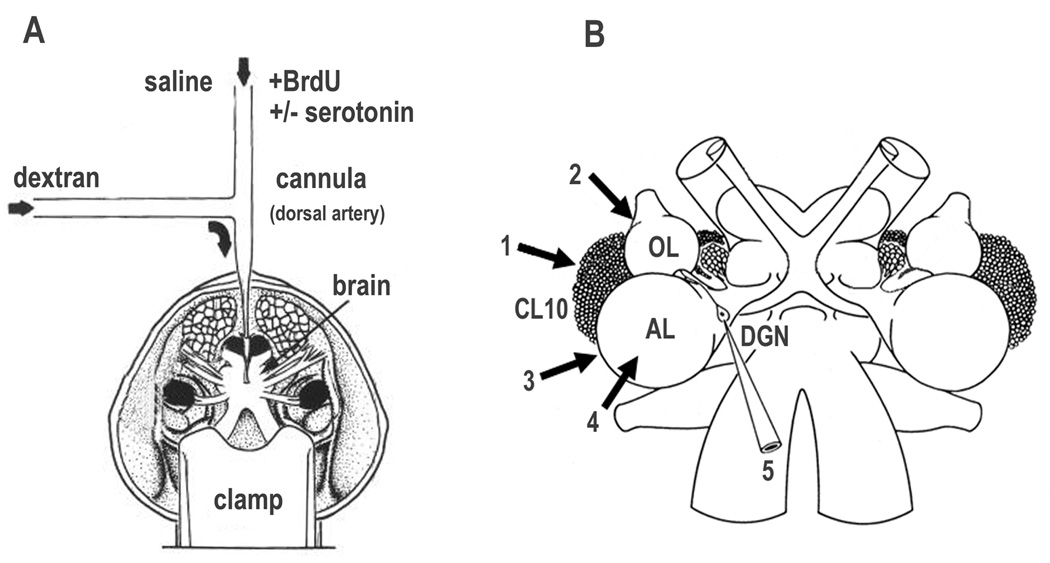
This preparation has been used in prior studies of the connectivity and physiology of the DGNs, and has been described in detail in previous publications (see Figure 1A and Sandeman and Sandeman, 1994). Briefly, the anterior part of the cephalothorax with the eyes, antennules, antennae and brain, is rapidly separated from the rest of the animal, mounted rostrum down in a small clamp and the main dorsal artery to the brain cannulated and immediately perfused with saline cooled to 18°C. Connective tissue is then removed, revealing the dorsal surface of the brain. In order to reach the AL, CL10 cell bodies, OL and DGN cell body for stimulation and recording, the sheath covering the brain is removed with small scissors and the underlying layer of glial cells washed away with a gentle stream of saline from a small-bore pipette. Such preparations, if the cannulation is prompt and perfusion is maintained, will remain physiologically stable and active for more than 24 hours.
Figure 1.
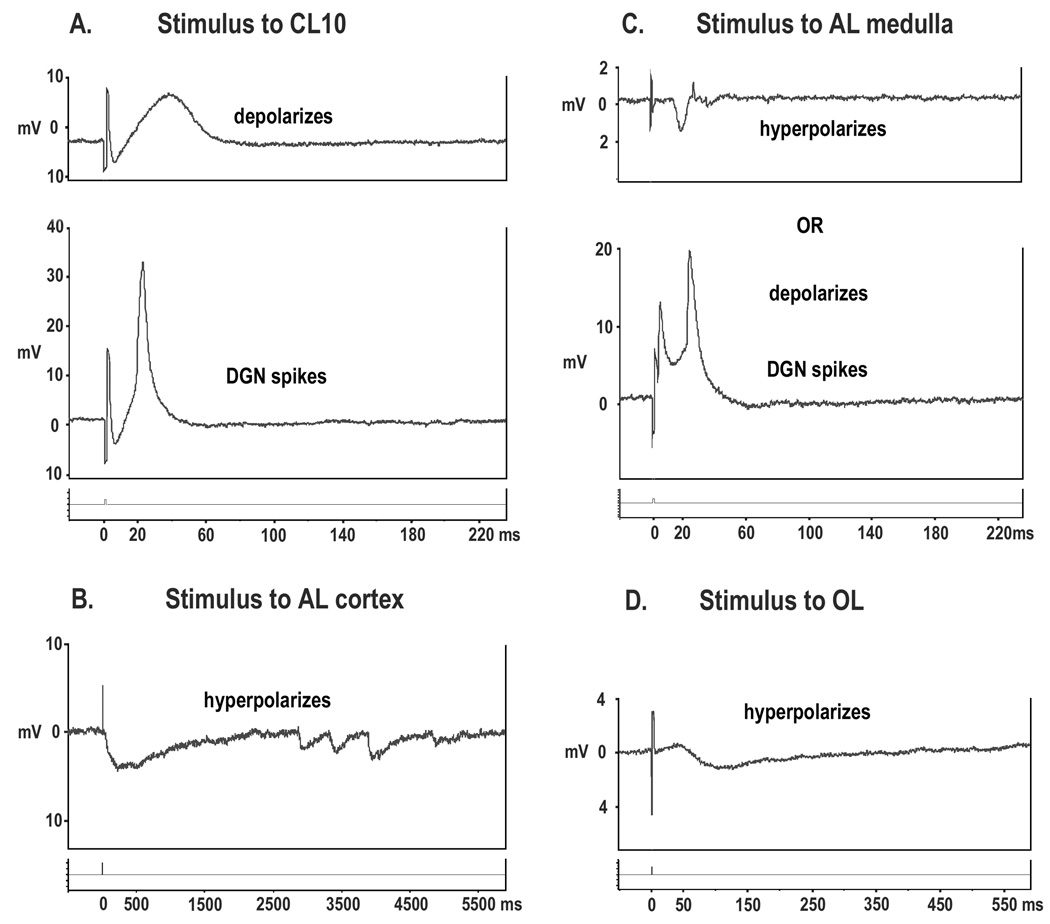
Figure 1A: The perfused brain preparation. The anterior end of the cephalothorax containing the brain is separated from the animal and mounted, rostrum down, in a clamp. The dorsal artery is cannulated and the brain perfused with cold saline. The brain is accessible from the dorsal side for intra- and extracellular recording and stimulating electrodes. Agents and dyes (e.g. dextran) can be introduced through a side arm connected to the perfusion cannula. 1B: Diagram of the brain of C. destructor to show sites of stimulation and recording. Extracellular stimuli (arrows) were delivered through a saline-filled glass electrode having an internal tip diameter of between 10 and 20µm. 1, CL10 cell somata; 2, OL; 3, AL cortex; 4, AL medulla. Intracellular recordings were made from the cell body of the DGN, 5.
Electrophysiological techniques
The electrical activity of the DGN was recorded with glass micropipettes filled with 1M KCl and having a resistance of between 30 and 50 megohms in saline. The cell body of the DGN on one side of the brain was visualized and impaled. Unstimulated DGNs were electrically silent but maintained robust resting potentials throughout the 6 hour stimulation and recording sessions, and resting potentials on removal of the electrode at the end of the experiment were between 70 and 80mV. Stimuli consisted of single, cathodal pulses of 1 msec duration every 30 seconds for the 6-hour duration of each experiment, delivered with a saline-filled suction electrode with an internal tip diameter of 10 to 20 microns. Pulses were applied via a stimulus isolation unit to the surface of the ipsilateral cell CL10, the surface of the OL or AL, or from deeper within the AL (Figure 1B).
Bromodeoxyuridine (BrdU) labeling
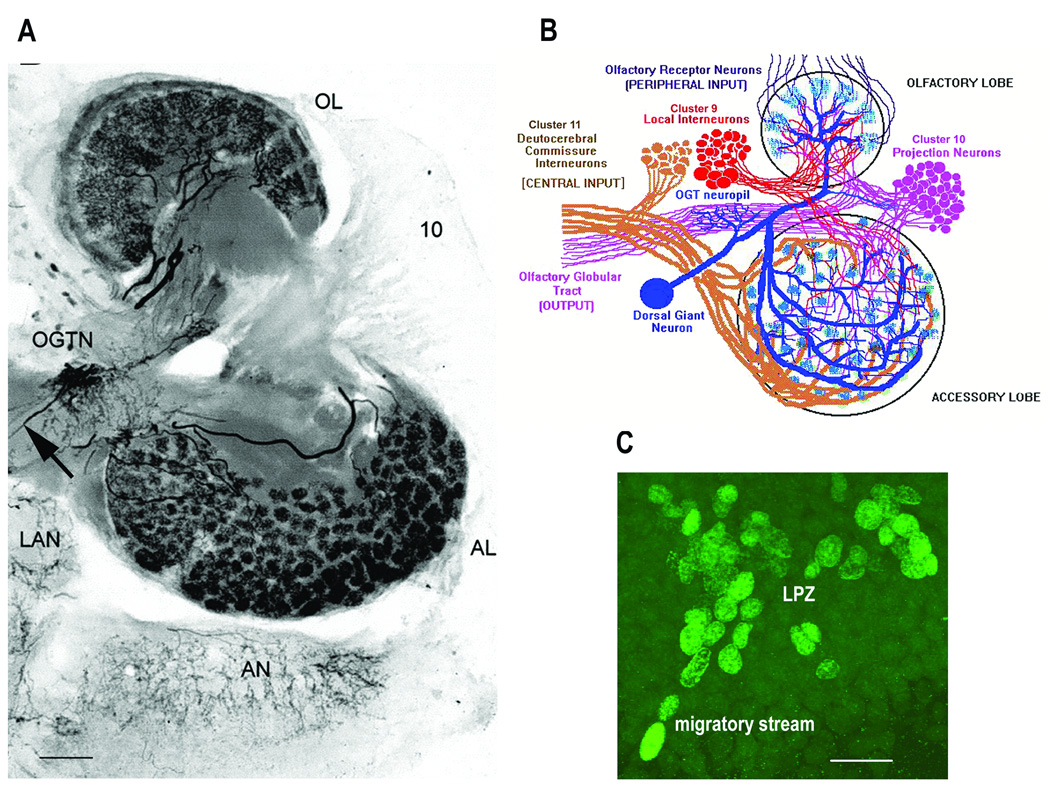
BrdU labeling of S-phase cells in the proliferation zone of CL10 was used as a quantitative assay for the influence of DGN activity on cell proliferation. To accomplish this, 0.5 mg/ml BrdU (Sigma) in crayfish saline was perfused through the isolated brains during the physiological manipulations. The experiments were run during the afternoon and early evening (12:00 to 18:00), in order to eliminate variables associated with time of day (Goergen et al., 2002). Cells in the migratory streams and in the proliferation zones were intensely labeled following this exposure (Figure 2C). At the end of the 6-hour experiment, the perfusion was stopped and the brains were immediately dissected out of the head capsule, desheathed on the ventral side, and pinned out in cold 4% paraformaldehyde. After 10 minutes the brains were transferred to vials containing a large volume of the fixative, and kept at 4°C overnight. The brains were then washed for 1–2 hours in 0.1M phosphate buffer with 0.3% Triton X-100 (PBTx), treated with 2N HCl for 45 minutes, washed in PBTx for the remainder of the day and placed in mouse anti-BrdU (Invitrogen USA) overnight at 4°C. The next day, the brains were again washed in PBTx before being placed in goat anti-mouse IgG conjugated to Alexa 488 (Invitrogen USA) overnight at 4°C. After washing in phosphate buffer, the brains were mounted ventral side up in Gelmount, coverslips were applied, and the brains gently compressed by stacking pennies on top of the coverslip. The finished preparations were kept at 4°C for two to three days and then imaged.
Figure 2.
Figure 2A: Photomicrograph of a thick section (100µm) of the right side of the brain of C. destructor. Immunolabeled for serotonin the projections of the DGN into the AL and OL were revealed. The DGN is the only serotonergic neuron to project into the AL, but shares its projections into the OL with at least two other serotonergic deutocerebral neurons. The large cell body of the DGN is dorsal and out of the section plane, but the thin primary neurite that connects it with the olfactory globular tract neuropil (OGTN) before it projects into the OL and AL, can be seen (arrow). 10 – CL10 cell bodies; LAN – lateral antennular neuropil; AN – antenna two neuropil. 2B: A diagram that summarizes the circuitry of the neural elements in the OL and AL. 2C: BrdU-labeled cells at the distal end of the migratory stream and in the lateral proliferation zone (LPZ) in CL10 in a brain of C. destructor after 6 hours of perfusion with saline containing 0.5mg/ml of BrdU. Scale bars: 2A - 100µm; 2C - 20µm. (2A from Sandeman and Sandeman, 2003; 2B from Beltz and Sandeman, 2003).
Confocal microscopy and cell counts
Labeled cells in CL10 were used as our quantitative assay, as BrdU labeling in this area is robust and reliable. To determine the number of cells that were labeled on each side of the brain in each individual animal, serial optical sections were taken through the proliferation zone of CL10 at 1µm intervals and saved as three-dimensional stacks using a Leica TCS SP laser-scanning confocal microscope equipped with argon, krypton and helium neon lasers. With a single confocal image projected on the monitor, BrdU-labeled cell profiles in CL10 were traced onto a transparent sheet, and then counted blindly. In cases where cells overlapped, several tracings were taken and then overlaid and compared to avoid counting the same profiles twice. Final counts are presented as numbers of labeled cell profiles per hemi-brain in each individual. Brains in which labeling was only present on one side or was indistinct, were rejected.
Statistical analysis
SPSS software was used for all statistical analyses. In this study, the right side of each brain served as the control for the left side. The numbers of BrdU-labeled cells in each cluster were then compared with one another using a Paired Samples T test. In cases where several groups of animals were compared, the individual scores were subjected to a one-way ANOVA. Differences between individual groups were estimated with a post hoc Tukey’s. The results of the comparisons between the two sides of the brain are represented graphically as boxplots in which the distribution of the data is shown as a shaded box with protruding lines (whiskers). The box contains half of the data (the interquartile range) and the whiskers show the smallest and largest values (the total range). The line across the box indicates the median value and provides an indication of the normality of the distribution. Outliers (i.e. scores that are more than 1.5 box lengths from the edge of the box), are shown as a small circle.
The blood vascular system
To reveal the relationship of the vascular system to the proliferating neurons, a small volume of 2mM dextran fluorescent dye, (D-3306, anionic, lysine fixable MW 3000; Invitrogen, USA), was introduced with the perfusate into brains that had been perfused for 6 hours with saline and BrdU. The brains were then immediately removed from the perfusion system, fixed and treated as described above. Analysis of the preparations was carried out with the Leica confocal microscope.
High performance liquid chromatography (HPLC)
HPLC was used for the determination of serotonin levels in (1) the homogenized tissue of brains that had been perfused, and (2) from the perfusate collected directly above the surface of the AL of perfused brains during periods of stimulation, or no stimulation, of the CL10 somata. (1): Whole brains or selected regions were dissected in cold saline and homogenized in 50µl of 0.1N perchloric acid. Homogenates were diluted in 100µl mobile phase (ESA Inc, MA, USA) and centrifuged at 14,000g and 18°C for 15min. The clear supernatant was decanted into Spin-X centrifuge tube filters (0.45µm Nylon, Corning, NY, USA) and centrifuged at 14,000g and 4°C for 15min. At least 60µl of the supernatant were transferred into the auto-sampler micro-vials for analysis. (2): Collected perfusate was added to mobile phase (2:1), centrifuged and filtered as for the brain tissue supernatant and transferred into auto-sampler micro-vials for analysis. Analysis in both cases was done with an HPLC (ESA Inc, MA, USA) fitted with an amperometric detector and a reverse phase C18 column (ESA Inc, 3µm, MD – 150x3.2).
Results
THE PERFUSED BRAIN PREPARATION
All of the electrophysiological experiments utilized the perfused brain preparation (see Methods and Figure 1A). In these studies, one side of the brain served as a control for the other, "experimental" side. It is therefore important to understand the neural and vascular connections between the two sides of the brain.
The vasculature
The dorsal artery enters the brain medially and passes through the brain to the ventral side before branching into left and right trunks that supply the neuropils and clusters of cell somata on the left and right sides of the brain, respectively. After passing through the brain the blood exits through the brain sheath into the surrounding cavity (Sandeman, 1967). Hence saline introduced into the brain via the dorsal artery passes separately through the left and right ALs and, once outside the brain is immediately drained away. A separate vascular system supplies the lateral protocerebra (eyestalks), and so saline passing into the brain from the dorsal artery will not reach the optic neuropils. Perhaps more importantly, no saline that has passed through the eyestalk and which may carry products from the sinus gland will enter the brain or come in contact with the proliferating neurons. The brain vasculature is therefore partitioned into left and right sides and only the deutocerebrum is accessed by our perfusion of the dorsal artery. These features are critical to the success of our experiments where one side of each individual brain serves as the control for the other experimental side.
Neural pathways
The normal connections between the brain and the rest of the nervous system are significantly attenuated in the perfused preparation. The esophageal connectives that normally connect the brain with the ventral nerve cord are severed, as are the protocerebral tracts which link the brain to the optic neuropils. Afferent input is therefore limited to the antennules and the antennae, which remain intact. The olfactory receptors on the antennules are known to become insensitive unless they are perfused by cannulating the lateral blood vessels from the heart (Ache and Sandeman, 1980); this was not done in the present study, and so with the exception of mechanoreceptors on the antennules and the antennae, the brain is virtually deafferented and there is a complete absence of neural signals between the brain and the ventral cord, optic ganglia or eyes.
NEUROGENESIS IN THE PERFUSED BRAIN PREPARATION
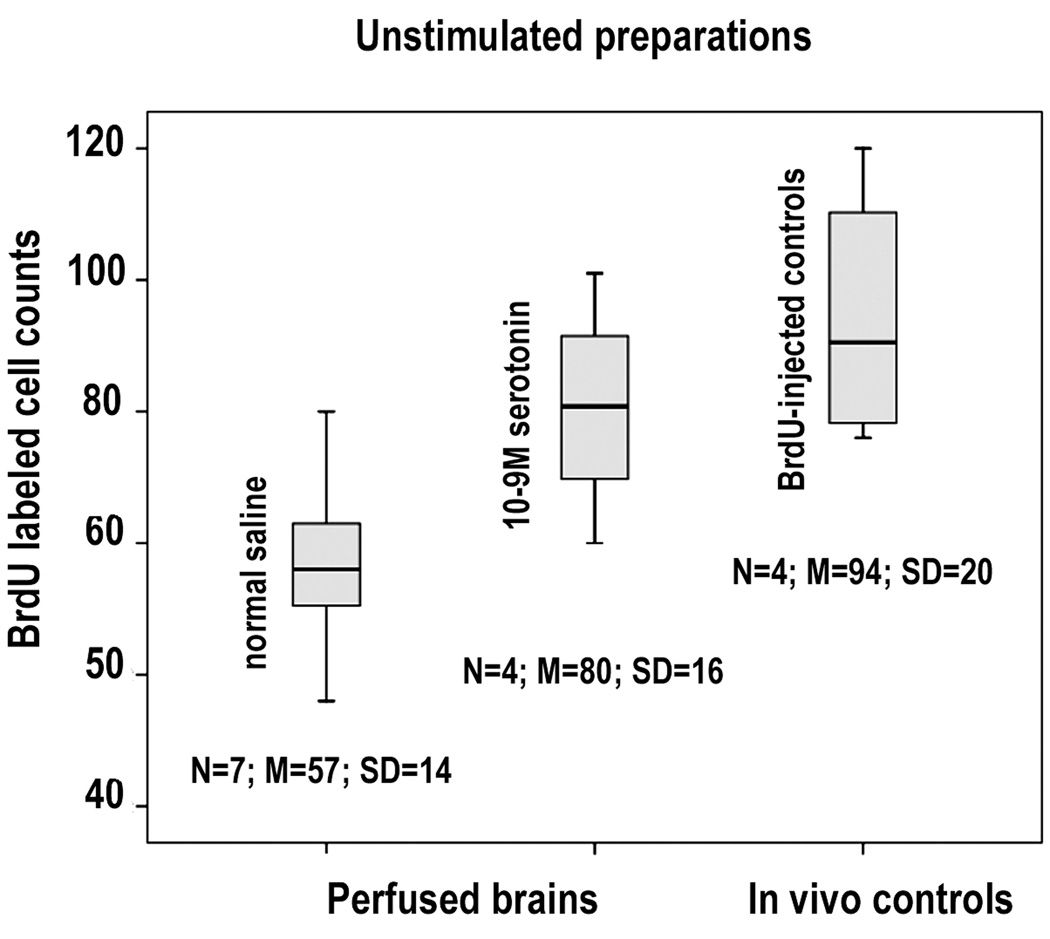
Neurogenesis continues in the crustacean brain even when the brain is isolated and maintained in short-term organ culture, although cells are generated at a lower rate (Benton et al., 2008). In order to use the perfused brain as a model, it was necessary to compare the number of cells that label over a period of perfusion with intact control animals exposed to BrdU over the same period. Brains were therefore perfused for 6 hours with saline containing BrdU (0.5mg/ml), fixed, processed, and the numbers of labeled cells in CL10 counted. This was compared with CL10 counts from in vivo controls that were injected with 0.1ml of 2mg/ml BrdU in saline (which, after dilution in the blood approximates the concentration of the brain perfusate), and then killed after 6 hours and processed. These experiments show that neurogenesis continues in the perfused brain preparation, but that counts of BrdU-labeled cells in the perfused preparations were generally lower than those of the injected controls (Figure 3), consistent with our findings in isolated organ-cultured brains. Of particular interest was the finding that serotonin added to the perfusate at a final concentration of 10−9M, raised the cell counts to the in vivo control levels. Serotonin is known to increase the rate of neurogenesis in the crustacean brain in both intact animals and in vitro (Benton et al., 2008).
Figure 3.
Comparison of BrdU-labeled cell counts in CL10 from unstimulated brains perfused with normal saline and saline containing 10−9M serotonin (first two columns), with in vivo controls (third column). Cell counts taken from in vivo controls contained significantly more labeled cells than those in the perfused brains, although no significant differences were found between the controls and the brains enriched with 5HT. (ANOVA, p = 0.01; Tukey, normal saline - serotonin, p = 0.097; normal saline - control, p = 0.009; serotonin - control, p = 0.494)
These results raised the possibility that serotonin was being lost from the preparation during the 6 hour perfusion, a situation that could undermine the goal of our experiments testing the influence of activation of the serotonergic DGNs on neurogenesis. To determine if this was the case, the serotonin content of brains from intact "control" animals and those that had been perfused over 6 hours (the "experimental" animals) were compared using HPLC. The result showed no significant differences between these brains, provided that the control and perfused brains were processed for analysis at the same time of the day. Control brains dissected at 09:00 and prepared for HPLC contained consistently less serotonin than perfused brains that were prepared for analysis at 15:00 following 6 hours of perfusion. Previous studies in lobsters demonstrated that the serotonin content in the brains of lobsters varies over the time of day in a circadian fashion (Wildt et al., 2004). We found that serotonin content in perfused C. destructor brains that were perfused over different time windows during a 24-hour period also varies with time of day with the highest values towards dusk (data not shown) indicating that circadian changes in serotonin content are maintained in the perfused preparation. To control for this variable, we carried out all of the experiments in the present study over the same 6-hour time period each day, i.e. from 12:00 to 18:00.
VARIABILITY OF CELL PROLIFERATION IN THE PERFUSED BRAIN
A characteristic of cell proliferation in the crustacean brain is the wide variation in the rate of neurogenesis even among siblings maintained in the same conditions. Growth in crustaceans also varies considerably among individuals, a feature well known to crayfish aquaculturists who select for “fast growers” in any batch of hatched individuals. This inherent variability presents a challenge when assessing the influence of endogenous (e.g., serotonin, molting hormones) or environmental (e.g., time of day, temperature) factors on neurogenesis.
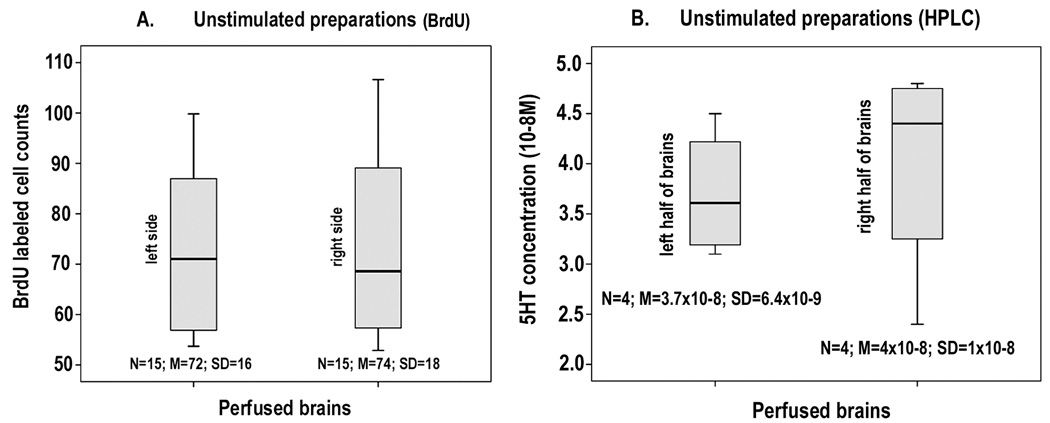
In these experiments the serotonergic DGN, which projects only ipsilaterally, was stimulated on one side of the brain, while the contralateral (unstimulated) side served as a control. This approach circumvented the issue of inter-animal variability, but raised another question concerning the variability in rates of neurogenesis between the two sides of the brain, which has not been systematically examined in crayfish. Neurogenesis in CL10 on the left and right sides of the brain was therefore compared in brains perfused for 6 hours with saline at 21°C. The results show that there is no statistical difference between the two sides of the brains of individual animals (Figure 4A). We also compared the levels of serotonin in the left and right halves of individual brains using HPLC analyses. After perfusing the brains for 6 hours with saline, they were removed from the head capsule, cut in half along the anterior/posterior midline and the left and right sides analyzed for serotonin content. We found no significant difference between the two sides of the brains (Figure 4B). Similar HPLC measurements on the crayfish P. clarkii confirmed both the level of serotonin and the left/right symmetry seen in C. destructor (Sandeman, Benton and Rose, unpublished data). Differences between the two sides of the brain following DGN stimulation, either in the rate of neurogenesis or in serotonin levels, should therefore be readily detected.
Figure 4.
Figure 4A: Comparison of the BrdU-labeled cell counts from the left (first column) and right (second column) CL10 of unstimulated, perfused brains. No significant difference was found between the numbers of BrdU-labeled cells on either side of the brains. (Paired samples T test: p = 0.685). 4B: Levels of serotonin contained in the left (first column) and right (second column) halves of unstimulated, C. destructor brains following 6 hours of perfusion. HPLC analysis revealed no significant difference between the two sides (Paired samples T test: p = 0.463)
RESPONSES OF THE DGN TO ELECTRICAL STIMULATION
The cell body of the DGN is large in diameter (80 to 100µm) and has a long thin primary neurite that extends ventrally towards the center of the AL and OL. Before branching into the OL and AL, it arborizes amongst the axons of a large tract of projection neuron axons (olfactory globular tract, OGT) that leave the OL/AL complex and target lateral protocerebral neuropils in the eyestalks (Figure 2A,B).
Electrically stimulating the projection neurons, either antidromically or through their cell bodies in CL10, produces an excitatory potential in the DGN cell body that increases gradually with increased stimulus intensity and which erupts into an action potential above a certain threshold (Figure 5A) (Sandeman and Sandeman, 1994). The amplitudes of the summed excitatory potential and action potentials recorded in the cell body are almost the same size, indicating that the synaptic input is closer to the cell body than the spike initiating zone. The absence of unitary epsps and the graded nature of the excitatory potential suggest that both are relatively remote from the cell body. This architecture would also explain why injecting current directly into the cell body of the DGN will occasionally, but not reliably, generate action potentials, even when these currents are very large. We chose, therefore, to activate the DGN via its synaptic inputs. This not only allowed us to achieve the primary goal of either depolarizing or hyperpolarizing the cell but also to more closely approximate its natural excitatory and inhibitory pathways. In the many hundreds of penetrations of this neuron in this and in previous studies, spike activity in the cell body of the DGN was never observed unless a stimulus was applied. Hence the electrical activity of the DGN, at least recorded at the cell body in the perfused brain preparation, is normally silent and limited to depolarizations (and action potentials) or hyperpolarizations, driven by electrical stimulation. We do not know if this is the case in an intact animal where the brain, and perhaps also the DGN, receive continuous inputs from the eyes and the rest of the body, all of which are absent in the isolated brain preparation.
Figure 5.
The electrical responses in the cell body of the DGN to stimulation of the surface of CL10 cell somata (5A), AL cortex (5B), the deeper medullary layers of the AL (5C), and the OL (5D). Stimulation of the CL10 cell bodies results in a depolarization (top trace, A) and, with increased stimulus intensity, a single action potential (bottom trace, A). The disproportionately small amplitude of the action potential in relation to the excitatory depolarization suggests that the spike initiating zone lies further from the cell body than the synaptic input to the cell. AL cortex stimulation (B) produces abrupt hyperpolarizations after a latency of about 25ms which were sometimes followed by a series of smaller hyperpolarizations. Stimulation of the deeper layers of the AL (C), produced either hyperpolarizations (top trace, C), or short latency action potentials, or depolarizations and an accompanying action potential (bottom trace, C). OL stimulation consistently produces a small hyperpolarization after a latency of about 50ms (D). Recordings of the entire stimulation period were stored on a digital device (AD Instruments, Powerlab/4SP). Vertical scales on the recordings depict the amplitude of the positive or negative changes of the cell’s membrane potential.
A second excitatory connection with the DGN is through axons in the deutocerebral commissure which extend over the surface of the AL before turning at right angles and projecting down into the lobe to end in glomeruli distributed throughout the AL; the DGN also sends branches to all AL glomeruli. The synaptology of the glomeruli is complex and electron micrographs of these show that the DGN receives inputs in the glomeruli but is also presynaptic to some elements (Sandeman RE et al., 1995).
There is also a functional segregation of areas within the AL (Sandeman DC et al., 1995; Sullivan and Beltz, 2005b). DiI tracing of OL projections showed that interneurons in the OL with their cell somata in CL9 arborize predominantly in the outer “cortical” glomeruli of the AL (Sullivan and Beltz, 2005b). Electrical stimulation of the surface of the OL consistently results in a small hyperpolarization of the DGN after a latency of about 50ms (Figure 5D). Electrical stimulation of the outer cortical layer of the AL also produces a hyperpolarization of the DGN (Figure 5B), albeit with a shorter latency than that achieved through OL stimulation, implying that the same inhibitory pathways are involved.
Tactile and visual information is processed in deeper, medullary AL glomeruli (Sandeman DC et al., 2005). Electrical stimulation of the deeper AL layers can lead to both depolarization and hyperpolarization of the DGN. In some cases, a very short latency action potential followed by a depolarization and a second action potential, suggest that the DGN was being activated directly and also indirectly via a synaptic pathway (Figure 5C).
The knowledge of synaptic relationships between the DGN and other areas allowed us to use these connections to depolarize or hyperpolarize the DGN. In order to be useful in the context of the present study, where effects of electrical stimulation must be restricted to one side of the brain, the pathways that are activated must be unilateral. The projection neurons from the OLs and ALs of each side ascend to lateral protocerebral neuropils in the eyestalks on both sides of the brain (Sullivan and Beltz 2005b). Bilateral, descending, inputs to the ALs from the eyestalks have not been demonstrated but cannot be ruled out, and bilaterally projecting deutocerebral commissure neurons carry visual information from the optic ganglia (Sandeman DC et al., 1995). The protocerebral tracts were therefore severed in all preparations to exclude the possibility of bilateral connections between the two sides of the brain via the optic ganglia.
A direct connection between the OLs has not been identified in any decapod crustacean, however in experiments in which we applied stimuli to the AL cortex, this could have resulted in antidromic action potentials in deutocerebral commissure neurons crossing and reaching the contralateral DGN (see Discussion and Sandeman DC et al., 1995).
CELL PROLIFERATION FOLLOWING DGN EXCITATION
i) Stimulation of projection neurons in CL10
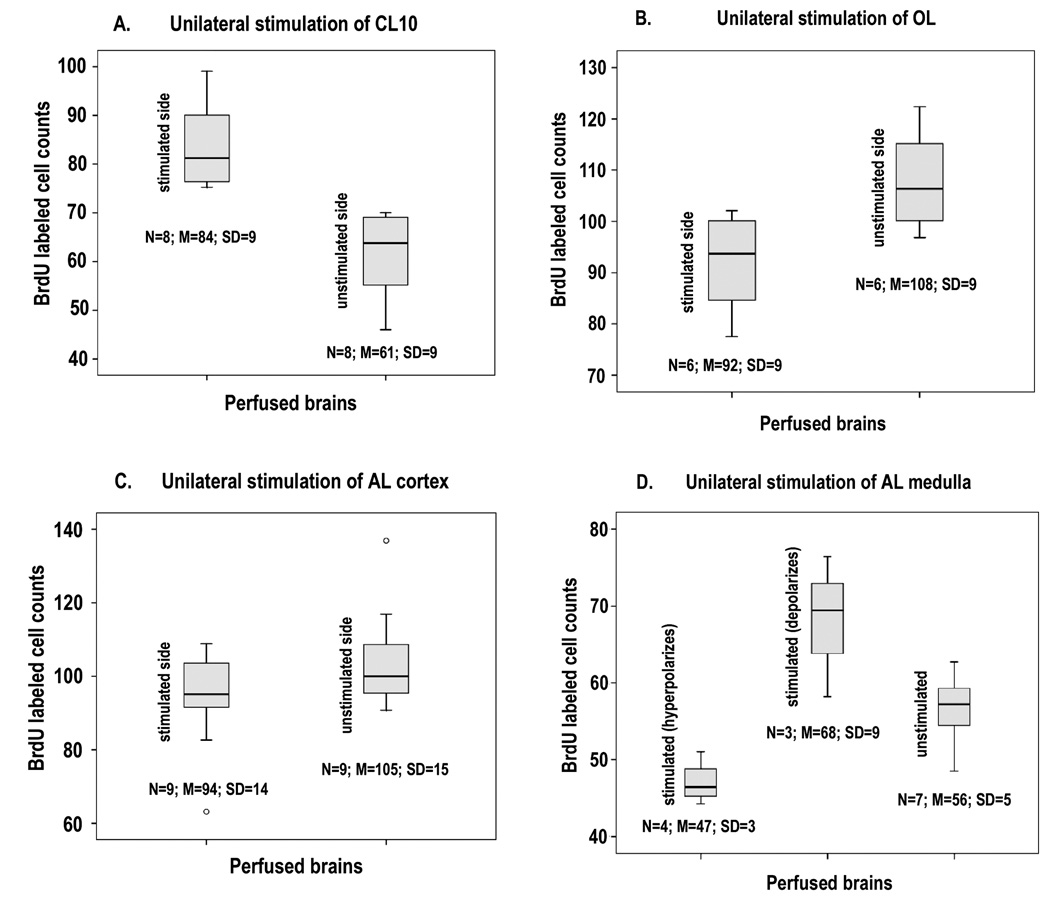
CL10 neurons were stimulated at an intensity sufficient to produce consistent action potentials in the DGN every 30 seconds for a period of 6 hours (Figure 5A). Responses to stimulation were recorded in the DGN throughout this period. Cell counts show that BrdU incorporation on the stimulated side is significantly greater than on the unstimulated side (Figure 6A). BrdU-labeled cell counts were also larger for the stimulated relative to the unstimulated side even in those occasional preparations where DGN action potentials were no longer present during the last one to two hours of the perfusion period and these data are included in Figure 6A. (see Discussion).
Figure 6.
Comparison of the counts of BrdU labeled cells in CL10 on the stimulated and unstimulated sides of brains in which stimulation was applied unilaterally to different areas of the brain. 6A: CL10. More cells were labeled on the stimulated side. (Paired samples T test: p = 0.008). 6B: OL. Fewer labeled cells were found on the stimulated side, but this difference was not statistically significant (Paired samples T test: p = 0.093). 6C: AL (cortex). No significant difference was observed between labeled cell counts on the two sides of the brain. (Paired samples T test; p = 0.271). 6D: AL (medulla). The results from the stimulated side are separated into preparations in which the DGN was hyperpolarized (first column) those in which it was depolarized (second column), and the unstimulated side (third column). Significant differences exist between the groups (ANOVA: p = 0.001) and within the groups (Tukey: hyperpolarized - depolarized, p = 0.001; hyperpolarized – unstimulated, p = 0.043; depolarized - unstimulated, p = 0.027)
ii) OL stimulation
Stimulation of the OL reliably produces a small hyperpolarization of the DGN (Figure 5D) and a decrease in cell proliferation on the stimulated side in comparison with the control side of the brain (Figure 6B). In spite of being clear to see in some animals, the decrease in the number of labeled cells on the stimulated side did not prove to be a consistent and statistically significant difference over the group of 6 animals that was tested (p = 0.093). This result may imply that a slow and ongoing release of serotonin occurs without stimulation and that this is reduced when the DGN is continuously inhibited. Alternatively, OL stimulation may cause the release of a factor that suppresses neurogenesis. For instance, nitric oxide is known to decrease the rate of neurogenesis in the lobster brain, and that under certain conditions the DGN contains nitric oxide synthase in addition to serotonin (Beltz and Sandeman, 2003; Benton et al., 2007).
iii) AL cortical stimulation
Stimulation of the surface of the AL, although producing a greater hyperpolarization of the DGN than that following OL stimulation, and sometimes accompanied by large unitary ipsps (Figure 5B), did not result in a significant decrease in BrdU-labeled cells in CL10 on the stimulated in comparison with the control side (Figure 6C) although counts on both sides tended to be lower than those in which no stimulation was applied (Figure 4A). An explanation for this may lie in activity in the deutocerebral commissure axons that lie at the surface of the AL, spreading to the contralateral DGN (see Discussion).
iv) AL medulla stimulation
Stimulation of the AL medulla produced a variety of responses in the DGN. These ranged from a hyperpolarization to those that resembled CL10 stimulation in which a large excitatory depolarization led to a small superimposed action potential, or a very short latency action potential not superimposed on a depolarization but followed by a longer latency depolarization crowned with an action potential (Figure 5C). We conclude that the current from medulla stimulation must spread to processes of the DGN itself, and also to the excitatory and inhibitory synaptic inputs on them. In assessing the cell proliferation from such experiments we separated the results according to the hyperpolarizing or depolarizing responses of the DGN, and compared the experimental and control sides of the brain in each. We find that hyperpolarization of the DGN was correlated with a decrease, and depolarization with an increase in cell proliferation, in comparison with the controls (Figure 6D).
v) Subthreshold stimulation of the DGN
In some preparations, stimulation of CL10 resulted in no response in the DGN or at most a small depolarization that did not generate an action potential. There are various possible reasons why some of the preparations failed, such as insufficient or slow perfusion, damage to the DGN during desheathing and exposing it for recording, or imprecise placement of the stimulating electrode, to name several. These preparations were nevertheless allowed to run the entire 6 hours as “default” controls. The brains were fixed, processed and the labeled cells on each side of the brain counted and compared. No significant difference was found between the numbers of labeled cells on the two sides of these brains (data not shown) just as in those preparations in which no stimulus had been applied (Figure 4A).
THE RELEASE OF SEROTONIN DURING ELECTRICAL STIMULATION
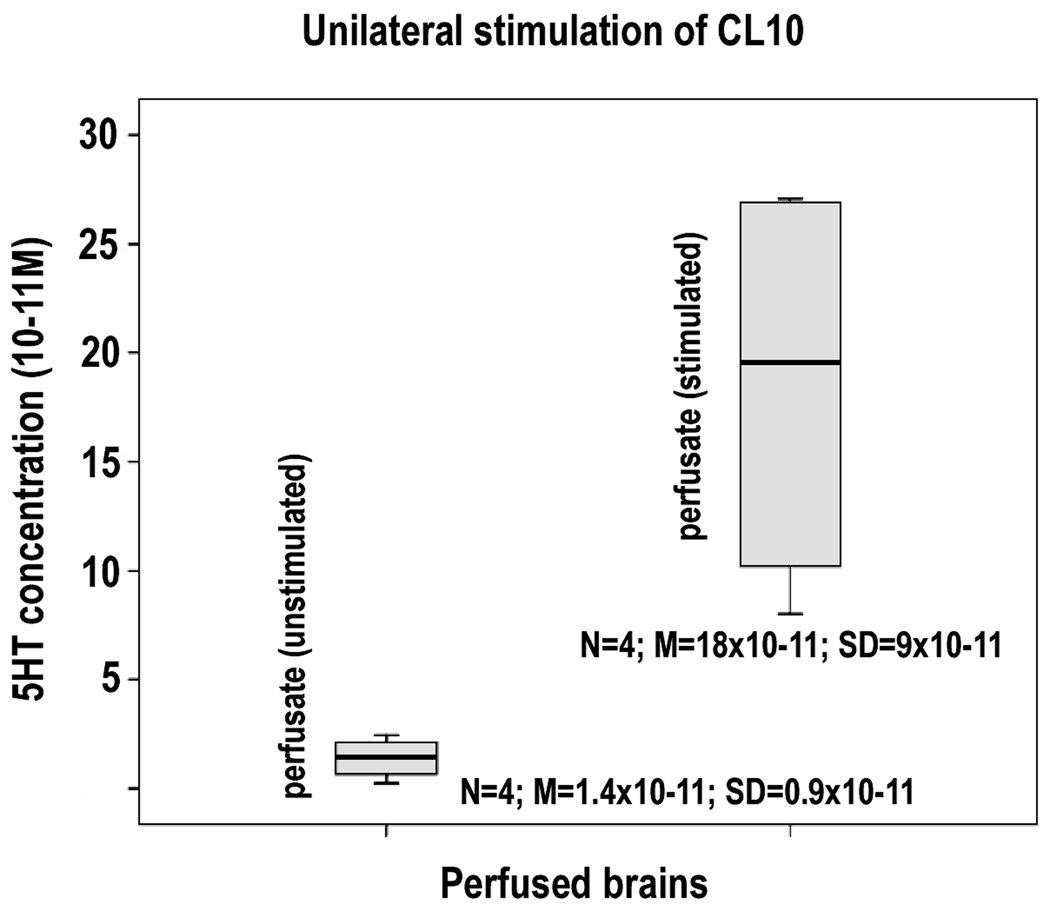
The fact that activation of the DGN reliably results in increases in the numbers of S-phase (BrdU-labeled) cells on the stimulated side suggests that the stimulated DGN releases serotonin. This serotonin then either directly or through some intermediate pathways, influences the number of proliferating cells in the CL10 proliferation zone. If this is the case then collecting the perfusate from the surface of the AL during electrical stimulation of the CL10 neurons might show the presence of serotonin in the perfusate. To examine this possibility we perfused crayfish brains and stimulated CL10 neurons at intensities known to be supra-maximal for the activation of the DGN, and with paired 1ms pulses, 30ms apart, which was found in earlier studies of the DGN (Sandeman and Sandeman 1994) to be a particularly effective stimulus. We simultaneously aspirated the perfusate from directly above the AL through a small bore suction cannula. Samples were collected for 10 minutes at the start of the experiment, prior to stimulation, and then during 10 minutes of stimulation. A 30-minute rest period followed each stimulation period in order to clear the perfusate contained in the preparation and the lines of the aspiration system. The stimulation and collection protocol was then repeated. The entire series including the 30 minute rest periods was run four times in each of 3 animals. The results from one animal are shown in Figure 7. Samples of the perfusate collected during the periods of stimulation were compared with those collected during periods of rest. We found that samples collected during stimulation contained levels of serotonin that were 8 to 10 times higher than those collected during the rest periods (Figure 7). Trace amounts of serotonin were found in some preparations even in the absence of stimulation, suggesting that serotonin may be released continuously at a very low level, a result that may explain why in some cases hyperpolarization of the DGN is associated with a decrease in cell proliferation (see Discussion). The level of 5HT in the samples collected over the 3 hour experiment did not vary in a consistent manner that would suggest depletion of the 5HT source or deterioration of the preparation during this period.
Figure 7.
Comparison between the levels of 5HT collected in the perfusate over 4 cycles from the crayfish brain during periods of no stimulation (first column), and stimulation (second column), of the CL10 cell bodies, measured by HPLC. 5HT levels in samples taken over 10-minute periods preceding the stimulation of CL10 are about ten times less than levels in samples taken over 10 minute periods during stimulation. (Independent samples T test: p < 0.013).
RELATIONSHIP OF THE BLOOD VASCULATURE AND THE LATERAL PROLIFERATION ZONE
Introducing dextran into the blood system of a perfused preparation (see Material and Methods) confirmed the presence of a complex network of blood vessels and fine capillaries that flow through the neuropil of the ALs (Sullivan et al., 2007a). In order to explore the association between the vasculature and the proliferating cells in CL10, we perfused the brains for 6 hours with saline and BrdU. At the end of this period, 3mls of dextran (2mM in saline) were injected into the perfusion system. The dextran was observed to immediately and cleanly enter the dorsal cerebral artery, travel deep into the brain and then emerge at its surface. At this point, only seconds after the introduction of the dye, perfusion was halted and the brains fixed immediately. Normal processing for the BrdU label followed.
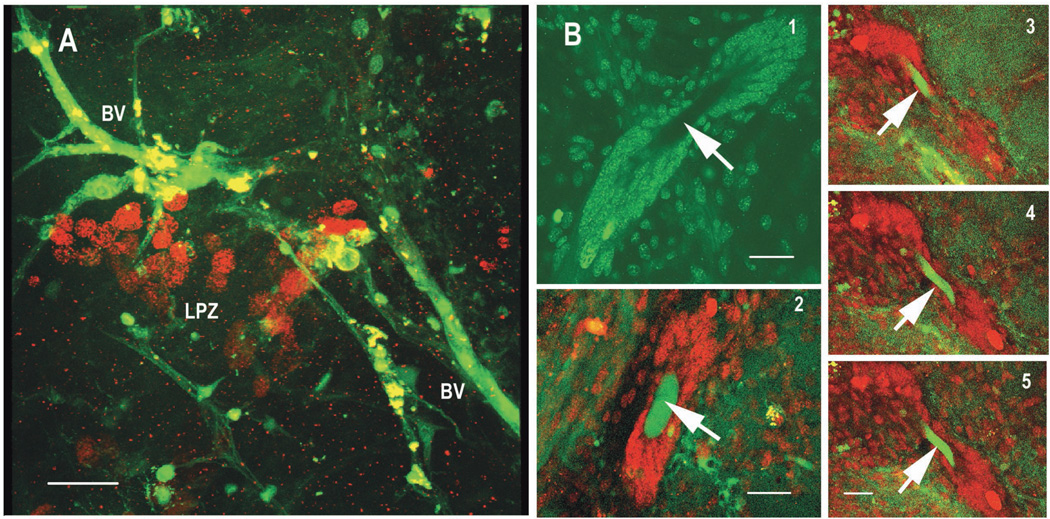
Dextran-filled vessels were found throughout the entire area of the AL neuropil and cell bodies in CL10 (Figure 8A). BrdU-labeled cells in the proliferation zone of CL10 lie close to, and in some cases even directly adjacent to dextran-labeled vessels. In addition, we found dextran label in the very center of the cluster of cells that form the neurogenic niche, a structure previously described in P.clarkii (Figure 8B). In C.destructor, as in P. clarkii (Sullivan et al., 2007a) this niche lies on the ventral surface of the AL. Given the rapidity with which dextran reaches this area of the niche in C. destructor (i.e. seconds), we have to assume direct access of the blood to this region of the niche in C. destructor as shown previously in P. clarkii (Sullivan et al., 2007a).
Figure 8.
Brain preparations perfused for 6 hours with saline containing BrdU and then injected with dextran dye immediately before fixation. The preparations were exposed to antibodies against BrdU, mounted and then imaged. 8A: Blood vessels (BV) filled with dextran (green) in the area around BrdU-labeled (red) cell nuclei in the proliferation zone (LPZ) of the CL10 cell bodies. The dextran was injected into the main cerebral (dorsal) artery as it entered the brain and would have passed through the capillary beds of the AL to reach these vessels in CL10. 8B: 1, the vascular cavity (arrow) in the centre of the neurogenic niche of C. destructor, surrounded by precursor cells. 2, seconds after dextran (green) is introduced into the main cerebral artery, it can be found in what we conclude is a blood vessel passing through the vascular cavity (arrow). Here the cells of the niche are red. 3,4,5, a series of optical sections taken through a niche showing a green dextran-filled blood vessel looping up through the vascular cavity (arrows). All the images in B have been brightened so that the non-specific background-labeled cells of the neurogenic niche can be seen. The niche cells in C. destructor surround the blood-filled cavity and are connected to the proliferation zones in Clusters 9 and 10 by long fibers as they are in P. clarkii and H. americanus (Sullivan et al., 2007b). Scale bars: 20µm.
Discussion
The system responsible for adult neurogenesis in the olfactory pathway of crayfish and clawed lobsters includes a neurogenic niche with clusters of precursor cells with glial properties associated with the blood vascular system (Sullivan et al., 2007a; Zhang et al., 2009). Daughters of these precursor cells migrate along glial strands to two proliferation zones, where they divide at least once more to produce cells that will differentiate into neurons (Sullivan and Beltz 2005a). The proliferation zones are located within clusters of cells (Clusters 9 and 10) that contain local olfactory interneurons and olfactory projection neurons that carry the output from the OL and the AL, respectively. A niche, its migratory streams and the associated proliferation zones constitute a production line generating new neurons in the adult crayfish brain. These systems are bilateral, and are located on the ventral surface of the brain. First described by Bazin (1970) in 10 different species of decapods, these structures were termed “deutocerebral organs”. Bazin noted the glial nature of cells that were associated with the organ and its strands, but was unable to determine their function. The neurogenic nature of this organ in crayfish has now been established (Sullivan et al., 2005a; Song et al., 2009; Zhang et al., 2009).
Serotonin exerts a powerful influence over the rate of adult neurogenesis in the crustacean brain (Benton and Beltz, 2001; Beltz et al., 2001; Benton et al., 2008). Even short-term exposure of the brain to hormonal levels (10−10M) of this monoamine results in significant increases in the numbers of BrdU-labeled cells (Benton et al., 2008). Our current experiments build on this knowledge by testing whether the DGN, the most prominent serotonergic neuron in the crustacean brain, is part of the endogenous pathway underlying serotonergic regulation of adult neurogenesis. This possibility has previously been proposed (Benton and Beltz, 2001) based on the widely distributed projections of this neuron throughout the OLs and ALs (Sandeman and Sandeman, 1994), and the demonstration in the lobster brain that the DGN sends a fiber directly to the proliferation zone in the projection neuron cell CL10 (Beltz et al., 2001). Our current work provides a direct test of the hypothesis that the serotonergic DGN is capable of regulating adult neurogenesis.
While it is possible that the DGN has an influence on the entire cell proliferation system, including the precursors in the niche, this study concentrated on the cells in the lateral proliferation zone contained within CL10; the final precursor divisions prior to differentiation into projection neurons occur in this proliferation zone. When the DGN is stimulated unilaterally, we find that BrdU incorporation into cells in this cluster is altered in comparison to CL10 on the contralateral side of the same brain. Depolarization and the presence of an action potential in the DGN are correlated with an increase in BrdU-labeled cells on the stimulated side relative to the unstimulated side. Hyperpolarization of the DGN on the other hand, is accompanied by a small decrease in the number of BrdU-labeled cells on the stimulated side in comparison to the control side.
An interpretation of these results is that suprathreshold excitation of the DGN results in the non-synaptic release of serotonin into the vascular system, which distributes the amine to the proliferation zone. Precursors that are actively in the cell cycle and nearing the end of G1 are accelerated into S-phase by the presence of serotonin, and thus are labeled in increasing numbers by the incorporation of the BrdU contained in the perfusate.
In support of this interpretation, we have shown that: (1) exposure of the perfused crayfish preparation to saline containing low levels of serotonin (10−9M) increases cell proliferation (Figure 3), in keeping with the demonstration in lobsters that such low concentrations of 5HT will significantly increase the rate of proliferation in the lateral proliferation zone in those animals (Benton et al., 2008); (2) stimulation of the CL10 neurons and the collection of perfusate from the brain shows that the level of serotonin contained in the perfusate increases during periods of stimulation when compared with levels collected during periods without stimulation; (3) the levels of serotonin collected in the perfusate during stimulation range between 10−11 to 10−13M, levels which, given the added dilution in the perfusate after release, would imply values at the sites of cell proliferation in the range of those shown to be optimal for influencing neurogenesis (Benton et al., 2008); (4) an extensive network of blood vessels is a feature of the AL and the CL10 cell body area, with fine capillaries passing close to areas containing proliferating cells; (5) the lack of a significant increase in CL10 proliferation when the DGN is stimulated but is not driven above its spike threshold, suggests that in order to release enough serotonin for an increase in neurogenesis over a short time period (6 hours), the DGN must undergo significant depolarization.
Support for the above interpretation also comes from previous studies on this system in C. destructor: (1) an isolated AL placed in saline containing a high level of potassium will release serotonin (Meunpol et al., 1998); (2) electron micrographs of the AL in which the DGN had been labeled with neurobiotin, showed that while the DGN rarely makes conventional synaptic contact with other neurons in this lobe, this cell receives many input synapses. The release of serotonin from this neuron then, in common with many serotonergic neurons in both vertebrates and invertebrates, may occur at non-synaptic sites (Christenson et al., 1990; Ridet et al., 1993; Sandeman RE et al., 1995); (3) non-synaptic exocytosis of vesicles into the extracellular space has been demonstrated in the central body of the crayfish brain (Schürmann et al., 1991), and studies with ferritin, a large electron-dense molecule, suggest there may be no blood-brain barrier between the vascular capillaries that pervade the brain and the extracellular space surrounding neurons (Abbot, 1972). It would appear, then, that substances released from neurons within the brain could easily enter the blood vessels.
In contrast to our interpretation, however, it could be argued that current from our relatively large extracellular stimulating electrode spread to the proliferation zone and directly influenced the precursor cells to enter S phase. Our results from stimulation of the surface of the OL, however, which is directly adjacent to the lateral proliferation zone, suggests that this is not likely; even in those preparations in which the stimulus intensity necessary to produce the OL-induced hyperpolarization of the DGN was doubled, no increase in cell proliferation was found on the stimulated side. Similarly, stimulation of the AL cortex also failed to increase the rate of proliferation on the stimulated side of the brain. Our data therefore strongly indicate that it is stimulation of the neural pathways, and not current spread from the stimulating electrode to the proliferation zone, that is altering the rate of cell proliferation in these experiments.
The excitation of the DGN was necessarily achieved in our studies through synaptic pathways and it may be argued that it was these presynaptic elements that induced the increase in neurogenesis and not the activation of the DGN itself. There are two reasons for this being less likely than our interpretation. The first is that subthreshold stimulation of the DGN did not significantly increase CL10 cell proliferation despite the presence of a small depolarization in the DGN showing that the presynaptic elements were active. Second, in C. destructor the branches of the DGN constitute the major, if not the entire, serotonergic projection to the AL; if serotonin is indeed the factor that produces the increase in neurogenesis, as indicated by the increase following the addition of serotonin to the perfusate, then the DGN is the only source that can be activated by stimulating the CL10 neurons or the AL medulla.
The decrease in cell proliferation following stimulation of the OL requires further examination. There is no identified pathway that explains the hyperpolarization of the DGN following OL stimulation. On the other hand, HPLC analysis of perfusate collected from over the AL in animals that were receiving no stimulation, contained small amounts of serotonin, suggesting a continuous release at low levels. Hyperpolarization of the DGN may reduce this, leading to the observed decrease in the cell proliferation. Stimulation of the AL cortex also hyperpolarizes the DGN and often more vigorously than stimulation of the OL, but produces an even smaller differential in BrdU-labeled cells on the two sides of the brain (Figure 6C). A possible explanation here may lie in the many deutocerebral axons that spread over the surface of the AL before projecting down into it. A stimulus electrode on the surface of the AL could initiate action potentials in these incoming axons that would spread antidromically across the brain to penetrate the contralateral AL, result in the hyperpolarization of the contralateral DGN and hence the decrease in the amount of serotonin on the contralateral side as well. A comparison of the cell proliferation of the control sides in all experiments does indeed reveal a tendency for the cell proliferation on the control side of brains that had received AL cortex stimulation, to be slightly lower than in the other experiments, although this difference is not statistically significant.
Despite the challenges of investigating a large and complex neuron such as the DGN in an isolated preparation, our studies do suggest that one role of the DGN in the crayfish, and perhaps in other decapods whose brains contain analogous serotonin-containing neurons, is to influence adult neurogenesis in these animals. This role of the DGN is interesting to examine in a broader context. Many studies on both vertebrate and non-vertebrate species have shown that adult neurogenesis in the brain is influenced by environmental, behavioral and endogenous inputs; these alterations in the production or survival of neurons in the adult brain may represent a plastic response of the central nervous system to a specific need, for example, maintenance through the turnover of neurons, or even to repair damage. In the decapod crustaceans, neurogenesis has been shown to be influenced by environmental inputs such as living conditions (C. destructor; Sandeman and Sandeman, 2000), the light/dark cycle (H. americanus; Goergen et al., 2002) and seasonality (Carcinus maenas; Hansen and Schmidt, 2004). In addition, cell proliferation during the day/night cycle in lobsters has been correlated to changes in brain levels of serotonin (Wildt et al., 2004).
The DGN in C. destructor is a very large and architecturally complex neuron, with a huge arbor of processes that infiltrate both the OLs and ALs, innervating each and every glomerulus in these regions. Its anatomy places this cell in a potentially unique position as an integrator of multi-modal input. For example, the DGN is the target of interneurons in the deutocerebral commissure that carry higher order information from visual and tactile inputs (Sandeman DC et al., 1995). These inputs converge on the DGN within the glomeruli of the AL and, when enough are active in a particular combination, may induce a general response from the DGN and a release of serotonin. The DGN also has a number of intriguing feedbacks within the AL and OL system. The single dominant input to the DGN, and the one we have exploited, is that which it receives from the primary output of the OL and AL neuropils – the CL10 projection neurons. It is this input which can lead to action potentials in the DGN which are probably responsible for the greatest release of serotonin. Released into the blood, serotonin would not only reach the proliferation zones in CL10 but also the inputs from the antennular chemoreceptors to the OL. Behavioral tests on the sensitivity of spiny lobsters to prey odors, for example, showed that sensitivity is influenced by their activity state. Resting animals need concentrations of the olfactory stimulant that are two orders of magnitude higher than that which will attract the attention of walking animals (Zimmer-Faust et al., 1996). Is this sensitivity modulation also part of DGN action? We do not yet know. Similarly, how much of the complex circuitry in the OLs and ALs is concerned with olfactory learning, an essential part of the normal behavioral requirement of animals that are predominantly night active, depending heavily on mechanoreceptive and chemosensitive inputs? If the DGN is indeed receiving all these inputs, which is highly likely, this neuron is in the best possible position to modulate adult neurogenesis among the many small projection and local interneurons that form the basis of OL and AL processing and which are ultimately responsible for the integration of multimodal inputs and the resulting behavioral outputs.
Acknowledgement
We thank Alice Rose for her assistance with the HPLC and P. Carey and V. Quinan for their care and maintenance of the animals. Supported by NIH R01 MH67157, NSF-IBN 0344448.
Contributor Information
D.C. Sandeman, Email: dsandema@wellesley.edu.
J.L. Benton, Email: jbenton@wellesley.edu.
B.S. Beltz, Email: bbeltz@wellesley.edu.
References
- Abbott J. Access of ferritin to the interstitial space of Carcinus brain from the intracerebral blood vessels. Tissue Cell. 1972;4:99–104. doi: 10.1016/s0040-8166(72)80010-7. [DOI] [PubMed] [Google Scholar]
- Ache BW, Sandeman DC. Olfactory induced central neural activity in the Murray crayfish, Euastacus armatus. J Comp Physiol. 1980;140:295–301. [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histologic studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137(4):43–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Bazin FM. Etude compare de l’organe deutocerebral des Macroures reptantia et des Anomures. (Crustaces Decapodes)1. Arch Zool exp gen. 1970;111:245–264. [Google Scholar]
- Bédard A, Parent A. Evidence of newly generated neurons in the human olfactory bulb. Brain Res Dev Brain Res. 2004;151(1–2):159–168. doi: 10.1016/j.devbrainres.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Beltz BS, Sandeman DC. Regulation of life-long neurogenesis in the decapod crustacean brain. Arth Struct Dev. 2003;32:39–60. doi: 10.1016/S1467-8039(03)00038-0. [DOI] [PubMed] [Google Scholar]
- Beltz BS, Benton JL, Sullivan JM. Transient uptake of serotonin by newborn olfactory projection neurons. PNAS. 2001;98(22):12730–12735. doi: 10.1073/pnas.231471298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton J, Beltz BS. Effects of embryonic serotonin depletion on olfactory interneurons in lobsters. J Neurobiol. 2001;46:193–205. doi: 10.1002/1097-4695(20010215)46:3<193::aid-neu1002>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Benton JL, Helluy S, Huber R, Beltz B. Serotonin depletion by 5,7-dihydroxytryptamine alters deutocerebral development in the lobster. J Neurobiol. 1997;33 doi: 10.1002/(sici)1097-4695(199710)33:4<357::aid-neu2>3.0.co;2-9. 357-353. [DOI] [PubMed] [Google Scholar]
- Benton JL, Sandeman DC, Beltz BS. Nitric oxide in the crustacean brain: Regulation of neurogenesis in the developing olfactory pathway. Dev Dynamics. 2007;236:3047–3060. doi: 10.1002/dvdy.21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton JL, Goergen EM, Rogan SC, Beltz BS. Hormonal and synaptic influences of serotonin on adult neurogenesis. Gen Comp Endocrinology. 2008;158:183–190. doi: 10.1016/j.ygcen.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Serotonin may stimulate granule cell proliferation in the adult hippocampus, as observed in rats grafted with fetal raphe neurons. Eur J Neurosci. 2000;12:391–396. doi: 10.1046/j.1460-9568.2000.00932.x. [DOI] [PubMed] [Google Scholar]
- Cayre M, Malaterre J, Scotto-Lomassese S, Strambi C. The common properties of neurogenesis in the adult brain: from vertebrates to invertebrates. Comp Biochem Physiol B Biochem Mol Biol. 2002;132:1–15. doi: 10.1016/s1096-4959(01)00525-5. [DOI] [PubMed] [Google Scholar]
- Christenson J, Cullheim S, Grillner S, Hökfelt T. 5-Hydroxytryptamine immunoreactive varicosities in the lamprey spinal cord have no synaptic specializations – An ultrastructural study. Brain Res. 1990;512:201–209. doi: 10.1016/0006-8993(90)90627-n. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Goergen EM, Bagay LM, Rehm K, Benton JL, Beltz BS. Circadian control of neurogenesis. J Neurobiol. 2002;53:90–95. doi: 10.1002/neu.10095. [DOI] [PubMed] [Google Scholar]
- Hansen A, Schmidt M. The influence of season and environment on adult neurogenesis in the central olfactory pathway of the shore crab, Carcinus maenas. Brain Res. 2004;1025:85–97. doi: 10.1016/j.brainres.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Harzsch S, Miller J, Benton J, Dawirs R, Beltz B. Development of the thoracic neuromeres in two crustaceans with different styles of metamorphic development. J Exp Biol. 1998;210:2465–2479. doi: 10.1242/jeb.201.17.2465. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, van Praag H, Gage FH. Adult brain neurogenesis and psychiatry: A novel theory of depression. Mol Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Adult neurogenesis. New York: Oxford University Press; 2006. 426 pp. [Google Scholar]
- Meunpol O, Sandeman R, Sandeman D, Kapoor V. Stimulus-coupled serotonin release from crayfish accessory lobes (C. destructor). Australian Soc Comp Physiol; Abstract, 1998 meeting; Melbourne, Australia. 1998. [Google Scholar]
- Paizanis E, Hamon M, Lanfumey L. Hippocampal neurogenesis, depressive disorders, and antidepressant therapy. Neural Plast. 2007:73754. doi: 10.1155/2007/73754. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridet JL, Rajaofetra N, Teihac JR, Geffard M, Privat A. Evidence for nonsynaptic serotonergic and noradrenergic innervation of the rat dorsal horn and possible involvement of neuron-glia interactions. Neuroscience. 1993;52:143–157. doi: 10.1016/0306-4522(93)90189-m. [DOI] [PubMed] [Google Scholar]
- Sandeman DC. Vascular circulation in the brain, optic lobes and thoracic ganglion of the crab Carcinus. Proc Roy Soc B. 1967;168:82–90. doi: 10.1098/rspb.1967.0052. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Sandeman R, Derby C, Schmidt M. Morphology of the brain of crayfish, crabs, and spiny lobsters: a common nomenclature for homologous structures. Biol Bull. 1992;183:304–326. doi: 10.2307/1542217. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Sandeman RE. Electrical responses and synaptic connections of giant serotonin-immunoreactive neurons in crayfish olfactory and accessory lobes. J Comp Neurol. 1994;341:130–144. doi: 10.1002/cne.903410111. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Beltz BS, Sandeman RE. Crayfish brain interneurons that converge with serotonin giant cells in accessory lobe glomeruli. J Comp Neurol. 1995;352:263–279. doi: 10.1002/cne.903520209. [DOI] [PubMed] [Google Scholar]
- Sandeman R, Sandeman D. “Impoverished” and “enriched” living conditions influence the proliferation and survival of neurons in crayfish brain. J Neurobiol. 2000;45:215–226. [PubMed] [Google Scholar]
- Sandeman R, Sandeman D. Development, growth, and plasticity in the crayfish olfactory system. Microsc Res Tech. 2003;60:266–277. doi: 10.1002/jemt.10266. [DOI] [PubMed] [Google Scholar]
- Sandeman RE, Watson AHD, Sandeman DC. Ultrastructure of the synaptic terminals of the dorsal giant serotonin-IR neuron and deutocerebral commissure neurons in the accessory and olfactory lobes of crayfish. J Comp Neurol. 1995;361:617–632. doi: 10.1002/cne.903610406. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Identification of putative neuroblasts at the base of adult neurogenesis in the olfactory midbrain of the spiny lobster, Panulirus argus. J Comp Neurol. 2007;503:64–84. doi: 10.1002/cne.21366. [DOI] [PubMed] [Google Scholar]
- Schürmann FW, Sandeman RE, Sandeman DC. Dense core vesicles and non-synaptic exocytosis in the central body of the crayfish brain. Cell Tiss Res. 1991;265:493–501. [Google Scholar]
- Song CK, Johnstone LM, Edwards DH, Derby CD, Schmidt M. Cellular basis of neurogenesis in the brain of crayfish Procambarus clarkii; Neurogenic complex in the olfactory midbrain from hatchlings to adults. Arth Struct Dev. 2009 doi: 10.1016/j.asd.2008.12.004. (in press) [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Newborn cells in the adult crayfish brain differentiate into distinct neuronal types. J Neurobiol. 2005a;65:157–170. doi: 10.1002/neu.20195. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Integration and segregation of inputs to higher-order neuropils of the crayfish brain. J Comp Neurol. 2005b;481:118–126. doi: 10.1002/cne.20346. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Benton JL, Beltz BS. Serotonin depletion in vivo inhibits the branching of olfactory projection neurons in the lobster deutocerebrum. J Neurosci. 2000;20:7716–7721. doi: 10.1523/JNEUROSCI.20-20-07716.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Benton JL, Sandeman DC, Beltz BS. Adult neurogenesis: A common strategy across diverse species. J Comp Neurol. 2007a;500:574–584. doi: 10.1002/cne.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Sandeman DC, Benton JL, Beltz BS. Adult neurogenesis and cell cycle regulation in the crustacean olfactory pathway: from glial precursors to differentiated neurons. J Mol Hist. 2007b;38:527–542. doi: 10.1007/s10735-007-9112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildt M, Goergen EM, Benton JL, Sandeman DC, Beltz BS. Regulation of serotonin levels by multiple light-entrainable endogenous rhythms. J Exp Biol. 2004;207:3765–3774. doi: 10.1242/jeb.01205. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Allodi S, Sandeman DC, Beltz BS. Adult neurogenesis in the crayfish brain: proliferation, migration and possible origin of precursor cells. Dev Neurobiol. 2009 doi: 10.1002/dneu.20717. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer-Faust RK, O'Neill PB, Schar DW. The relationship between predator activity state and sensitivity to prey odor. Biol Bull. 1996;190(1):82–87. doi: 10.2307/1542677. [DOI] [PubMed] [Google Scholar]