Abstract
The genomes of Listeria spp. encode all but one of 25 enzymes required for the biosynthesis of adenosylcobalamin (AdoCbl; coenzyme B12). Notably, all Listeria genomes lack CobT, the nicotinamide mononucleotide:5,6-dimethylbenzimidazole (DMB) phosphoribosyltransferase (EC 2.4.2.21) enzyme that synthesizes the unique α-linked nucleotide N1-(5-phospho-α-D-ribosyl)-DMB (α-ribazole-5′-P, α-RP), a precursor of AdoCbl. We have uncovered a new pathway for the synthesis of α-RP in Listeria innocua that circumvents the lack of CobT. The cblT and cblS genes (locus tags lin1153 and lin1110) of L. innocua encode an α-ribazole (α-R) transporter and an α-R kinase, respectively. Results from in vivo experiments indicate that L. innocua depends on CblT and CblS activities to salvage exogenous α-R, allowing conversion of the incomplete corrinoid cobinamide (Cbi) into AdoCbl. Expression of the L. innocua cblT and cblS genes restored AdoCbl synthesis from Cbi and α-R in a Salmonella enterica cobT strain. LinCblT transported α-R across the cell membrane, but not α-RP or DMB. UV-visible spectroscopy and mass spectrometry data identified α-RP as the product of the ATP-dependent α-R kinase activity of LinCblS. Bioinformatics analyses suggest that α-R salvaging occurs in important Gram-positive human pathogens.
INTRODUCTION
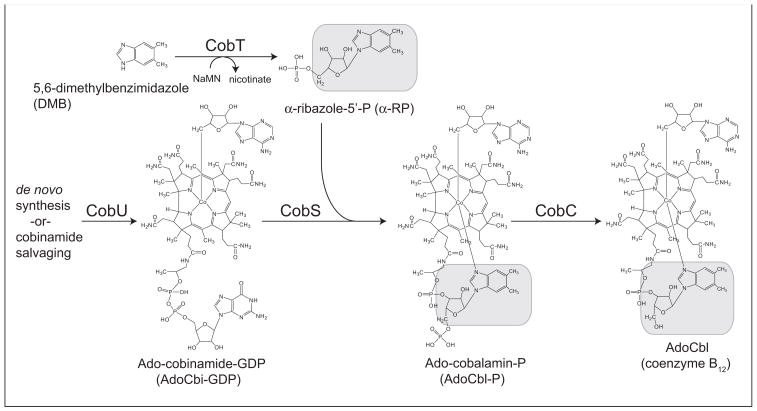
Cobamides, such as adenosylcobalamin (AdoCbl, Fig. 1) are complex cobalt-containing cyclic tetrapyrroles whose biosynthesis is restricted to bacteria and archaea (Escalante-Semerena & Warren, 2008). The lower ligand of AdoCbl is 5,6-dimethylbenzimidazole (DMB), a purine analog tethered to the corrin ring via a structure known as the nucleotide loop. Salmonella enterica assembles the nucleotide loop in four steps. One enzyme activates DMB, another activates the corrin ring, a third condenses the activated precursors, and a fourth yields AdoCbl, the final product of the pathway (Escalante-Semerena & Warren, 2008).
Figure 1. De novo assembly of the lower ligand of coenzyme B12 in S. enterica.
The α-R moiety is indicated with grey boxes. Abbreviations: CobT, nicotinate mononucleotide:5,6-dimethylbenzimidazole phosphoribosyltransferase; CobS, adenosylcobalamin-phosphate synthase; CobC, adenosylcobalamin-phosphate phosphatase; NaMN, nicotinate mononucleotide; Ado, 5′-deoxyadenosine; P, phosphate; GDP, guanosine diphosphate.
Most relevant to the work reported here is the activation of DMB. As shown in Fig. 1, the nicotinate mononucleotide (NaMN):DMB phosphoribosyltransferase (CobT, EC: 2.4.2.21) enzyme activates DMB into N1-(5-phospho-α-D-ribosyl)-DMB (α-ribazole-5′-P, α-RP). (Trzebiatowski et al., 1994; Cameron et al., 1991; Friedmann & Harris, 1965). Notably, the genomes of bacteria of the genus Listeria lack a cobT homolog (Hain et al., 2006; Buchrieser et al., 2003), raising the question of how Listeria compensates for the absence of CobT. Bioinformatics analysis performed by others (Rodionov et al., 2003) noted the lack of CobT in Listeria, and proposed that two genes of unknown function (dubbed cblT and cblS) might encode non-orthologous replacements for CobT. The authors of these studies hypothesized that the putative CblS protein might have CobT-like activity, and that the putative CblT protein might be a DMB transporter (Rodionov et al., 2009; Rodionov et al., 2003).
We have identified the biochemical activities of CblT and CblS from Listeria innocua, and provide a physiological framework for their activities. We show that LinCblT is an α-ribazole (αR) transporter and LinCblS is α-R kinase. Together, LinCblT and LinCblS comprise a new pathway for salvaging α-R and for the CobT-independent synthesis of α-RP. The distribution and possible implications of this new pathway amongst AdoCbl producers is discussed.
RESULTS
L. innocua cblT and cblS functions allow a S. enterica cobT strain to synthesize α-RP
We initially took a genetic approach to investigate the function of LinCblT and LinCblS. In these experiments, we used S. enterica cobT strains to block the synthesis of α-RP (Trzebiatowski et al., 1994). All S. enterica strains lacked the Cbl-independent methionine synthase (MetE) enzyme, so methionine synthesis depended on the Cbl-dependent methionine synthase (MetH) enzyme (Jeter et al., 1984). S. enterica strains were grown under aerobic conditions to block de novo synthesis of the corrin ring (Escalante-Semerena & Roth, 1987), but the medium was supplemented with dicyanocobinamide ([CN]2Cbi), a precursor of AdoCbl whose conversion to AdoCbl required the synthesis of α-RP.
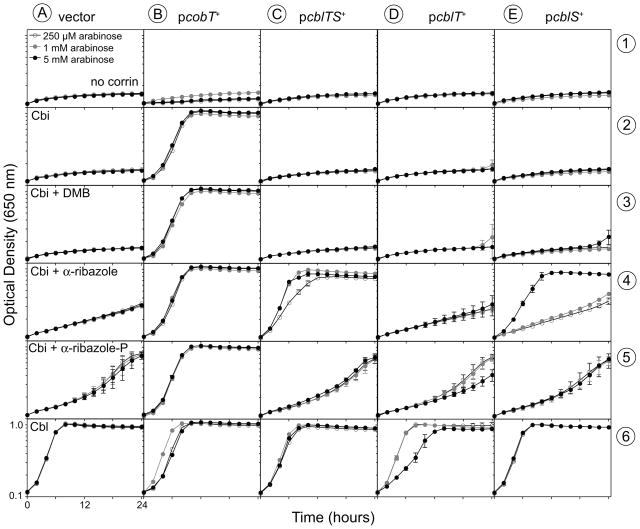
The Lin cblT and cblS genes (locus tags lin1153 and lin1110, respectively) were cloned individually or together into plasmid pBAD24, placing them under the control of the arabinose-inducible PBAD promoter (Guzman et al., 1995). The resulting plasmids were transformed into S. enterica cobT strain JE1244 (Table S1), a strain unable to make α-RP (Fig. 1). As expected, robust growth of strain JE1244 carrying plasmid pBAD24 was restored by the addition of CNCbl to the medium (Fig. 2, column A, row 6), but not in medium containing (CN)2Cbi (Fig. 2, column A, row 2), or (CN)2Cbi + DMB (Fig. 2, column A, row 3). Poor growth was obtained in medium containing (CN)2Cbi + α-R (Fig. 2, column A, row 4). A culture of strain JE1244 reached the same density in medium supplemented with (CN)2Cbi + α-RP or CNCbl. However, growth with (CN)2Cbi + α-RP occurred at a slower rate (Fig. 2, column A, rows 5, 6), suggesting that α-RP was inefficiently taken up by S. enterica. Positive control experiments showed that a plasmid-encoded cobT+ allele restored AdoCbl synthesis in strain JE1244, allowing growth in medium supplemented with (CN)2Cbi, with or without addition of DMB, α-R, or α-RP (Fig. 2, column B).
Figure 2. α-R salvaging in S. enterica expressing cblT and cblS alleles from L. innocua.
Corrinoid-dependent aerobic growth of S. enterica JE1244 (metE205 ara-9 cobT10::Tn10d[tet+]) derivatives in NCE containing glycerol (22 mM), MgSO4 (1 mM), trace minerals, ampicillin (100 μg ml−1), and arabinose (as indicated). Optical density at 650 nm was measured for 24 h at 37°C. Corrinoids were added at 15 nM. DMB, α-R, and α-RP were added at 250 nM. Plasmids used were: vector, pBAD24; pcobT+, pCOBT48 (S. enterica cobT+); pcblTS+, pCBLTS1 (Lin cblT+ cblS+); pcblT+, pCBLT1 (Lin cblT+); pcblS+, pCBLS4 (Lin cblS+). Growth curves were obtained using an ELx808 Ultra Microplate reader (Bio-Tek Instruments). Each growth curve was performed in triplicate. Error bars of one standard deviation are indicated.
A plasmid encoding Lin cblT+ and cblS+ (pCBLTS1) did not improve the growth of JE1244 in medium containing (CN)2Cbi, (CN)2Cbi + DMB, or (CN)2Cbi + α-RP, even at high levels of induction (Fig. 2, column C, rows 2, 3, and 5). The latter results indicated that the LinCblT and LinCblS proteins did not have CobT-like activity. In contrast, even at a low concentration of inducer (250 μM arabinose), plasmid pCBLTS1 (Lin cblTS+) restored AdoCbl synthesis in strain JE1244 growing in medium containing (CN)2Cbi + α-R (Fig. 2, column C, row 4). Plasmid pCBLT1 (Lin cblT+) did not support growth of strain JE1244 under the conditions tested (Fig. 2, column D), and even caused a slight inhibitory effect when expressed at high levels (5 mM arabinose) in medium containing CNCbl (Fig. 2, column D, row 6). Expression of plasmid-encoded Lin cblS+ (pCBLS4) restored AdoCbl synthesis in strain JE1244 in medium supplemented with (CN)2Cbi + α-R, but only when high level of inducer (5 mM arabinose) was present in the medium (Fig. 2, column E, row 4).
L. innocua cannot synthesize α-RP, but can salvage α-R
The above results suggested that L. innocua, which naturally lacks CobT, might rely on exogenous α-R for AdoCbl biosynthesis. To test this hypothesis, we developed a nitrogen-limited defined medium for growth of L. innocua (MLM, Table S2). The only nitrogen sources in MLM were low concentrations of adenine and the required amino acids cysteine, leucine, isoleucine, and valine. We tested whether wild-type L. innocua could use ethanolamine as a nitrogen source, which would require the activity of ethanolamine ammonia-lyase, an AdoCbl-dependent enzyme (Babior, 1982).
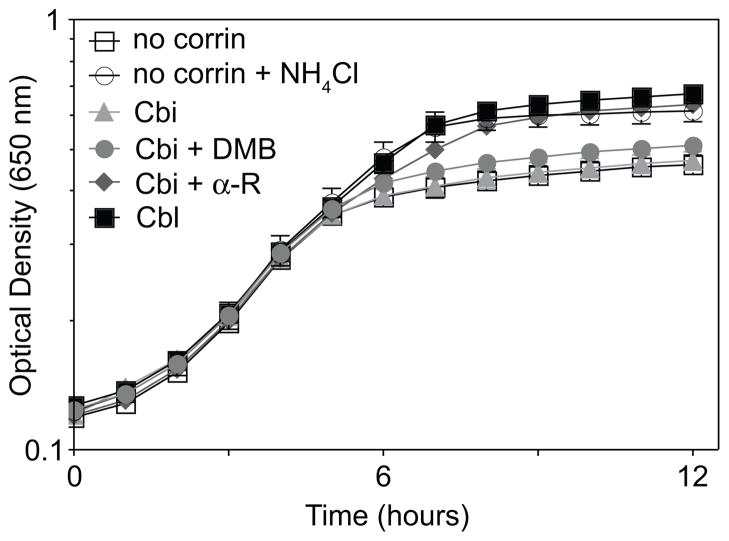
Under the conditions used, the cell density of a culture of L. innocua growing with ethanolamine as a nitrogen source was substantially higher when the medium was supplemented with CNCbl or NH4Cl (Fig. 3, black squares, open circles vs. open squares). When (CN)2Cbi substituted for CNCbl in the medium, L. innocua grew poorly (Fig. 3, light grey triangles), suggesting that L. innocua could not synthesize α-RP de novo. Addition of DMB had only a very slight stimulatory effect (Fig. 3, grey circles). L. innocua grew well when provided with (CN)2Cbi and α-R (Fig. 3, dark grey diamonds), supporting the hypothesis that L. innocua contained a pathway for salvaging α-R from its environment.
Figure 3. Corrinoid-dependent ethanolamine utilization in L. innocua.
Aerobic growth of L. innocua DD680 in MLM containing ethanolamine (100 mM). Optical density at 650 nm was measured for 12 h at 37°C. Corrinoids were added at 15 nM, α-R was added at 500 nM, and NH4Cl was added at 1 g l−1. Growth curves were obtained using an ELx808 Ultra Microplate reader (Bio-Tek Instruments). Each growth curve was performed in triplicate. Error bars of one standard deviation are indicated.
A model for α-R salvaging
Ideas about the possible roles of the LinCblT and LinCblS proteins emerged from bioinformatics analyses. From the literature we knew that LinCblT was a member of the ECF class of vitamin transporters (Rodionov et al., 2003; Rodionov et al., 2009), and our own PSI-BLAST searches (Altschul et al., 1997) identified LinCblS as a member of the phosphoribosylaminoimidazole synthetase (PurM) ATP-binding protein superfamily (McCulloch et al., 2008, Li et al., 1999). From this information, we surmised that LinCblT and LinCblS might comprise a system for the uptake of α-R and its conversion to α-RP. To our knowledge, an α-R salvaging pathway has not been described in any organism. The putative functions of LinCblT and LinCblS proteins were investigated in vitro and in vivo.
LinCblT transports α-R across the cell membrane
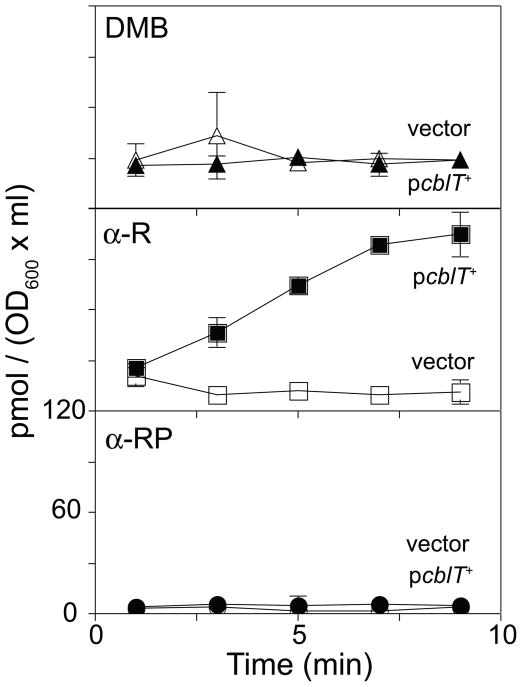
A S. enterica cobT strain expressing Lin cblT+ from a plasmid was grown to mid-log phase and tested for its ability to take up extracellular [14C, C-2]DMB, [14C, C-2]α-R, or [14C, C-2]α-RP. Expression of Lin cblT+ allowed S. enterica to accumulate α-R, but not DMB or α-RP (Fig. 4), indicating that the LinCblT protein was a specific α-R transporter. No DMB, α-R, or α-RP accumulated in a strain lacking Lin cblT (Fig. 4).
Figure 4. LinCblT is an α-R transporter.
Accumulation of radiolabeled compounds by S. enterica strains JE8511 (vector; open symbols; metE205 ara-9 cobT10::Tn10d[tet+]/pBAD24 [bla+]) and JE12550 (pcblT+; filled symbols; metE205 ara-9 cobT10::Tn10d[tet+]/pCBLT1 [cblT+ bla+]) in NCE containing glycerol (22 mM), MgSO4 (1 mM), trace minerals, ampicillin (100 μg ml−1), and arabinose (250 μM). At time zero, [14C]DMB (top panel), [14C]α-R (middle panel), or [14C]α-RP (bottom panel) was added to exponentially growing cells to a concentration of 250 nM. Each accumulation curve was performed in triplicate. Error bars of one standard deviation are indicated.
The LinCblS protein catalyzes the ATP-dependent phosphorylation of α-R
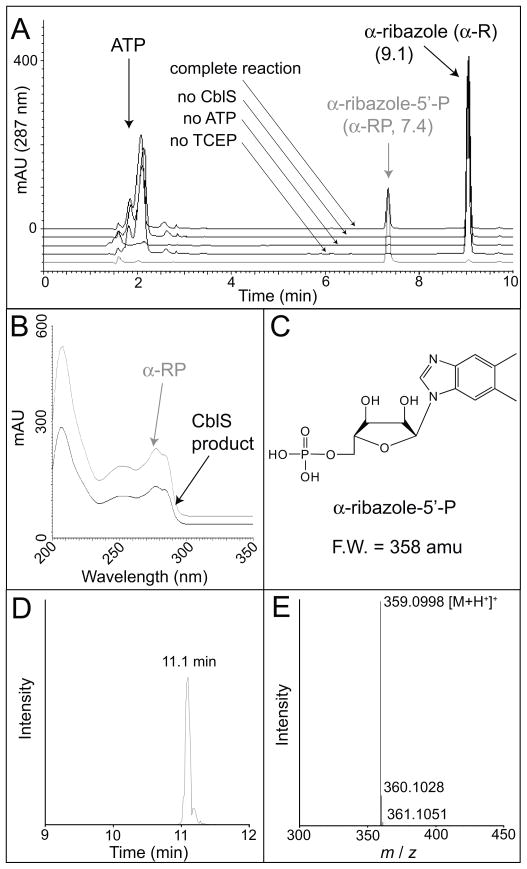
The LinCblS protein was overproduced in Escherichia coli as a fusion protein with an N-terminal H6 tag. H6-LinCblS protein was purified by Ni affinity chromatography, and the H6 tag was removed using rTEV protease (Rocco et al., 2008). A second Ni affinity chromatographic step yielded homogenous LinCblS with three non-native N-terminal residues (Gly-Ala-Ser) (Fig. S1). LinCblS (10 μg) was incubated with α-R (30 μM) and ATP (1 mM) for 3 h at 37°C in a 200-μl reaction mixture containing Tris-HCl (100 mM, pH 7.5 @ 25°C), MgCl2 (1 mM), KCl (50 mM), and TCEP (1 mM). Components of the reaction mixture were separated by reverse-phase HPLC. The product that accumulated had an elution time, UV-visible absorbance spectrum, and mass spectrum identical to those of authentic α-RP (Fig. 5). No detectable product was formed (<0.04 nmol min−1 mg−1 protein) in reactions mixtures lacking LinCblS, ATP, or TCEP (Fig. 5).
Figure 5. LinCblS is an ATP-dependent α-R kinase.
Reactions containing LinCblS (10 μg), Tris-HCl (100mM, pH 7.5), MgCl2 (1 mM), KCl (50 mM), α-R (30 μM), ATP (1 mM), and TCEP (1 mM) were incubated 3 h at 37°C. A. Products were separated by RP-HPLC with a 10-min linear gradient from 97% ammonium acetate (20 mM, pH 4.5) + 3% acetonitrile (CH3CN) to 40% CH3CN. Control reactions lacking LinCblS, ATP, or TCEP are indicated. The authentic α-RP control is shown in grey. B. UV-visible spectra of the LinCblS product (black trace) and authentic α-RP (grey trace). C. Structure and formula weight of α-RP. D. ESI-TOF LC/MS elution time of the LinCblS product, separated with a 20-min linear gradient from 100% formic acid in H2O (0.1% v/v), to 15% formic acid (0.1% v/v) in CH3CN. E. Mass spectrum of the LinCblS product, which was identical to that of authentic α-RP (not shown).
We optimized reaction conditions for the α-R kinase activity of LinCblS (Fig. S2). Optimal pH was pH 7.0 with highest activity at 35°C. KCl was required, with optimal activity measured at 750 mM KCl; the activity of the enzyme varied at > 750 mM KCl. LinCblS activity was optimal with 50 mM MgCl2; reduced activity was observed when CoCl2 or MnCl2 (50 mM) were used instead of MgCl2; activity was not observed with CaCl2, CuCl2, NiCl2, or ZnCl2. Under optimal conditions, product formation was linear up to 20 min, with 3, 5, or 7 μg of LinCblS in the reaction mixture.
DISCUSSION
CobT-independent synthesis of α-RP
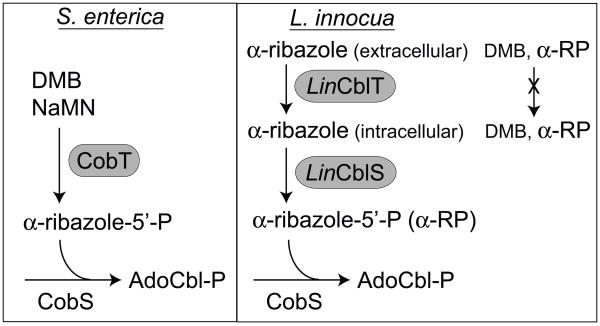
Prior to this work, there was no precedent in the literature for CobT-independent synthesis of α-RP. The genome of L. innocua does not encode a CobT homolog, the enzyme that synthesizes α-RP from DMB and NaMN. To circumvent this problem, L. innocua relies on an α-R transporter (LinCblT, encoded by lin1153) and an α-R kinase (LinCblS, encoded by lin1110) to synthesize α-RP. Thus LinCblT and LinCblS define a previously unknown pathway for α-R salvaging and α-RP synthesis (Fig. 6).
Figure 6. Comparison of lower ligand assembly in S. enterica and L. innocua.
Abbreviations: LinCblT, α-R transporter; LinCblS, α-R kinase; AdoCbl-P, adenosylcobalamin-phosphate. The right panel reflects the findings reported in figure 4, which indicate that, most likely, in L. innocua CblT does not transport DMB nor α-ribazole-P (α-RP) into the cell.
Not all bacteria encoding CblT or CblS homologs lack CobT (Table S3), suggesting that α-riboside salvaging and endogenous α-ribotide synthesis are not mutually exclusive. In fact, some genomes (e.g. Propionibacterium acnes, Moorella thermoacetica) encode two homologs of CobT; the significance of this apparent redundancy is unknown.
The results shown in Fig. 4 indicate that S. enterica, which is known to use exogenous DMB in AdoCbl biosynthesis (Escalante-Semerena & Warren, 2008), does not have a dedicated DMB importer. Based on results shown in figure 4, we also conclude that CblT does not function as a DMB importer, as previously proposed (Rodionov et al., 2003). Thus, the existence of an active DMB transporter in any organism remains an open question.
Genes encoding functions of the α-R salvaging pathway are found in Gram-positive human pathogens
Bioinformatics searches identified CblT and CblS homologs only in a subset of Gram-positive bacteria, among which are a number of significant human pathogens, including L. monocytogenes, Clostridium botulinum, C. tetani, and C. perfringens (Tables S3) (Markowitz et al., 2006). The cblT and cblS homologs of different species exist in different genetic contexts, and in many instances cblT and cblS are found in loci containing putative AdoCbl synthesis genes (Fig. S3).
We found three genomes (Bacillus halodurans, Desulfitobacterium hafniense, P. acnes), which encode CblS homologs, but not CblT homologs, and one (B. coahuilensis) that encodes a CblT homolog, but no CblS homolog. It is unclear why in some cases only one of these proteins is synthesized. It is possible that genomes that encode one but not the other protein contain non-orthologous replacements of the missing protein.
Multiple routes to the lower ligand
Some genomes that encode CblT or CblS homologs also encode BluB (O2-dependent, FMNH2-degrading DMB synthase) homologs (Table S3) (Taga et al., 2007; Gray & Escalante-Semerena, 2007). The presence of BluB suggests that, in the presence of oxygen, these bacteria synthesize DMB, and that α-RP is endogenously synthesized by CobT. The presence of CblT and CblS suggests an alternative means of synthesizing α-RP, possibly in response to changing oxygen levels. Eight genomes encoding CblT or CblS homologs also encoded corrinoid amidohydrolase (cobyric acid-forming) CbiZ homologs (Table S3), suggesting that these bacteria have cobamide remodeling capabilities to ensure that the correct lower ligand base is incorporated into the final product of the pathway (Gray et al., 2008; Gray & Escalante-Semerena, 2009).
What does the existence of CblTS tell us about the environment?
The existence of CblT and CblS, especially in bacteria that lack CobT, implies that there is a reliable supply of α-R and other α-ribosides to support cobamide biosynthesis in the habitats occupied by these bacteria. This idea needs to be investigated.
How do CblT and CblS work?
Alignment of 29 CblT homologs (Fig. S4) reveals a conserved Gly-Phe-Pro-Leu motif in a predicted cytoplasmic loop of CblT. We hypothesize that this motif may be involved in substrate recognition. CblS is homologous to the PurM protein superfamily, but alignment of LinCblS with the E. coli proteins of this family (PurM, ThiL, HypE, and SelD) shows few conserved residues (Fig. S5). Alignment of 32 CblS homologs revealed conserved motifs (Fig. S6), but their function is not clear. Detailed structure-function analyses of CblS are needed to understand the mechanism and substrate recognition properties of CblS. A better understanding of CblT and CblS function would be needed for the development of antimicrobial drugs targeting these proteins.
EXPERIMENTAL PROCEDURES
Bacterial strains and growth conditions
Strains and plasmids used in this study are listed in Table S1. E. coli strains were grown at 37°C in lysogenic broth (LB, Difco) (Bertani, 1951; Bertani, 2004). S. enterica strains were derived from strain TR6583 (metE205 ara-9). S. enterica strains were grown at 37°C in nutrient broth (NB, Difco) or no-carbon essential (NCE) minimal medium (Berkowitz et al., 1968) containing MgSO4 (1 mM), glycerol (22 mM), and trace minerals (Balch & Wolfe, 1976). Listeria innocua DD680 was grown at 37°C in brain heart infusion (BHI, Difco) or in MLM defined medium (minimal Listeria medium, Table S2). For growth curves, starter cultures were grown aerobically overnight in NB containing ampicillin (for S. enterica) or BHI (for L. innocua) and used to inoculate fresh medium (5% v/v). Growth curves were obtained using an ELx808 Ultra Microplate Reader (Bio-Tek Instruments) in a volume of 200 μl per well. When present, ampicillin was at 100 μg ml−1. When added, corrinoids were at 15 nM and DMB, α-R, and α-RP were at 250 or 500 nM, as indicated. Synthesis of α-R and α-RP is described below. All other chemicals were purchased from Sigma.
Preparation of α-R and α-RP
α-RP was prepared enzymatically from DMB and NaMN, using purified S. enterica NaMN:DMB phosphoribosyltransferase (CobT) enzyme, as described (Maggio-Hall & Escalante-Semerena, 1999; Trzebiatowski & Escalante-Semerena, 1997). α-R was prepared by alkaline hydrolysis of CNCbl by a modification of the method of Pakin et al. (Pakin et al., 2005); 0.1 g of CNCbl was incubated for 50 min at 100°C in 20 ml of 2.5 N NaOH, then neutralized by addition of 10 ml 5 N HCl. Alkaline phosphatase (1000 U) was added in 30 ml of 1 M Tris-HCl (pH 8.0), and incubated 48 h at 37°C. α-R was purified by reverse-phase liquid chromatography by binding to a 70-ml column of LiChroprep® RP-8 resin (EM Separations), previously equilibrated at 5 ml min−1 with H2O. The column was rinsed with 250 ml H2O, and then α-R was eluted off the column with a 450 ml linear gradient from 0 to 100% methanol (MeOH). Fractions containing α-R were identified by their characteristic fluorescence (excitation wavelength 250 nm, emission wavelength 312 nm) using a SpectraMAX Gemini EM spectrofluorimeter (Molecular Devices), dried under vacuum, and resuspended in 10 ml of dimethlysulfoxide (DMSO). Yield of α-R was approximately 8 mg. Samples of α-R were further purified by RP-HPLC, using a Beckman Coulter System Gold® 126 HPLC system equipped with a 250 × 10 mm Luna 5μ C18(2) column (Phenomenex); elution off the column was monitored using a photodiode array detector (λ 200 – 600 nm). The column was equilibrated at 3 ml min−1 with 70% H2O and 30% MeOH. Column development started 20 min after injection, with a 50-min linear gradient to 60% MeOH, followed by a 10-min linear gradient to 100% MeOH. Fractions containing α-R were collected, dried under vacuum, and re-suspended in DMSO. The identity of α-R was confirmed by mass spectrometry (University of Wisconsin-Madison Biotechnology Center) (Fig. S7). ESI mass spectra were obtained using an Applied Biosystems 3200 Q TRAP mass spectrometer. The molar extinction coefficients for DMB in DMSO (ε280 = 6027 M−1 cm−1) or in MeOH (ε280 = 5260 M−1 cm−1) (Maggio-Hall, 2001) were used to estimate the concentrations of purified α-R and α-RP in DMSO and MeOH.
Preparation of radiolabeled DMB, α-RP, and α-R
[C14, C-2]DMB (43.24 μCi/μmol) was prepared as described (Claas et al., 2010). [C14]α-RP (9.51 μCi/μmol) was synthesized from [C14-2]DMB and NaMN using S. enterica CobT as described (Maggio-Hall & Escalante-Semerena, 1999; Trzebiatowski & Escalante-Semerena, 1997). [C14, C-2]α-R was prepared from 10 nmoles of [C14, C-2]α-RP by addition of alkaline phosphatase (10 U) in 1 ml of Tris-HCl buffer (1 M, pH 8.0), incubation 24 h at 37°C, then bound to a 1 ml C18 Sep-Pak cartridge (Waters), rinsed with 40 ml H2O, eluted with MeOH, and dried under vacuum, and resuspended in H2O.
Genetic and molecular techniques
DNA manipulations were performed using described methods (Bloch, 1995; Moore & Dowhan, 2002; Struhl, 1987). Restriction and modification enzymes were purchased from Fermentas (Ontario, Canada) and used according to the manufacturer’s instructions. All DNA manipulations were performed in E. coli DH5α (Raleigh et al., 1989; Woodcock et al., 1989). Plasmid DNA was isolated using the Wizard Plus SV Plasmid Miniprep kit (Promega). PCR products were purified with the Wizard SV Gel and PCR Clean-Up System kit (Promega). Genomic DNA was isolated from bacterial cultures using the Wizard SV Genomic DNA Purification kit (Promega). DNA sequencing reactions used non-radioactive BigDyeR protocols (ABI PRISM; Applied Biosystems) and were resolved at the Biotechnology Center of the University of Wisconsin-Madison. Primers used in this study are listed in Table S4. The identity of all inserts cloned into plasmid pBAD24 (Guzman et al., 1995) was confirmed by sequencing with primers [1] and [2].
Construction of Lin cblT+ plasmid
The Lin cblT+ coding sequence was amplified using primers [3] and [4], and the resulting product was cloned into the EcoRI and XbaI sites of plasmid pBAD24 to yield plasmid pCBLT1.
Construction of Lin cblS+ plasmids
The Lin cblS+ coding sequence was amplified using primers [5] and [6], and the resulting product (with an ATG rather than the native TTG start codon) was cloned into the NheI and HindIII sites of plasmid pKLD116 (Rocco et al., 2008) to yield plasmid pCBLS3. The identity of the insert was confirmed by sequencing with primers [7] and [8]. The Lin cblS+ coding sequence plus 6 bp of 3′ sequence was excised from plasmid pCBLS3 and sub-cloned into the NheI and NotI sites of plasmid pTEV5 (Rocco et al., 2008) to yield plasmid pCBLS5. The identity of the insert was confirmed by sequencing with primers [8] and [9]. The Lin cblS+ coding sequence (with ATG start codon) was amplified using primers [10] and [11], and the resulting product was cloned into the EcoRI and HindIII sites of plasmid pBAD24 to yield plasmid pCBLS4.
Construction of Lin cblT+ cblS+ plasmid
The Lin cblT+ coding sequence was amplified using primers [12] and [13], and the Lin cblS+ coding sequence (with ATG start codon) was amplified using primers [14] and [15]. The resulting PCR products were cloned into the EcoRI and PstI sites of pBAD24 using the In-Fusion™ PCR Cloning System (Clontech) according to the manufacturer’s instructions, yielding plasmid pCBLTS1.
Transport assays
Overnight cultures of S. enterica strains grown in NB containing ampicillin were sub-cultured (10% v/v) into 5 ml NCE glycerol medium containing ampicillin, arabinose (250 μM), and CNCbl (15 nM), then grown at 37°C with shaking to an optical density (OD600) of 0.5 – 0.6. [14C]DMB, [14C]α-R, or [14C]α-RP was added to 250 nM, and cultures were incubated at 37°C. 200-μl samples were removed at intervals, filtered through 0.45-μm filter discs (Pall Life Sciences) under vacuum, and washed with 5 ml of ice-cold NCE medium. Filter discs were placed in 8 ml of Scinti-Safe scintillation fluid (Fisher Scientific), and cell-associated radioactivity was quantified with a Tri-Carb 2100TR liquid scintillation counter (Packard).
Purification of LinCblS protein
LinCblS protein fused to a rTEV protease cleavable N-terminal H6 tag was overproduced using plasmid pCBLS5 in E. coli BL21(DE3) (Novagen). 40 ml of an overnight culture of the overexpressing strain was inoculated into 2 liters of LB broth containing ampicillin. Cultures were grown 2 hours at 37°C with shaking, isopropyl-β-D-thiogalactopyranoside was added to 1 mM, and cultures were incubated for 20 h at 15°C with shaking. Cells were harvested by centrifugation (15 min at 5,000 × g at 4°C), resuspended in 10 ml of Tris-HCl buffer (20 mM, pH 7.9 at 4°C) containing NaCl (0.5 M) and imidazole (5 mM), and broken by sonication with a Fisher Scientific Sonic Dismembrator 550 (5 min at half duty). Cell lysate was cleared by centrifugation (1 h at 14,000 g at 4°C) and filtered through a 0.45-μm syringe filter (Nalgene). Tagged LinCblS protein was purified using His-Bind® resin (Novagen) according to the manufacturer’s instructions. The H6 tag was removed by incubation for 3 h at 30°C with rTEV protease (Kapust & Waugh, 2000) present in the buffer at a 1:10 ratio of LinCblS:rTEV protease. H6-rTEV protease was resolved from de-tagged LinCblS protein by passing the protein mixture over His-Bind® resin. The purity of proteins was monitored by SDS-PAGE (Laemmli, 1970) and Coomassie Blue staining (Sasse, 1991). Fractions containing de-tagged LinCblS protein were pooled, dialyzed (MWCO = 10,000 membrane, Pierce) at 4°C against 2 liters of Tris-HCl buffer (20 mM, pH 7.9 at 4°C) containing NaCl (50 mM) and glycerol (10% v/v) with three buffer changes, and stored at −80°C after flash freezing in liquid N2. Protein purity was assessed using the TotalLab software package (Nonlinear Dynamics Ltd).
α-R kinase activity assays
LinCblS activity was assayed in 200-μl reaction mixtures containing 3 – 10 μg of LinCblS. Reactions contained Tris-HCl buffer (100 mM, pH 7.0 @ 25°C), tris(2-carboxyethyl)phosphine (5 mM), KCl (0.75 M), MgCl2 (50 mM), α-R (60 μM), and ATP (1 mM), unless otherwise indicated. Where indicated, MgCl2 was replaced with 50 mM CaCl2, CoCl2, CuCl2, MnCl2, NiCl2, or ZnCl2. Buffers (100 mM) used to assess pH optimum were: 2-(N-morpholino)ethanesulfonic acid for pH 5.5, 6, and 6.5; Tris-HCl for pH 7, 7.5, 8, 8.5, and 9. Reactions were incubated at the indicated temperatures and stopped by incubation at 100°C for 10 min. Precipitated protein was removed by centrifugation (2 min at 7,500 × g) and samples were filtered with Spin-X filtration columns (2-μm pore size) (Costar).
HPLC analysis of LinCblS reaction products
The product of the LinCblS reaction was resolved by a modification of Phenomenex HPLC application #15754 using a Beckman Coulter System Gold® 126 HPLC system equipped with a Beckman Coulter System Gold® 508 autosampler and a Phenomenex 150 × 4.6 mm Synergi 4μ Hydro-RP column. Products were detected by their absorbance at 287 nm using a photodiode array detector. The column was equilibrated at 1 ml min−1 with 97% 20 mM ammonium acetate, pH 4.5 and 3% acetonitrile (CH3CN). After injection, the column was developed for 10 min with a linear gradient to 40% CH3CN, then developed for 5 min with a linear gradient to 100% CH3CN. α-RP was quantified by comparison with a standard curve of α-R (limit of detection = 10 pmol). The identity of the LinCblS reaction product was confirmed by liquid chromatography mass spectrometry (University of Wisconsin-Madison Biotechnology Center), using a using an Agilent LC/MSD ESI-TOF with a mass accuracy of greater than 3 ppm. Separation was performed with an Agilent 1100 LC with a 2.1 × 50 mm Zorbax SB-C18 column (1.8 μm particle size), using a 20 min gradient of 100% solvent A (0.1% formic acid in water) to 15% solvent B (0.1% formic acid in acetonitrile). Reference masses of 121.05087 and 922.0098 amu from the Agilent API TOF reference mass solution kit were used as a lock mass standard.
Supplementary Material
Acknowledgments
This work was supported by USPHS grant GM40313 (to J.C.E.-S.). We thank Kathryn Boor (Cornell University) for the gift of L. innocua DD680, and Kyle Hasenstein for technical assistance; Kathy Claas synthesized radiolabeled DMB.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Miller W, Lipmann DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior BM. Ethanolamine ammonia-lyase. In: Dolphin D, editor. B12. New York: John Wiley & Sons; 1982. pp. 263–288. [Google Scholar]
- Balch WE, Wolfe RS. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl Environ Microbiol. 1976;32:781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz D, Hushon JM, Whitfield HJ, Jr, Roth J, Ames BN. Procedure for identifying nonsense mutations. J Bacteriol. 1968;96:215–220. doi: 10.1128/jb.96.1.215-220.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani G. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J Bacteriol. 2004;186:595–600. doi: 10.1128/JB.186.3.595-600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch KD. Restriction endonucleases. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. Hoboken, NJ: John Wiley & Sons, Inc; 1995. pp. 3.1.1–3.1.21. [Google Scholar]
- Buchrieser C, Rusniok C, Kunst F, Cossart P, Glaser P. Comparison of the genome sequences of Listeria monocytogenes and Listeria innocua: clues for evolution and pathogenicity. FEMS Immunol Med Microbiol. 2003;35:207–313. doi: 10.1016/S0928-8244(02)00448-0. [DOI] [PubMed] [Google Scholar]
- Cameron B, Blanche F, Rouyez MC, Bisch D, Famechon A, Couder M, Cauchois L, Thibaut D, Debussche L, Crouzet J. Genetic analysis, nucleotide sequence, and products of two Pseudomonas denitrificans cob genes encoding nicotinate-nucleotide: dimethylbenzimidazole phosphoribosyltransferase and cobalamin (5′-phosphate) synthase. J Bacteriol. 1991;173:6066–6073. doi: 10.1128/jb.173.19.6066-6073.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claas KR, Parrish JR, Maggio-Hall LA, Escalante-Semerena JC. Functional analysis of the nicotinate mononucleotide:5,6-dimethylbenzimidazole phosphoribosyltransferase (CobT) enzyme, involved in the late steps of coenzyme B12 biosynthesis in Salmonella enterica. J Bacteriol. 2010;192:145–154. doi: 10.1128/JB.01159-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante-Semerena JC, Roth JR. Regulation of cobalamin biosynthetic operons in Salmonella typhimurium. J Bacteriol. 1987;169:2251–2258. doi: 10.1128/jb.169.5.2251-2258.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante-Semerena JC, Warren MJ. Biosynthesis and Use of Cobalamin (B12) In: Böck A, Curtiss R III, Kaper JB, Karp PD, Neidhardt FC, Nyström T, Slauch JM, Squires CL, editors. EcoSal - Escherichia coli and Salmonella: cellular and molecular biology. Washington, D. C: ASM Press; 2008. [Google Scholar]
- Friedmann HC, Harris DL. The formation of α-glycosidic 5′-nucleotides by a single displacement trans-N-glycosidase. J Biol Chem. 1965;240:406–412. [PubMed] [Google Scholar]
- Gray MJ, Escalante-Semerena JC. Single-enzyme conversion of FMNH2 to 5,6- dimethylbenzimidazole, the lower ligand of B12. Proc Natl Acad Sci U S A. 2007;104:2921–2926. doi: 10.1073/pnas.0609270104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MJ, Escalante-Semerena JC. The cobinamide amidohydrolase (cobyric acid- forming) CbiZ enzyme: a critical activity of the cobamide remodelling system of Rhodobacter sphaeroides. Mol Microbiol. 2009;74:1198–1210. doi: 10.1111/j.1365-2958.2009.06928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MJ, Tavares NK, Escalante-Semerena JC. The genome of Rhodobacter sphaeroides strain 2.4.1 encodes functional cobinamide salvaging systems of archaeal and bacterial origins. Mol Microbiol. 2008;70:824–836. doi: 10.1111/j.1365-2958.2008.06437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hain T, Steinweg C, Kuenne CT, Billion A, Ghai R, Chatterjee SS, Domann E, Karst U, Goesmann A, Bekel T, Bartels D, Kaiser O, Meyer F, Puhler A, Weisshaar B, Wehland J, Liang C, Dandekar T, Lampidis R, Kreft J, Goebel W, Chakraborty T. Whole-genome sequence of Listeria welshimeri reveals common steps in genome reduction with Listeria innocua as compared to Listeria monocytogenes. J Bacteriol. 2006;188:7405–7415. doi: 10.1128/JB.00758-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter RM, Olivera BM, Roth JR. Salmonella typhimurium synthesizes cobalamin (vitamin B12) de novo under anaerobic growth conditions. J Bacteriol. 1984;159:206–213. doi: 10.1128/jb.159.1.206-213.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapust RB, Waugh DS. Controlled intracellular processing of fusion proteins by TEV protease. Protein Expr Purif. 2000;19:312–318. doi: 10.1006/prep.2000.1251. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li C, Kappock TJ, Stubbe J, Weaver TM, Ealick SE. X-ray crystal structure of aminoimidazole ribonucleotide synthetase (PurM), from the Escherichia coli purine biosynthetic pathway at 2.5Å resolution. Structure. 1999;7:1155–1166. doi: 10.1016/s0969-2126(99)80182-8. [DOI] [PubMed] [Google Scholar]
- Maggio-Hall LA. Synthesis and incorporation of the lower ligand base of cobalamin. Department of Bacteriology. Madison: University of Wisconsin; 2001. p. 155. [Google Scholar]
- Maggio-Hall LA, Escalante-Semerena JC. In vitro synthesis of the nucleotide loop of cobalamin by Salmonella typhimurium enzymes. Proc Natl Acad Sci U S A. 1999;96:11798–11803. doi: 10.1073/pnas.96.21.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz VM, Korzeniewski F, Palaniappan K, Szeto E, Werner G, Padki A, Zhao X, Dubchak I, Hugenholtz P, Anderson I, Lykidis A, Mavromatis K, Ivanova N, Kyrpides NC. The integrated microbial genomes (IMG) system. Nucleic Acids Res. 2006;34:D344–348. doi: 10.1093/nar/gkj024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch KM, Kinsland C, Begley TP, Ealick SE. Structural studies of thiamin monophosphate kinase in complex with substrates and products. Biochemistry. 2008;47:3810–3821. doi: 10.1021/bi800041h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DD, Dowhan D. Preparation and analysis of DNA. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. Hoboken, NJ: John Wiley & Sons, Inc; 2002. pp. 2.0.1–2.12.17. [Google Scholar]
- Pakin C, Bergaentzle M, Aoude-Werner D, Hasselmann C. α-Ribazole, a fluorescent marker for the liquid chromatographic determination of vitamin B12 in foodstuffs. J Chromatogr A. 2005;1081:182–189. doi: 10.1016/j.chroma.2005.05.066. [DOI] [PubMed] [Google Scholar]
- Raleigh EA, Lech K, Brent R. Selected topics from classical bacterial genetics. In: Ausubel FA, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: Wiley Interscience; 1989. p. 1.4. [DOI] [PubMed] [Google Scholar]
- Rocco CJ, Dennison KL, Klenchin VA, Rayment I, Escalante-Semerena JC. Construction and use of new cloning vectors for the rapid isolation of recombinant proteins from Escherichia coli. Plasmid. 2008;59:231–237. doi: 10.1016/j.plasmid.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov DA, Hebbeln P, Eudes A, ter Beek J, Rodionova IA, Erkens GB, Slotboom DJ, Gelfand MS, Osterman AL, Hanson AD, Eitinger T. A novel class of modular transporters for vitamins in prokaryotes. J Bacteriol. 2009;191:42–51. doi: 10.1128/JB.01208-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS. Comparative genomics of the vitamin B12 metabolism and regulation in prokaryotes. J Biol Chem. 2003;278:41148–41159. doi: 10.1074/jbc.M305837200. [DOI] [PubMed] [Google Scholar]
- Sasse J. Detection of proteins. In: Ausubel FA, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: Wiley Interscience; 1991. pp. 10.16.11–10.16.18. [Google Scholar]
- Struhl K. Construction of hybrid DMA molecules. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. Hoboken, NJ: John Wiley & Sons, Inc; 1987. pp. 3.16.11–13.16.11. [Google Scholar]
- Taga ME, Larsen NA, Howard-Jones AR, Walsh CT, Walker GC. BluB cannibalizes flavin to form the lower ligand of vitamin B12. Nature. 2007;446:449–453. doi: 10.1038/nature05611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzebiatowski JR, Escalante-Semerena JC. Purification and characterization of CobT, the nicotinate-mononucleotide:5,6-dimethylbenzimidazole phosphoribosyltransferase enzyme from Salmonella typhimurium LT2. J Biol Chem. 1997;272:17662–17667. doi: 10.1074/jbc.272.28.17662. [DOI] [PubMed] [Google Scholar]
- Trzebiatowski JR, O’Toole GA, Escalante-Semerena JC. The cobT gene of Salmonella typhimurium encodes the NaMN: 5,6-dimethylbenzimidazole phosphoribosyltransferase responsible for the synthesis of N1-(5-phospho-alpha-D-ribosyl)-5,6-dimethylbenzimidazole, an intermediate in the synthesis of the nucleotide loop of cobalamin. J Bacteriol. 1994;176:3568–3575. doi: 10.1128/jb.176.12.3568-3575.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock DM, Crowther PJ, Doherty J, Jefferson S, De Cruz E, Noyer-Weidner M, Smith SS, Michael MZ, Graham MW. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucl Acids Res. 1989;17:3469–3478. doi: 10.1093/nar/17.9.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.