Summary
The cellular mechanisms driving mammalian epithelial morphogenesis are of significant fundamental and practical interest. Historically, these processes have been difficult to study directly, owing to the opacity and relative inaccessibility of mammalian tissues. Recent experimental advances in time-lapse imaging and in 3D organotypic culture have enabled direct observation of epithelial morphogenesis. In the mammary gland, branching morphogenesis is observed to proceed through a novel form of collective epithelial migration. The active unit of morphogenesis is a multilayered epithelium with reduced apico-basal polarity, within which cells rearranged vigorously. From within this multilayered state, new ducts initiate and elongate into the matrix without leading cellular extensions or dedicated leaders. We discuss the implications of these findings on our understanding of epithelial morphogenesis in other organs and in cancer progression.
Introduction
Epithelia constitute an essential component of branching mammalian organs. Epithelial structure is established during embryonic development and then dysregulated in epithelial cancers. Despite tremendous fundamental and practical interest, the cellular mechanisms driving mammalian epithelial morphogenesis have been, until recently, essentially unknown.
The mammary gland is an important model of mammalian branching morphogenesis. Mammary development begins with the formation of an ectodermal placode in the mid-gestation embryo, and proceeds to form a rudimentary ductal tree in the fetus. Unlike other branched organs, the majority of branching morphogenesis is elaborated in the postnatal female, and further modulated by reproductive hormonal signals during estrus, pregnancy, lactation and involution [1]. The postnatal development of the mammary gland, coupled with the development of mammary specific Cre transgenic mice, has enabled evaluation of the loss of function phenotypes of dozens of genes and significant insights into the genetic regulation of branching morphogenesis [2-4].
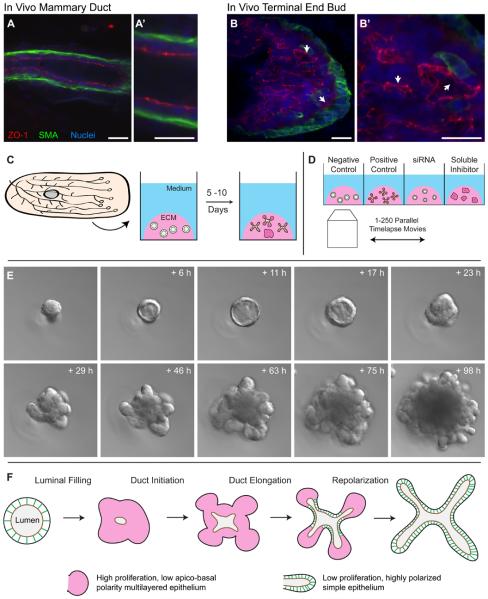
However, despite these advances, the cellular basis of mammary branching morphogenesis has until recently been inferred. Histologic and ultrastructural analysis strongly suggested that the major events of ductal elongation and bifurcation occur at the ends of mammary ducts, in specialized structures known as terminal end buds (TEBs) [5,6]. Quiescent mammary ducts have a bilayered organization with a single luminal epithelial cells layer and basally positioned myoepithelial cells. The luminal cells organize in a simple epithelium, with tight junctions defining a fluid filled lumen (Figure 1A). In contrast, the TEB is composed of multiple luminal epithelial cell layers (body cells) and an outer layer of myoepithelial-like cap cells (Figure 1B) [1,5,6]. Direct observation of cell behaviors within in vivo mammary ducts or TEBs is made difficult by an extracellular matrix (ECM) and adipocyte rich stroma that is highly diffractive for standard fluorescent and confocal imaging techniques.
Figure 1. 3D primary organotypic culture makes mammary branching morphogenesis observable.
(A, B) Confocal images of anti-body stained sectioned in vivo mammary duct (A) and terminal endbud (B), illustrating the normal in vivo composition of apical tight junctions (ZO-1; red) and the basal myoepithelial cell layer (SMA; green). (C) The mammary “organoid” assay involves isolation of epithelial fragments each initially consisting of 100-500 cells (“organoids”) from mammary glands through a combination of mechanical and enzymatic disruption [14,15,35]. These organoids are then subsequently embedded in Matrigel – a laminin, collagen IV, heparin sulfate rich matrix- and fed a serum-free media containing defined growth factors (e.g. FGF2). Fragments then develop over a period of 5-10 days and undergo a complex program of branching morphogenesis. (D) Mice can yield 1-5,000 organoids each, thereby enabling organoids from the same mouse to be cultured in different microenvironmental conditions [13,14]. The large scale enables parallel experimental design in which epithelium from the same mouse is allocated to different ECM, solution or perturbation (e.g. siRNA) conditions and enables the consequences of these variables to be assessed individually or parametrically with genetically identical starting material. Advances in timelapse imaging automation make it possible to image 100-500 movies in parallel and monitor individual cell behaviors within branching mammalian epithelia over 10s to 100s of hours. (E) Organoids first clear their lumen to a simple, bilayered architecture, then fill their lumen with cells prior to initiating, elongating and bifurcating new ducts. Images courtesy of Kim-Vy Nguyen-Ngoc, Johns Hopkins Medical School. (F) Mammary branching morphogenesis involves large, but transient, changes in apico-basal polarity, proliferation and epithelial organization. The functional unit of morphogenesis has high proliferation, low polarity and multilayered organization (pink). As elongation ceases the epithelium reorganizes and restores highly polarized simple epithelial organization (green). Images from A-B are reprinted with permission from [10]. Scale bars are 20 microns.
Importantly, advances on two fronts have made the real-time study of the cellular basis of mammary epithelial development feasible. First, 3D organotypic culture techniques have enabled a reasonable model of mammary epithelial development to occur in vitro in a highly observable and manipulable format [7-9]. Second, long-term multi-position timelapse imaging culture, has enabled robust observation of the cell movements and behaviors driving the development of these cultures [10,11]. In this review, we focus on recent progress toward a cellular description of mammary branching morphogenesis arising from these techniques, and discuss their implications for our understanding of mammalian epithelial development in other organ systems and in the invasion strategies of epithelial tumors.
The organoid model of mammary epithelial morphogenesis
Although the timing of mammary branching morphogenesis is controlled by systemic steroid hormone signals, these signals are interpreted in the context of a signal-rich extracellular matrix (ECM) and cellular stroma [1]. Decades of work have identified critical ECM-epithelial and stroma-epithelial signaling interactions capable of modulating mammary development and the progression of mammary tumors [9,12-14]. These studies have established that tissue architecture and microenvironmental context can critically influence gene expression, cell behavior and invasive potential [12,15,16].
The normal tissue architecture of the mammary epithelium is incompletely recapitulated in classic 2D cultures and so extensive efforts have been directed at developing more organotypic models of mammary development and neoplasia [7,17]. The “organoid assay” was developed in the Bissell and Werb laboratories as a model for normal mammary development [10,11,18-20]. In the organoid assay, epithelial fragments are isolated from the mammary gland by chopping and enzymatic digestion, embedded in laminin I and collagen IV rich Matrigel and fed a serum-free media containing defined growth factors (e.g. FGF2) (Figure 1C). Organoids undergo a rich program of branching morphogenesis that recapitulates multiple cell biological and histological characteristics observed in vivo, despite greatly reduced levels of adipocytes, mesenchymal cells and collagen I. Branching epithelial ducts in 3D culture contain multiple luminal epithelial cell layers, lack forward oriented protrusions and contain convoluted luminal structures within the multilayered region, observed both in vivo and in vitro (Figure 1B vs. 2B-D) [10]. This close similarity is the basis of the term “organotypic” and the assay enables the observation of the normal cellular mechanisms of branching morphogenesis in a convenient and realistic model.
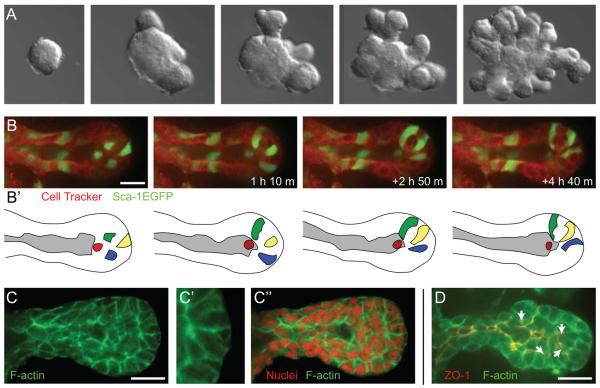
Figure 2. The basic program of mammary branching morphogenesis.
(A) Epithelial fragments (“organoids”) isolated from the mammary gland will undergo a complex program of branching morphogenesis in 3D ECM culture. This program involves the de novo initiation, elongation and bifurcation of new ducts. (B-B′) Cells within the elongation front rearrange vigorously and there do not appear to be dedicated leader cells [14]. (C-C″) Elongating mammary ducts have a multilayered organization at the elongation front and do not have actin based subcellular protrusions into the ECM [14]. (D) Within the multilayered endbud there is typically a tight junction (ZO-1) lined main lumen as well as isolated microlumens within the multilayered region (arrows) [14]. ECM = extracellular matrix, ZO-1 = zona occludens 1, a tight junction marker. Images from B-D are reprinted with permission from [10]. Scale bars are 20 microns.
Biomedical engineering based assays complement the organoid assay and have enabled more precise control of the ECM microenvironment. For example, culture of mammary epithelial cells in precise, lithographically-patterned ECM enabled researchers to show that the geometry of the ECM can determine the pattern of branching [21]. This approach has been highly productive for isolating the influence of specific experimental variables, such as the effect of local geometry on the diffusion of morphogenetic molecules (e.g. TGFβ [22], the importance of competition among epithelial cells within a restrained geometry based on differential expression of matrix metalloproteinases [23] and the ability of local alignment of collagen I to promote tumor invasion [24]. Engineering based approaches provide a powerful complement to more cell biology oriented assays and enable independent modulation of experimental variables that are present, but difficult to manipulate, in vivo.
The cellular program of mammary branching morphogenesis
Advances in microscope automation have enabled serial imaging of experimentally perturbed epithelia under identical microenvironmental and imaging conditions (Figure 1D), and enabled reliable identification and tracking of individual cell behaviors during branching (Figure 2B) [10,11]. By combining organotypic culture and advanced microscopy, the normal sequence of events underlying mammary branching morphogenesis has been defined [10]. Organoids embedded in 3D Matrigel first clear their lumens and establish a polarized bilayered epithelial architecture. Then, surprisingly, they fill their lumens with cells (Figure 1E). Luminal filling can be partial or complete but is always observed to precede the initiation and elongation of new ducts (Figure 1E [10]). Addition of pharmacologic inhibitors established that luminal filling requires proliferation[10] and that growth factor induced proliferation requires Erk1/2 [10,11]. New ducts initiate, elongate, and bifurcate as a multilayered epithelium and eventually spontaneously stop and recanalize to adopt a bilayered organization (Figure 1A-B, E and 2A). During morphogenesis the multilayered mammary epithelium exhibits high levels of proliferation and reduced apico-basal polarity, both in organotypic culture and in vivo (Figure 1B and 2D) [10].
Mammary branching involves the coordinate, and strikingly different, motility of two epithelial cell types, the luminal and myoepithelial cells [10]. Luminal epithelial cells contribute to the elongation of ducts while myoepithelial cells surround and define the basal surface of the luminal epithelium [10]. Luminal cells within initiating and elongating mammary ducts shared three key features: the end of the elongating duct was multilayered, there were no evident subcellular protrusions into the ECM and there were extensive cell rearrangements during elongation (Figure 2B; [10]). Subsequent staining for F-actin revealed no evidence for actin-based protrusions extending into the ECM (Figure 2C, [10]). Though ductal elongation appeared orderly and processive at the tissue level (Figure 2A), analysis of individual cell behaviors revealed chaotic rearrangements and extensive mixing of cells in the elongation front (Figure 2B).
Though no direct timelapse analysis of ductal elongation has been performed in vivo, previous histological and ultrastructural studies of elongating mammary ducts in vivo also revealed a multilayered epithelial organization within the TEB and a smooth elongation front, without subcellular protrusions [6]. Myoepithelial cells are in direct contact with the basally located basement membrane and ECM, while the most basally positioned luminal epithelial cells make infrequent ECM contact, through gaps in myoepithelial coverage [10,25]. However, the multilayered epithelial organization that underlies ductal elongation shows reduced apico-basal polarity; as many cells are located outside of contact with ECM. (Figure 2D, [10]).
Cellular mechanisms of mammalian branching morphogenesis
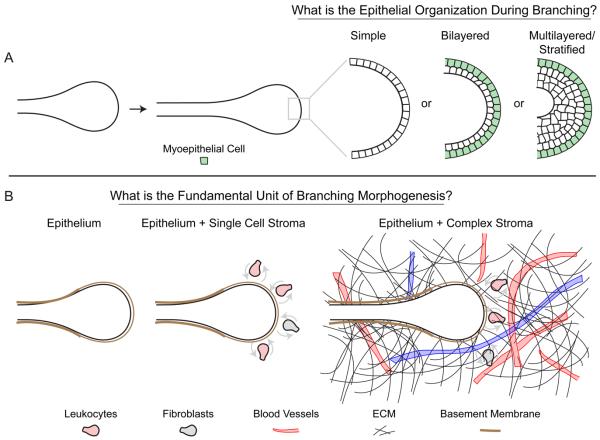
Real-time, organotypic imaging of branching morphogenesis has also been described in the salivary gland [26], kidney [27-29], and pancreas [30]. Among these organs, the salivary gland appears most similar to the mammary gland [31]; both organs undergo branching morphogenesis as multilayered/stratified epithelia (Figure 3A), but ultimately produce simple tubes [10,26]. Timelapse movies of epithelial cells within branching salivary gland explants revealed a surprising degree of dynamic cell movements and rearrangements [26]. Similarly, epithelial cells within the distal Wolffian duct of the kidney were observed to move actively and to rearrange dynamically [27]. They then condensed to form a pseudostratified epithelium, which extends collectively as ureteric bud (UB) epithelium [27]. While, the cell cycle is locally active in regions preceding UB formation, directed migration of cells from distal regions of the Wolffian duct appears also to contribute to UB formation [27]. Moreover, subtle changes in the level of Ret signaling drive the migration and clustering of cells forming the UB placode [27]. These results highlight the importance of local mitosis, cell migration and cell shape change and potentially differences in cell adhesion as mechanisms of endbud formation.
Figure 3. Conserved developmental mechanisms for branching morphogenesis.
(A) Most mammalian epithelial tubes have a simple organization. However, during active morphogenesis both the mammary [14] and salivary [26] epithelia are multilayered or stratified. By contrast lung appears to retain a simple organization during branching morphogenesis [40]. (B) During branching morphogenesis in each of the epithelial organs there is a rich diversity of ECM proteins and stromal cell types, including diverse leukocytes and fibroblasts. Functional evidence has been provided for the importance of macrophages during mammary development [22,26], but it remains a relatively open question which stromal cell types are required for, or assist in, the normal development of the epithelial organs. ECM = extracellular matrix.
Epithelial bifurcation or ‘clefting’ involves the division of one epithelial unit into two or more units. Although the molecular and physical mechanisms of cleft formation are largely undefined, we know most about the progression of these events in the salivary gland. Cleft formation and progression is hypothesized to result from the exchange of cadherin mediated cell-cell adhesions for integrin mediated cell-matrix adhesions through the translocation of fibronectin into the deepening cleft [26,32]. Interestingly, a recent study observed physical indentations of the plasma membrane into the cytoplasm in luminal epithelial cells during cleft formation and deepening [33]. These indentations are connected to cytoplasmic actin fibers and are observed to shift to neighboring cells as the cleft progresses [33].
Two key features are observed during cleft initiation in the salivary gland; deposition of basally positioned ECM proteins such as fibronectin, collagen I, III and IV [26,34,35] and the presence of mesenchymal or myoepithelial cells in the branching cleft [33,34]. Robust branching morphogenesis and deposition of collagen IV in the cleft is dependent on MT2-MMP modifications of collagen IV matrix [35]. Thus, localized expression or activation of ECM cleaving proteases may be regulating clefting. Recent work has shown that progression of salivary clefts requires ROCK1 signaling to MLC2 [36]. One interpretation of this study, coupled with observations of cellular indentations observed in luminal epithelial cells [33], suggests that cleft formation deepen based on ROCK1/MLC2 dependent contraction of a cytoplasmic indentation, possibly anchored to sites of integrin-fibronectin adhesion. Downstream signaling is becoming clearer, with a recent study showing fibronectin dependent induction of Btbd7, leading to Snail induction and E-cadherin supression; this loss of E-cadherin allows local separation of the epithelium and generation of a cleft [37]. It will be important to determine the cellular source and the molecular mechanisms regulating spatially-selective deposition of ECM (Figure 3B).
Basal type cells in other branching organs
Basal cell populations are observed in adult branched organs of the prostate, breast, salivary gland and lungs. These cells are contractile myoepithelial cells (salivary and breast), nonmyoepithelial basal cells (prostate) or myofibroblasts (lung) [31]. During prostate morphogenesis all epithelial cells express both luminal and basal cell markers, a subset of which differentiate in the adult prostate to definitive basal cells [38]. The lung epithelium has basally localized myofibroblasts. Moreover, these smooth muscle actin positive cells are observed covering early lung epithelial buds yet are absent from the distal elongating endbud tips. In addition, these cells are present in the clefts of branching lung epithelial endbuds [39,40]. The location of these cells suggests that myofibroblasts may restrict or pattern lung branching in an analogous manner to that of myoepithelial cells in mammary branching [10]. The dynamics and contributions of these basal cell populations for branching morphogenesis of the lung, salivary gland and prostate remain largely unexplored. A major outstanding question across the branching organs is the extent to which branching is autonomous to the luminal epithelium or requires the instructive contributions of the ECM and/or specific stromal cell populations (Figure 3B).
Patterns of branching morphogenesis
Separate from the question of the formation and elongation of ducts is the question of the patterning of duct number, geometry and location. Fixed time series analysis of lung development demonstrated deeply stereotyped branching patterns and proposed that complex branching patterns can be generated from three simpler mechanisms: domain branching, planar bifurcation and orthogonal branching [40]. By contrast, mammary branching morphogenesis both in vivo and in organotypic cultured appears non-stereotyped. Imaging of branching morphogenesis in the kidney revealed that that dipodial terminal branching dominates over lateral budding [27-29]. Similar analysis in the pancreas illustrates a predominance of lateral branching behaviors [30]. While future research may reveal unifying themes in the patterning of all branched organs, at present there appear to be fundamental differences in the branching patterns of different mammalian organs. It is also possible that geometric or ECM aspects of the culture conditions employed could be influencing the pattern of branching [22].
Mammary branching morphogenesis and its relevance to cancer pathogenesis
Insights from real-time imaging of mammary branching morphogenesis may also be instructive for our understanding of at least three stages of cancer pathogenesis: development of in situ carcinoma, transition to the invasive state, and dissemination.
Luminal filling is an early step in ductal carcinoma in situ
A critical event in carcinoma progression is the development of the preinvasive state. Breast tumors frequently originate in the terminal duct lobular unit (TDLU), a developmental analogue of the mouse terminal end bud [41]. One of the earliest steps in breast cancer progression is the development of a preinvasive lesion called ductal carcinoma in situ (DCIS). DCIS is defined histologically by the accumulation of cells within the ductal lumen. These cells are often abnormal in appearance, but are confined within the lumen and are surrounded by myoepithelial cells and basement membrane [42]. Significantly, luminal filling and loss of apico-basally polarized epithelial architecture are both long-established features of epithelial cancers and are frequently observed in both benign and invasive breast lesions [7,43]. Studies in MCF-10A cells have revealed that overexpression of constitutively active growth factor receptors, or related signaling molecules, is sufficient to induce a persistently filled lumen [7]. Separately, it was observed that activation of Erk1/2 was sufficient to induce luminal filling and motility in luminally confined cells [44]. In this context, the transient, reduced-polarity, multilayered state seen in mammary organoid culture may represent a common tissue architectural motif recapitulated in the MCF10A cell line and aberrantly maintained in carcinoma in-situ [10].
Myoepithelial loss correlates with the in situ to invasive transition
The transition from in situ to invasive carcinoma is pathologically defined by loss of basement membrane and the myoepithelial cell border at sites of invasion [45]. In addition to this pathological correlation, there is accumulating evidence that loss of myoepithelial cells has functional consequences [46-49]. A human breast cancer cell line initially forms DCIS lesions that subsequently progress to invasive carcinoma [50]. However, when this cell line is co-injected with myoepithelial cells, progression to invasive carcinoma is significantly reduced [50]. One proposed mechanism for these observations is that myoepithelial cells may exert direct effects on the proper polarity of luminal cells in a collagen matrix [51]. Real-time imaging of mammary branching morphogenesis has revealed highly dynamic interactions between luminal and myoepithelial cells [10]. These data are consistent with the hypothesis that myoepithelial cells actively restrict luminal cell movement and direct the growth of normal ducts [10] and raise the possibility that a similar cellular mechanism may restrain the DCIS to invasive transition in breast cancer [10,46-50].
Organotypic modeling of cancer invasion
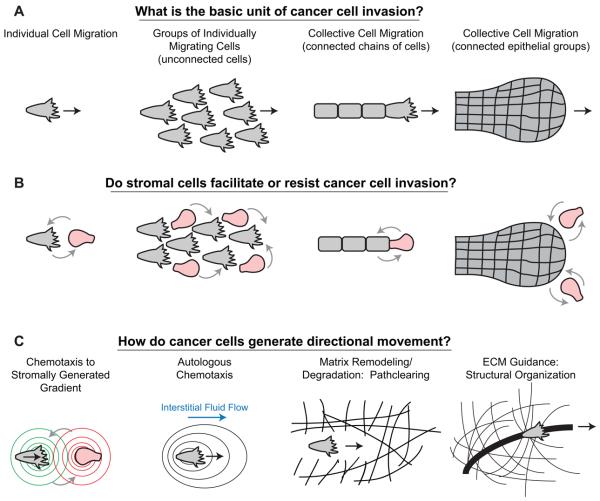
Cancer cells clearly demonstrate the capacity to invade tissues, disseminate, and metastasize. More recently, advances in confocal and 2-photon microscopy have enabled direct visualization of the cellular basis of cancer invasion. These studies have revealed a diversity of invasion strategies(Figure 4A) [52,53]. Cancer cells have been found to invade singly into tissue, but also to migrate collectively with intact cell-cell junctions [52] (Figure 4A). These different modes of invasion can also coexist within the same tumor [54]. Importantly, cancer cells can also transition between invasion modes. For example, TGF-beta activation has been associated with single cell movement in breast cancer, and blockade of TGF-beta signaling reverts cancer cells to a collective invasion strategy [55]. In addition, individual invasive breast cancer cells can proteolytically expand tracks through the ECM to allow collective invasion [56] (Figure 4C). In contrast to these findings, during normal mammary branching morphogenesis, disseminative invasion is not observed, despite dramatic reductions in apico-basal cell polarity and active epithelial motility [10]. In this context, it is clear that additional cellular and molecular mechanisms must be dysregulated in invasive cancers.
Figure 4. Cellular mechanisms driving epithelial cancer invasion.
(A) Cancer cell invasion has been observed to proceed by both individual and collective mechanisms. Collective migration strategies can include: chains, files and connected, largely epithelial groups such as pushing boundaries [52]. In addition, single cell invasion can involve large numbers of individually migrating cells, but the term collective cell migration is reserved for connected groups of cells. (B) Stromal cells, including macrophages [58] and fibroblasts [69], have been observed to assist in the invasion of tumors. It remains an open question which stromal cell types in vivo serve to assist or resist the invasion and dissemination of cancer cells. Stromal cells could influence the invasion of singly or collectively migrating cancer cells. (C) A diversity of mechanisms have also been reported to explain the guidance of cancer cell invasion, including: reciprocal gradients of EGF and CSF-1 between macrophages and cancer cells [58], autologous chemotaxis whereby molecules released by cancer cells are shifted towards the lymphatics by interstitial fluid flow [60], pathclearing through the ECM, for example by fibroblasts ahead of squamous cell carcinoma cells [69] or alternately reorganization of the ECM to promote the invasion of cancer cells [63]. ECM = extracellular matrix.
In mammary branching morphogenesis, invasion through the fat pad is dependent on luminal cell interactions with stromal components such as fibroblasts and macrophages, as well as with extracellular matrix including laminin and collagen [1]. These observations suggest that components of the tumor microenvironment also may be essential for disseminative styles of invasion [14,57]. In support of this concept, diverse stromal-epithelial interactions have been observed in cancer invasion (Figure 4B); these include cancer cell interactions with fibroblasts, pericytes, endothelium, and bone marrow derived cells, most prominently macrophages [57,58]. It remains an open question to what extent these stromal cell types serve to assist or resist the invasion and dissemination of cancer cells in human patients.
Directional Guidance in Cancer Invasion
Histologic studies and intravital imaging suggest that stromal components interact with tumor to promote productive movement toward lymph nodes and blood vessels [57,58]. Various guidance mechanisms have been identified, and include reciprocal gradients between stromal components and cancer cells, path-clearing through the ECM, and reorganization of the ECM matrix (Figure 4C). For example, tumor cells can secrete colony-stimulating factor and attract macrophages, which in turn secrete epidermal growth factor to guide tumor cells toward blood vessels, in a process termed macrophage-led tumor invasion [58,59]. In contrast to these findings, cell-non-autonomous chemoattractant may not be necessary for productive movement; a self generated chemotaxis gradient can be generated in the presence of even a small flow rate toward a draining lymph node [60] (Figure 4C). A non-paracrine cellular mechanism has also been described for stromal fibroblasts. In this form of single cell invasion, cancer cells undergo strand migration along tracks of extracellular matrix remodeled by leading fibroblast [61]. The importance of matrix remodeling is further underscored by observations that alignment and rigidity of collagen are key determinants of direction of tumor invasion [16,62]. Live cell imaging studies suggest that tumor cells traverse in parallel rather than perpendicular to collagen tracks as determined by second harmonic generation [62-64]. Some of these pathologic guidance mechanisms may also function to facilitate, restrict or direct normal branching process.
Conclusions
Real-time imaging of branching morphogenesis has enabled resolution of this complex tissue process into a series of events (Figure 1E-F and 2A). Insights from these studies raise a number of important questions. First, it remains an open question whether morphogenesis is autonomous to the luminal epithelial cells or requires the contributions of other cell types (Figure 3B). Second, it remains to be determined how similar the programs of branching morphogenesis are in different organs. Real-time imaging and organotypic culture provide a convenient framework within which to resolve the program of branching morphogenesis further, into discrete, molecularly-regulated changes in the behavior and properties of individual cells. The potential for stromal-epithelial recombinations and loss-of-function experiments ex vivo will enable identification of the critical cellular and molecular regulators of branching morphogenesis (Figure 3B). The combination of advanced cellular imaging and molecular genetics will enable us to distinguish whether there is a common developmental program unifying branching morphogenesis across organ systems.
Organotypic culture and time-lapse imaging also have the potential to elucidate the cellular and molecular basis of cancer invasion. As in the normal setting, it will be critical to build suitably realistic organotypic models for specific cancer subtypes and to develop reliable means to validate conclusions from these studies in human tissue samples. Just as developmental programs can differ between tissues, there may be different programs for epithelial morphogenesis in different cancers and cancer subtypes. Understanding the specific differences between the mechanisms driving cancer and developmental invasion may allow the identification of novel cancer-specific molecular regulators (Figure 4). More broadly, understanding the cellular mechanisms driving epithelial branching and invasion will enable a more nuanced understanding of the molecular mechanisms guiding these processes. Our goal is to determine how individual genes regulate branching morphogenesis through modification of discrete cellular behaviors and properties, within the context of a developing tissue and organ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sternlicht MD, Kouros-Mehr H, Lu P, Werb Z. Hormonal and local control of mammary branching morphogenesis. Differentiation. 2006;74:365–381. doi: 10.1111/j.1432-0436.2006.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;6:715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- 3.Howlin J, McBryan J, Martin F. Pubertal mammary gland development: insights from mouse models. J Mammary Gland Biol Neoplasia. 2006;11:283–297. doi: 10.1007/s10911-006-9024-2. [DOI] [PubMed] [Google Scholar]
- 4.Sternlicht MD. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res. 2006;8:201. doi: 10.1186/bcr1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinck L, Silberstein GB. Key stages in mammary gland development: the mammary end bud as a motile organ. Breast Cancer Res. 2005;7:245–251. doi: 10.1186/bcr1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams JM, Daniel CW. Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol. 1983;97:274–290. doi: 10.1016/0012-1606(83)90086-6. [DOI] [PubMed] [Google Scholar]
- 7.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 8.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 9.Weigelt B, Bissell MJ. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Semin Cancer Biol. 2008;18:311–321. doi: 10.1016/j.semcancer.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fata JE, Mori H, Ewald AJ, Zhang H, Yao E, Werb Z, Bissell MJ. The MAPK(ERK-1,2) pathway integrates distinct and antagonistic signals from TGFalpha and FGF7 in morphogenesis of mouse mammary epithelium. Dev Biol. 2007 doi: 10.1016/j.ydbio.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagios C, Lochter A, Bissell MJ. Tissue architecture: the ultimate regulator of epithelial function? Philos Trans R Soc Lond B Biol Sci. 1998;353:857–870. doi: 10.1098/rstb.1998.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radisky D, Hagios C, Bissell MJ. Tumors are unique organs defined by abnormal signaling and context. Semin Cancer Biol. 2001;11:87–95. doi: 10.1006/scbi.2000.0360. [DOI] [PubMed] [Google Scholar]
- 14.Egeblad M, Nakasone ES, Werb Z. Tumors as Organs: Complex Tissues that Interface with the Entire Organism. Developmental Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson CM, Bissell MJ. Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin Cancer Biol. 2005;15:342–352. doi: 10.1016/j.semcancer.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development. 2001;128:3117–3131. doi: 10.1242/dev.128.16.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005;132:3923–3933. doi: 10.1242/dev.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, Soloway P, Itohara S, Werb Z. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J Cell Biol. 2003;162:1123–1133. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson CM, Inman JL, Bissell MJ. Three-dimensional lithographically defined organotypic tissue arrays for quantitative analysis of morphogenesis and neoplastic progression. Nat Protoc. 2008;3:674–678. doi: 10.1038/nprot.2008.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mori H, Gjorevski N, Inman JL, Bissell MJ, Nelson CM. Self-organization of engineered epithelial tubules by differential cellular motility. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0901269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Provenzano PP, Inman DR, Eliceiri KW, Trier SM, Keely PJ. Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophys J. 2008;95:5374–5384. doi: 10.1529/biophysj.108.133116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taddei I, Deugnier MA, Faraldo MM, Petit V, Bouvard D, Medina D, Fassler R, Thiery JP, Glukhova MA. Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol. 2008;10:716–722. doi: 10.1038/ncb1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsen M, Wei C, Yamada KM. Cell and fibronectin dynamics during branching morphogenesis. J Cell Sci. 2006;119:3376–3384. doi: 10.1242/jcs.03079. [DOI] [PubMed] [Google Scholar]
- 27.Chi X, Michos O, Shakya R, Riccio P, Enomoto H, Licht JD, Asai N, Takahashi M, Ohgami N, Kato M, et al. Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev Cell. 2009;17:199–209. doi: 10.1016/j.devcel.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shakya R, Watanabe T, Costantini F. The role of GDNF/Ret signaling in ureteric bud cell fate and branching morphogenesis. Dev Cell. 2005;8:65–74. doi: 10.1016/j.devcel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Srinivas S, Goldberg MR, Watanabe T, D'Agati V, al-Awqati Q, Costantini F. Expression of green fluorescent protein in the ureteric bud of transgenic mice: a new tool for the analysis of ureteric bud morphogenesis. Dev Genet. 1999;24:241–251. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<241::AID-DVG7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 30.Puri S, Hebrok M. Dynamics of embryonic pancreas development using real-time imaging. Dev Biol. 2007;306:82–93. doi: 10.1016/j.ydbio.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayward SW, Brody JR, Cunha GR. An edgewise look at basal epithelial cells: three-dimensional views of the rat prostate, mammary gland and salivary gland. Differentiation. 1996;60:219–227. doi: 10.1046/j.1432-0436.1996.6040219.x. [DOI] [PubMed] [Google Scholar]
- 32.Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–881. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- 33.Kadoya Y, Yamashina S. Cellular dynamics of epithelial clefting during branching morphogenesis of the mouse submandibular gland. Dev Dyn. 239:1739–1747. doi: 10.1002/dvdy.22312. [DOI] [PubMed] [Google Scholar]
- 34.Fukuda Y, Masuda Y, Kishi J, Hashimoto Y, Hayakawa T, Nogawa H, Nakanishi Y. The role of interstitial collagens in cleft formation of mouse embryonic submandibular gland during initial branching. Development. 1988;103:259–267. doi: 10.1242/dev.103.2.259. [DOI] [PubMed] [Google Scholar]
- 35.Rebustini IT, Myers C, Lassiter KS, Surmak A, Szabova L, Holmbeck K, Pedchenko V, Hudson BG, Hoffman MP. MT2-MMP-dependent release of collagen IV NC1 domains regulates submandibular gland branching morphogenesis. Dev Cell. 2009;17:482–493. doi: 10.1016/j.devcel.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daley WP, Gulfo KM, Sequeira SJ, Larsen M. Identification of a mechanochemical checkpoint and negative feedback loop regulating branching morphogenesis. Dev Biol. 2009;336:169–182. doi: 10.1016/j.ydbio.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onodera T, Sakai T, Hsu JC, Matsumoto K, Chiorini JA, Yamada KM. Btbd7 regulates epithelial cell dynamics and branching morphogenesis. Science. 329:562–565. doi: 10.1126/science.1191880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Hayward S, Cao M, Thayer K, Cunha G. Cell differentiation lineage in the prostate. Differentiation. 2001;68:270–279. doi: 10.1046/j.1432-0436.2001.680414.x. [DOI] [PubMed] [Google Scholar]
- 39.Kim N, Vu TH. Parabronchial smooth muscle cells and alveolar myofibroblasts in lung development. Birth Defects Res C Embryo Today. 2006;78:80–89. doi: 10.1002/bdrc.20062. [DOI] [PubMed] [Google Scholar]
- 40.Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008;453:745–750. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wellings SR, Jensen HM, Marcum RG. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975;55:231–273. [PubMed] [Google Scholar]
- 42.Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350:1430–1441. doi: 10.1056/NEJMra031301. [DOI] [PubMed] [Google Scholar]
- 43.Ewing J. Lectures on tumor pathology. edn 2nd Cornell University Medical School, Class of 1934; New York: 1933. [Google Scholar]
- 44.Pearson GW, Hunter T. Real-time imaging reveals that noninvasive mammary epithelial acini can contain motile cells. J Cell Biol. 2007;179:1555–1567. doi: 10.1083/jcb.200706099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lerwill MF. Current practical applications of diagnostic immunohistochemistry in breast pathology. Am J Surg Pathol. 2004;28:1076–1091. doi: 10.1097/01.pas.0000126780.10029.f0. [DOI] [PubMed] [Google Scholar]
- 46.Gudjonsson T, Adriance MC, Sternlicht MD, Petersen OW, Bissell MJ. Myoepithelial cells: their origin and function in breast morphogenesis and neoplasia. J Mammary Gland Biol Neoplasia. 2005;10:261–272. doi: 10.1007/s10911-005-9586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polyak K, Hu M. Do myoepithelial cells hold the key for breast tumor progression? J Mammary Gland Biol Neoplasia. 2005;10:231–247. doi: 10.1007/s10911-005-9584-6. [DOI] [PubMed] [Google Scholar]
- 48.Sternlicht MD, Barsky SH. The myoepithelial defense: a host defense against cancer. Med Hypotheses. 1997;48:37–46. doi: 10.1016/s0306-9877(97)90022-0. [DOI] [PubMed] [Google Scholar]
- 49.Sternlicht MD, Kedeshian P, Shao ZM, Safarians S, Barsky SH. The human myoepithelial cell is a natural tumor suppressor. Clin Cancer Res. 1997;3:1949–1958. [PubMed] [Google Scholar]
- 50.Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, Richardson A, Violette S, Nikolskaya T, Nikolsky Y, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13:394–406. doi: 10.1016/j.ccr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 53.Sahai E, Wyckoff J, Philippar U, Segall JE, Gertler F, Condeelis J. Simultaneous imaging of GFP, CFP and collagen in tumors in vivo using multiphoton microscopy. BMC Biotechnol. 2005;5:14. doi: 10.1186/1472-6750-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedl P, Noble PB, Walton PA, Laird DW, Chauvin PJ, Tabah RJ, Black M, Zanker KS. Migration of coordinated cell clusters in mesenchymal and epithelial cancer explants in vitro. Cancer Res. 1995;55:4557–4560. [PubMed] [Google Scholar]
- 55.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–1296. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 57.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 59.Patsialou A, Wyckoff J, Wang Y, Goswami S, Stanley ER, Condeelis JS. Invasion of human breast cancer cells in vivo requires both paracrine and autocrine loops involving the colony-stimulating factor-1 receptor. Cancer Res. 2009;69:9498–9506. doi: 10.1158/0008-5472.CAN-09-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–538. doi: 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 61.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 62.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- ••.Onodera T, Sakai T, Hsu JC, Matsumoto K, Chiorini JA, Yamada KM. Btbd7 regulates epithelial cell dynamics and branching morphogenesis. Science. 2010 Jul 30;329(5991):562–5. doi: 10.1126/science.1191880. This paper demonstrates the novel expression of Btbd7 within the clefts of salivary epithelium. The authors suggests a molecular mechanism for epithelial clefting whereby fibronectin induces Btbd7, which in turn suppresses E-cadherin dependant cell adhesions and induces Snail dependent epithelial motility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009 Nov;11(11):1287–96. doi: 10.1038/ncb1973. Epub 2009 Oct 18. This paper demonstrates that single cell and collective invasion can coexist in the same tumor, and that TGFbeta signaling is necessary and sufficient to induce transition to single cell motility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••.Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, Richardson A, Violette S, Nikolskaya T, Nikolsky Y, Bauerlein EL, Hahn WC, Gelman RS, Allred C, Bissell MJ, Schnitt S, Polyak K. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008 doi: 10.1016/j.ccr.2008.03.007. In this paper, a model of human DCIS is used to demonstrate functional evidence for myoepithelial cells in restraining tumor progression from preinvasive DCIS to invasive ductal carcinoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nature Cell biology. 2007 doi: 10.1038/ncb1658. In a series of elegant experiments in organotypic culture, this study describes a mechanism by which the fibroblast can facilitate invasion, via remodeling of the extracellular matrix. [DOI] [PubMed] [Google Scholar]
- ••.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007 Aug;9(8):893–904. doi: 10.1038/ncb1616. In this paper, the transition from single cell to collective invasion is imaged by time-lapse microscopy, and a role for pericellular proteolysis is established to be critical to the formation of wide tracks for collective movement. [DOI] [PubMed] [Google Scholar]
- ••.Chi X, Michos O, Shakya R, Riccio P, Enomoto H, Licht JD, Asai N, Takahashi M, Ohgami N, Kato M, Mendelsohn C, Costantini F. Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev Cell. 2009 Aug;17(2):199–209. doi: 10.1016/j.devcel.2009.07.013. This paper discusses molecular evidence for Ret signaling in the clustering of pre-bud initiation in the kidney and illustrates the cell behaviors associated with initiation of the ureteric bud epithelium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. This paper demonstrated that mammary ductal elongation utilizes a novel form of collective cell migration and uncovers dynamic interactions of luminal and myo- epithelial cell types during branching morphogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••.Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008 Jun 5;453(7196):745–50. doi: 10.1038/nature07005. This paper uses careful analysis of fixed whole lung tissues to suggest that lung branching morphogenesis is highly stereotyped and that a complex global branching pattern can be generated through iterative deployment of a series of simplified branching modules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••.Larsen M, Wei C, Yamada KM. Cell and fibronectin dynamics during branching morphogenesis. J Cell Sci. 2006 Aug 15;119(Pt 16):3376–84. doi: 10.1242/jcs.03079. Epub 2006 Aug 1. PubMed PMID: 16882689. These references was the first report of the dynamic cellular rearrangements that underlie salivary gland epithelial morphogenesis. [DOI] [PubMed] [Google Scholar]
- ••.Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. This paper illustrates that physical geometries and diffusion gradients can instruct branching morphogenesis and developed novel bioengineering based 3D culture assays. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009 Nov 25;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. This paper describes how matrix stiffening promotes integrin mediated invasion, and shows that loss of stiffening via inhibition of LOX impedes invasion and metastasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007 Mar 15;67(6):2649–56. doi: 10.1158/0008-5472.CAN-06-1823. This paper describes the use of multiphoton microscopy to provide direct time-lapse evidence of tumor cell intravasation in association with perivascular macrophages. [DOI] [PubMed] [Google Scholar]
- •.Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, Sahai E, Condeelis JS, Gertler FB. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell. 2008 Dec;15(6):813–28. doi: 10.1016/j.devcel.2008.09.003. This paper demonstrates that a protein isoform of Mena can magnify response to EGF-mediated cell invasion thus facilitating productive chemotaxis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007 Jun;11(6):526–38. doi: 10.1016/j.ccr.2007.04.020. In this study, fluid flow potentiated by lymphatic drainage is shown to be sufficient for productive cancer cell invasion using only autocrine signaling. [DOI] [PubMed] [Google Scholar]
- •.Provenzano Paolo P., Inman David R., Eliceiri Kevin W., Trier Steven M., Keely Patricia J. Contact Guidance Mediated Three-Dimensional Cell Migration is Regulated by Rho/ROCK-Dependent Matrix Reorganization. Biophys J. 2008 December 1;95(11):5374–5384. doi: 10.1529/biophysj.108.133116. This paper provides evidence in 3D culture that collagen fibril orientation is a major variable in tumor cell invasion and migration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Patsialou A, Wyckoff J, Wang Y, Goswami S, Stanley ER, Condeelis JS. Invasion of human breast cancer cells in vivo requires both paracrine and autocrine loops involving the colony-stimulating factor-1 receptor. Cancer Res. 2009 Dec 15;69(24):9498–506. doi: 10.1158/0008-5472.CAN-09-1868. This paper demonstrates that paracrine signaling from macrophages and autocrine signaling from tumor cells play dual roles in supporting invasion in a human mammary tumor model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Kadoya Y, Yamashina S. Cellular dynamics of epithelial clefting during branching morphogenesis of the mouse submandibular gland. Dev Dyn. 2010 Jun;239(6):1739–47. doi: 10.1002/dvdy.22312. PubMed PMID: 20503369. This paper uncovers a novel cellular mechanism associated with salivary gland clefting. [DOI] [PubMed] [Google Scholar]
- •.Puri S, Hebrok M. Dynamics of embryonic pancreas development using real-time imaging. Dev Biol. 2007 Jun 1;306(1):82–93. doi: 10.1016/j.ydbio.2007.03.003. This paper provides the first description of pancreas morphogenesis in culture and further highlights clustering behaviors of B-cells during pancreas morphogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Mori H, Gjorevski N, Inman JL, Bissell MJ, Nelson CM. Self-organization of engineered epithelial tubules by differential cellular motility. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0901269106. This paper highlight that differential expression of matrix metalloproteases can direct epithelial cell branching morphogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Rebustini IT, Myers C, Lassiter KS, Surmak A, Szabova L, Holmbeck K, Pedchenko V, Hudson BG, Hoffman MP. MT2-MMP-dependent release of collagen IV NC1 domains regulates submandibular gland branching morphogenesis. Dev Cell. 2009;17:482–493. doi: 10.1016/j.devcel.2009.07.016. This paper highlights a mechnism of active collagen IV processing during the formaiton and progression of salivary epithelial bifurcations. [DOI] [PMC free article] [PubMed] [Google Scholar]